Professional Documents
Culture Documents
Test For Proteins
Test For Proteins
Uploaded by
diamonddia145Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Test For Proteins
Test For Proteins
Uploaded by
diamonddia145Copyright:
Available Formats
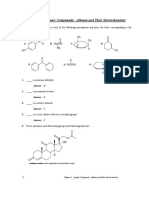
Test for proteins
DISCUSSION
• Proteins are AMPHOTERIC (can act as base and
an acid)- D. Reaction with concentrated Acids
• Acts as BUFFER (neutral pH) • 1ml of filtered albumin solution, HCl,
• High MP and soluble in POLAR solvents like ionic HNO3.
substances
• Denatured in heat, addition of acids, bases or
alcohols
o Denaturation-pagkamatay/sira ng
protein
• Flocculation is a reversible process while
E. Reaction with alcohol
coagulation in albumin is irreversible
• Increasing concentration, greater
coagulation
• 10ml of 95%, 70% and 40% ethanol, 2ml
A. Reaction of heat
egg albumin
+ coagulation
➢ experts say 70% (concentration) is
actually better for disinfecting. It has
more water, which helps it to dissolve
more slowly, penetrate cells, and kill
bacteria
B. Reaction with Salts of Heavy Metals
Metals: CHROMIUM, LEAD, MERCURY, SPECIFIC TESTS
CADMIUM, ARSENIC
• Biuret Test
• Ninhydrin Test
Heavy metals cause precipitation of proteins • Xanthoproteic Test
• The more alkalinic the protein, • Hopkins Cole Test
greater chance of precipitation • Millon's Test
• 1% AgNO3, 5% HgCl2
A. Biuret Test
➢ General test for Proteins based on the
peptide bonds present.
o Reagent: 1% cupric sulfate and 10%
NaOH
(+) formation of Violet to pink complex due to the
coordination of cupric ion to basic solution
C. Reaction with Alkaloidal reagents
• 2ml of egg albumin, 1ml of saturated
picric acid, 10% tannic acid and TCA
• Place 1ml of the following solution: 1% gelatin, 1% egg
albumin, and 1% glycine
• Add 1ml of the biuret reagent (1ml of 10% NaOH and
2gtts of 1% cupric sulfate)
B. Ninhydrin Test
➢ Involves several complex reactions
between amino group and alpha Carbon
• Mix 10gtts of Hopkin's Cole Reagent to
and the ninhydrin solution.
1ml of the solution to be tested. 1%
(+: Appearance of intense blue colored solution)
albumin, 1% Casein, 1% Gelatin and 1%
Reagent: Ninhydrin reagent (0.1gm ninhydrin in 100ml
Tryptophan
of water)
• Add 10gtts of concentrated sulfuric acid
down the side of the inclined test tube.
E. Millon's Test
• Mix 1ml of 0.2% ninhydrin solution with 10 gtts of egg ➢ Specific test for proteins containing phenol
albumin, and alanine group in the side chains such as tyrosine
• Heat for several minutes in a water bath + formation of flesh to red-colored precipitate
Reagent: Millons Reagent: Dissolve 160 grams of
C. Xanthoproteic Test mercuric nitrate and 160 grams of mercurous
➢ Positive to Tyrosine, Tryptophan and nitrate in 400 ml concentrate nitric acid solution.
Phenylalanine. The reagent is then made to 1000 ml by the
➢ Specific test for Amino acids with addition of 600ml distilled water. The formula can
aromatic ring be adjusted to suit the performance parameters.
