Professional Documents
Culture Documents
Determinationofcellconstant 191023045558
Determinationofcellconstant 191023045558
Uploaded by
saurabh07777777Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Determinationofcellconstant 191023045558
Determinationofcellconstant 191023045558
Uploaded by
saurabh07777777Copyright:
Available Formats
SHREE MALLIKARJUN COLLEGE CLASS: SYBSC
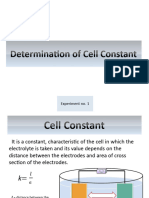
Aim: Determination of cell constant.
Chemicals: Potassium chloride.
Apparatus: Beakers, standard flask and glass rod.
Instrument: Conductivity cell
Theory: Conductivity (mho or ohm -1) of any solution depends on the nature of ions present in it.
The specific conductance is measure of conductance of solution with electrodes of unit area that
are place 1 cm apart. The specific conductance (K) is given by
K = (1/R) (l/a) as 1/R = C i.e. conductance
K = C (l/a)
l/a = K/C as l/a is constant called as ‘cell constant’
Cell constant = Specific conductance (ohm -1cm -1) / measured conductance (ohm -1)
Thus, the unit of cell constant is cm -1.
Procedure:
i) Calibrate the conductivity cell
Refer the instrument manual.
ii) Preparation of 0.1 M and 0.01N KCl solution
Prepare 0.1 N KCl by dissolving _____g in 100 mL distilled water. Pipette out 10 mL of 0.1 N
KCl and dilute to 100 mL to prepare 0.01N KCl solution
iii) Measuring conductance.
a) Measure the conductance of distilled water.
b) Dip the conductivity cell in 40 mL 0.1N KCl and record the conductance observed. Note down
the temperature. Wash the conductivity cell with distilled water and measure the conductance
for 0.01 N KCl. Tabulate the readings in Table 1.
Observations:
Room temperature =____ ◦C.
Conductance of distilled water ‘A’=______ ohm -1.
Table 1
Concentration of Specific Measured Observed Cell constant
KCl conductance conductance conductance (cm -1)
(mohm -1 cm -1) (ohm -1) (ohm -1)
‘B’ =B-A
0.1 12.88
0.01 1.413
Result: Cell constant =______ cm -1.
Further Reading
Experiments in Applied Chemistry, S. Rattan, S. K Kataria and Sons.
Dr. Mithil Fal Desai
You might also like
- Investigating The Kinetics of A Crystal Violet Reaction Prelab QuestionsDocument9 pagesInvestigating The Kinetics of A Crystal Violet Reaction Prelab QuestionsconnieNo ratings yet
- LMS Solutions ElectrochemistryDocument200 pagesLMS Solutions ElectrochemistrySai Rithvik Kanakamedala80% (10)
- SL Chemistry Ia 4Document12 pagesSL Chemistry Ia 4api-37363504650% (2)
- Faculty of Applied Sciences Electrochemistry Chm578 Laboratory Report Experiment 1: Galvanic CellDocument14 pagesFaculty of Applied Sciences Electrochemistry Chm578 Laboratory Report Experiment 1: Galvanic CellimizzNo ratings yet
- Cell ConductivityDocument3 pagesCell ConductivityKajal KadamNo ratings yet
- ConductivityDocument6 pagesConductivitydrag000nNo ratings yet
- Electro-Chemistry 2020 2022Document28 pagesElectro-Chemistry 2020 2022Vincent AnzoNo ratings yet
- All-Experiments 3RDDocument5 pagesAll-Experiments 3RDHawta AbdullaNo ratings yet
- ElectrochemistryDocument21 pagesElectrochemistryessaNo ratings yet
- Chemistry ChapterDocument8 pagesChemistry Chaptermaster .Rahul gautamNo ratings yet
- Experiment No. 6 Variation of Conductance With ConcentrationDocument16 pagesExperiment No. 6 Variation of Conductance With ConcentrationrizaNo ratings yet
- Electrochemistry Answer KeyDocument6 pagesElectrochemistry Answer KeyAriesMascarhenasNo ratings yet
- Expected Questions For Board Examination 2013Document34 pagesExpected Questions For Board Examination 2013Harsh AgarwalNo ratings yet
- ExampleDocument6 pagesExampleAbhinavNo ratings yet
- Shambhugowda Lecturer in ChemistryDocument30 pagesShambhugowda Lecturer in ChemistryAryan Sai ANo ratings yet
- 12th Unit 9 ElectrochemistryDocument6 pages12th Unit 9 ElectrochemistryThahseen AfzalNo ratings yet
- Conductivity of Water: Based On ISO Standard 7888:1985Document4 pagesConductivity of Water: Based On ISO Standard 7888:1985Sadagopan RajaNo ratings yet
- Cbse Test Paper-03 Class 12 Chemistry (Electrochemistry)Document8 pagesCbse Test Paper-03 Class 12 Chemistry (Electrochemistry)raghupredator2No ratings yet