Concentrated Nitric Acid
+ appearance of yellow precipitate
• Add 5 gtts of concentrated nitric acid to 10 • Into each test tubes, place 1ml of the sample
drops of solution to be used in separate test • Add 10 gtts of Millon's Reagent, shake and boil in
tubes. Heat for several minutes in boiling water bath for 10 minutes
water bath
• Let it cool in cold water and add 10% NaOH
until the solution turns into alkaline
using litmus paper
D. Hopkins Cole Test
➢ Specific test for Trypthophan (indole-
ring containing)
Reagent: Hopkins Cole reagent (Glyoxylic
acid- It can be prepared by exposing
glacial acetic acid to sunlight for a few days
and Concentrated H2SO4)
+ Presence of purple ring
You might also like
- SSS 2 E-Note 3rd Term ChemistryDocument61 pagesSSS 2 E-Note 3rd Term ChemistryDave Blessed83% (6)
- EXPERIMENT 5 Robinson Annulation ReactionDocument13 pagesEXPERIMENT 5 Robinson Annulation ReactionMuhammad Amirul Afifi100% (1)
- Quiz On Lipid Metabolism - BIOCHEMISTRYDocument12 pagesQuiz On Lipid Metabolism - BIOCHEMISTRYAlliah Turingan100% (1)
- Laboratory Experiment 6 - Proteins (GROUP 4)Document14 pagesLaboratory Experiment 6 - Proteins (GROUP 4)Renee Dwi Permata Messakaraeng100% (1)
- Colour Reactions of ProteinsDocument7 pagesColour Reactions of ProteinsTARIQNo ratings yet
- 5550 Tannin and Lignin (2010) PDFDocument2 pages5550 Tannin and Lignin (2010) PDFCristian Perla MartinezNo ratings yet
- 4500-B Boron (2000) PDFDocument3 pages4500-B Boron (2000) PDFAntoninaPontes100% (1)
- Chem 528 - Activity 6Document4 pagesChem 528 - Activity 6Aries Jay ReyesNo ratings yet
- Biochemistry Lab Con ProteinsDocument47 pagesBiochemistry Lab Con Proteinsriana santosNo ratings yet
- Chemical Test For The Components of Nucleic Acid LABREPORTDocument4 pagesChemical Test For The Components of Nucleic Acid LABREPORT19 - CELENDRO ADVINNNo ratings yet
- GUID - 1 en-USDocument1 pageGUID - 1 en-USDilawar BakhtNo ratings yet
- Activity 6 Laboratory ProcedureDocument7 pagesActivity 6 Laboratory ProcedureAinie JuripaeNo ratings yet
- Principle:-Urease Specifically Acts On Urea To Liberate Ammonium CarbonateDocument17 pagesPrinciple:-Urease Specifically Acts On Urea To Liberate Ammonium CarbonateAmit kumarNo ratings yet
- Xanthan GumDocument2 pagesXanthan GumKasidit SornchaiNo ratings yet
- 7 Activity Protein PrecipitationDocument7 pages7 Activity Protein PrecipitationNicole Dane100% (1)
- GUID - 1 en-USDocument1 pageGUID - 1 en-USDilawar BakhtNo ratings yet
- 2011 02 25povidoneDocument3 pages2011 02 25povidonedini hanifaNo ratings yet
- Normal UrineDocument6 pagesNormal Urineabhay GiriNo ratings yet
- ANALYSIS AND DENATURATION OF PROTEINS AnswersDocument5 pagesANALYSIS AND DENATURATION OF PROTEINS AnswersdgfdgsdfgsdsdgNo ratings yet
- BL NurBio Activity 7 - Proteins Precipitation (REVISED 6.25.20)Document8 pagesBL NurBio Activity 7 - Proteins Precipitation (REVISED 6.25.20)Niño PadacaNo ratings yet
- Biochem Expt 3 4Document45 pagesBiochem Expt 3 4Lance FloresNo ratings yet
- Normal - Urine - Analysis 22 11 20018Document30 pagesNormal - Urine - Analysis 22 11 20018Vipin TakNo ratings yet
- Biochemistry Laboratory 1Document2 pagesBiochemistry Laboratory 1Janine Aura JarilloNo ratings yet
- Abx096002 IfuDocument3 pagesAbx096002 IfuLinh ĐỗNo ratings yet
- Frormal Report GlutenDocument3 pagesFrormal Report GlutenDeza SantosNo ratings yet
- CHEM330 Lab Manual With Video LinksDocument12 pagesCHEM330 Lab Manual With Video LinksWajeeha MumtazNo ratings yet
- Normal Constituents of UrineDocument4 pagesNormal Constituents of UrineVeshalineeNo ratings yet
- Dextrin: AssayDocument79 pagesDextrin: AssayChemicals MolarNo ratings yet
- Exp 2 Specific Test of A.ADocument19 pagesExp 2 Specific Test of A.AFahad BashirNo ratings yet
- Experiment 2 GRANDA JOHNDocument7 pagesExperiment 2 GRANDA JOHNKENT BENEDICT PERALESNo ratings yet
- GUID - 4 en-USDocument1 pageGUID - 4 en-USDilawar BakhtNo ratings yet
- Experiement 4Document6 pagesExperiement 4JharaNo ratings yet
- 4 Activity Carbohydrates IIDocument9 pages4 Activity Carbohydrates IIFelica Delos ReyesNo ratings yet
- Normal Constituents of UrineDocument5 pagesNormal Constituents of Urinenawejil351No ratings yet
- Acid Number of Petroleum Products InflectionDocument3 pagesAcid Number of Petroleum Products InflectionKeng JungNo ratings yet
- MZXCDocument4 pagesMZXCvodachemicals20No ratings yet
- Reactions of Protein-01!11!2018Document26 pagesReactions of Protein-01!11!2018Rdh MnbNo ratings yet
- CarbohydratesDocument13 pagesCarbohydrateser dequilatoNo ratings yet
- Vegetable TanninDocument6 pagesVegetable TanninAditi SharmaNo ratings yet
- Appendix-1 Estimation of Total CarbohydrateDocument46 pagesAppendix-1 Estimation of Total CarbohydrateRanjith Kumar100% (1)
- USP Calcium CarbonateDocument2 pagesUSP Calcium CarbonateAnnastasia PiyogoNo ratings yet
- Verhoeff's Iron Haematoxylin BMLT 5th SEMDocument1 pageVerhoeff's Iron Haematoxylin BMLT 5th SEMSuman MandalNo ratings yet
- Zinc GluconateDocument2 pagesZinc GluconateKasidit SornchaiNo ratings yet
- 10.3 Module 10 Lab Report Group 3Document6 pages10.3 Module 10 Lab Report Group 3princessfarah hussinNo ratings yet
- GUID - 4 en-USDocument1 pageGUID - 4 en-USDilawar BakhtNo ratings yet
- Midterm - CC1 LabDocument11 pagesMidterm - CC1 LabHazel Joyce Gonda RoqueNo ratings yet
- Histology Department, Our Lady of Victory Hospital, Lackawanna, New YorkDocument3 pagesHistology Department, Our Lady of Victory Hospital, Lackawanna, New YorksoundharyaNo ratings yet
- Ninhydrin TestDocument17 pagesNinhydrin TestAchrya KailashNo ratings yet
- Usp42-Nf37 2599Document1 pageUsp42-Nf37 2599RestiNo ratings yet
- Organic Benzyl AcetateDocument4 pagesOrganic Benzyl Acetatemoaz ahmadNo ratings yet
- Scheme For Organic AnalysisDocument3 pagesScheme For Organic Analysisameenahmed10927No ratings yet
- Biochem Lab Con NucleicDocument27 pagesBiochem Lab Con NucleicChristine Ho70% (10)
- Act 9Document3 pagesAct 9Rei Deller TagiobonNo ratings yet
- Qualitative Tests For Amino AcidsDocument15 pagesQualitative Tests For Amino AcidsA sisonNo ratings yet
- Barecuatro-Angelica Claire-Bsn11l-MidtermsDocument12 pagesBarecuatro-Angelica Claire-Bsn11l-MidtermsAngelica Claire BarecuatroNo ratings yet
- 1972-1973 Pregelatinized StarchDocument2 pages1972-1973 Pregelatinized StarchMaterial Science DivisionNo ratings yet
- Organic Lab Final Syllabus MergeDocument95 pagesOrganic Lab Final Syllabus MergeBisma Shafiq100% (1)
- Chemical Analysis of Proteins: Activity No. 5Document41 pagesChemical Analysis of Proteins: Activity No. 5Francis ValdezNo ratings yet
- FR2-Isolation of Proteins and Color ReactionDocument4 pagesFR2-Isolation of Proteins and Color ReactionKriziaoumo P. OrpiaNo ratings yet
- Act6 MCPH30Document3 pagesAct6 MCPH30masorNo ratings yet