- Electrolytic ConductanceDocument8 pagesElectrolytic Conductancevijaye36100% (1)
- Practic Le 6Document3 pagesPractic Le 6Mehul KhimaniNo ratings yet
- Unit1 ElectrochemistryDocument18 pagesUnit1 ElectrochemistryRajeshNo ratings yet
- 3.electrochemistry FDocument30 pages3.electrochemistry Fchemistrymohan123No ratings yet
- Conductometry SSzENDocument4 pagesConductometry SSzENbara copyNo ratings yet
- AbirDocument13 pagesAbirizarul islamNo ratings yet
- Redox Lab Report CompleteDocument15 pagesRedox Lab Report CompleteJackson KasakuNo ratings yet
- ElectrochemistryDocument6 pagesElectrochemistryaxiliya6No ratings yet
- Chm524 Experiment 5Document26 pagesChm524 Experiment 52022608166100% (1)
- Experiment 2 Cmt555Document8 pagesExperiment 2 Cmt555DIey ChokiEyNo ratings yet
- ECRE Lab Manual - 2021!1!4 ExperimentsDocument19 pagesECRE Lab Manual - 2021!1!4 ExperimentsRajachedambaram RajachedambaraNo ratings yet
- Instrumental AnalysisDocument15 pagesInstrumental AnalysisAbhiram ChandranNo ratings yet
- A. BS-6th-Ch1 (2022-09-19) (Conductometry)Document15 pagesA. BS-6th-Ch1 (2022-09-19) (Conductometry)Gulraiz JuttNo ratings yet
- 2023 JC2 H1 Chem BT MCQ MSDocument7 pages2023 JC2 H1 Chem BT MCQ MSemman tzhNo ratings yet
- CMT610 Eksp2Document21 pagesCMT610 Eksp2syahidnovovic4No ratings yet
- Potentiometry 2023 - PL - BPDocument58 pagesPotentiometry 2023 - PL - BPfojirof555No ratings yet
- ConductometryDocument9 pagesConductometryMuhammad FahmiNo ratings yet
- ConductivityDocument1 pageConductivityMicrotesting labNo ratings yet
- Experiment I1 Preparation of Some Cobaltammine ComplexesDocument7 pagesExperiment I1 Preparation of Some Cobaltammine ComplexesIftitah HauriyahNo ratings yet
- Chapter 7-2 ConductivityDocument17 pagesChapter 7-2 ConductivityNajmul Puda PappadamNo ratings yet
- BCP 503Document28 pagesBCP 503Vineet JhambNo ratings yet
- #4 Chem Lab Report - AgustinDocument6 pages#4 Chem Lab Report - AgustinSeth Jarl G. AgustinNo ratings yet
- Numerical ElectrochemistryDocument2 pagesNumerical ElectrochemistryS KhanNo ratings yet
- Unit-I Electrochemistry & CorrosionDocument38 pagesUnit-I Electrochemistry & CorrosionNitish ReddyNo ratings yet
- Experiment 9 ProtocolDocument7 pagesExperiment 9 ProtocolMANINo ratings yet
- Conductometry - Dr. Hisham Ezzat AbdellatefDocument21 pagesConductometry - Dr. Hisham Ezzat AbdellatefEka PratistaNo ratings yet
- Calibration and MaintainanceDocument6 pagesCalibration and MaintainancePranyusha VeluriNo ratings yet
- ConductivityDocument28 pagesConductivityTiee TieeNo ratings yet
- Worksheet No 8 Electrochemistry G D Goenka Public School Model Town DelhiDocument2 pagesWorksheet No 8 Electrochemistry G D Goenka Public School Model Town DelhiMINI SINGHNo ratings yet
- Chemistry Assignment Electro GGDocument4 pagesChemistry Assignment Electro GGyashNo ratings yet
- Experiment 4 Electrochem CMT555Document10 pagesExperiment 4 Electrochem CMT555Amar Safwan100% (1)
- Worksheet ElectroChemistry-2Document2 pagesWorksheet ElectroChemistry-2Rishi ChatterjeeNo ratings yet
- PART-2 ElectrochemistryDocument32 pagesPART-2 ElectrochemistrySangeetha RajaNo ratings yet
- Chemistry Department Third Class Second Course Electrical ChemistryDocument23 pagesChemistry Department Third Class Second Course Electrical ChemistryyazachewyizengawNo ratings yet
- Chapter 20 - ElectrochemistryDocument5 pagesChapter 20 - ElectrochemistrySai SanigepalliNo ratings yet
- 8a ElectrochemistryDocument7 pages8a ElectrochemistryjukoninjaNo ratings yet
- ElectrochemistryDocument29 pagesElectrochemistryAditya PandeyNo ratings yet
- 1018 Combo Batch PDFDocument4 pages1018 Combo Batch PDFAnjali GuptaNo ratings yet
- CHEM 334L - Conductance of Solutions - Estimating K For A Weak AcidDocument4 pagesCHEM 334L - Conductance of Solutions - Estimating K For A Weak Acidfdobonat613100% (1)
- Electrochemical Processes in Biological SystemsFrom EverandElectrochemical Processes in Biological SystemsAndrzej LewenstamNo ratings yet
- Physical Chemistry of Polyelectrolyte SolutionsFrom EverandPhysical Chemistry of Polyelectrolyte SolutionsMitsuru NagasawaNo ratings yet