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresFrom EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresRating: 5 out of 5 stars5/5 (1)
- The Chemistry of Dairy Products - A Chemical Analysis of Milk, Cream and ButterFrom EverandThe Chemistry of Dairy Products - A Chemical Analysis of Milk, Cream and ButterNo ratings yet
- Development Formulation: Lux Shampoo With APISCALP™ ( ) and Capilectine™ SP ( ) SED1410179JDocument2 pagesDevelopment Formulation: Lux Shampoo With APISCALP™ ( ) and Capilectine™ SP ( ) SED1410179JaliNo ratings yet
- Permatex Battery Protector & Sealer SDSDocument10 pagesPermatex Battery Protector & Sealer SDSMiles HoltNo ratings yet
- Vitamin Analysis: Definition and ImportanceDocument18 pagesVitamin Analysis: Definition and ImportanceManjusha KondepudiNo ratings yet
- Azo PigmentDocument9 pagesAzo PigmentTechn TecNo ratings yet
- IsomerismDocument21 pagesIsomerismkaransharma690No ratings yet
- AppNote 191Document6 pagesAppNote 191benyNo ratings yet
- Classification of Man-Made Fibers and Their CodesDocument2 pagesClassification of Man-Made Fibers and Their CodesFalgon IslamNo ratings yet
- IChO 2015 Preparatory ProblemsDocument73 pagesIChO 2015 Preparatory ProblemsTudor PipirigNo ratings yet
- Metals, Polymers, Ceramics and CompositesDocument80 pagesMetals, Polymers, Ceramics and Compositestharushisewwanndi43No ratings yet
- Green ChemistryDocument27 pagesGreen ChemistryMonika11Sharma100% (1)
- 24-Sour Gas ProcessingDocument24 pages24-Sour Gas ProcessingGlory Adekunle Tokede100% (2)
- Qualitative Salt AnalysisDocument11 pagesQualitative Salt AnalysisAditya ChoudharyNo ratings yet
- Analysis of SugarsDocument29 pagesAnalysis of SugarsFendi Zola HendrawanNo ratings yet
- Pfas Waste Water ADocument17 pagesPfas Waste Water AMohamedNo ratings yet
- 80 Most Repeated Botany MCQ Oc Competitive ExamDocument15 pages80 Most Repeated Botany MCQ Oc Competitive ExamNahid BukhariNo ratings yet
- 1.introduction To MetabolismDocument7 pages1.introduction To MetabolismProtusha RakshitNo ratings yet
- Pharmaceutical Packaging FinalDocument78 pagesPharmaceutical Packaging Finalmonoj5859No ratings yet
- QP Chemistry Pb2 Xii Set2Document13 pagesQP Chemistry Pb2 Xii Set2Yug GandhiNo ratings yet
- Simposium Prof KimDocument31 pagesSimposium Prof KimAsa Étudier La-DienNo ratings yet
- Çhapter 3Document6 pagesÇhapter 3Kelsi Kyla PeraltaNo ratings yet
- Genetic Mutations Practice WorksheetDocument3 pagesGenetic Mutations Practice WorksheetNoah HolznerNo ratings yet
- Fdocuments - in - Edaplan Metolat Guide Formulations For Organic Pigments Edaplan MetolatDocument26 pagesFdocuments - in - Edaplan Metolat Guide Formulations For Organic Pigments Edaplan MetolatLong An ĐỗNo ratings yet
- SECTION 22 15 00 General Service Compressed-Air SystemsDocument8 pagesSECTION 22 15 00 General Service Compressed-Air Systemsm2110No ratings yet
- Biomass EnergyDocument9 pagesBiomass EnergyHj JayatheerthaNo ratings yet
- Composition of Oil-Based Muds PDFDocument0 pagesComposition of Oil-Based Muds PDFA MahmoodNo ratings yet
- 05 Conformational Anal 2Document11 pages05 Conformational Anal 2Swati GautamNo ratings yet
- Evonik Sunmide CX 1151 UploadDocument6 pagesEvonik Sunmide CX 1151 UploadEpox by Epx polymers pvt ltdNo ratings yet
- What Are Organic ManuresDocument34 pagesWhat Are Organic ManuresPrit Ranjan Jha100% (4)