Professional Documents
Culture Documents
Kinetics of Ethylbenzene Hydrogenation On Ni-Al2O3 - 8
Kinetics of Ethylbenzene Hydrogenation On Ni-Al2O3 - 8
Uploaded by
Mourad kharbachCopyright:
Available Formats
You might also like
- Principle To The Aquatic Chemistry 2nd Edition Morel HeringDocument18 pagesPrinciple To The Aquatic Chemistry 2nd Edition Morel Heringcsh8911290% (1)
- ArticleDocument12 pagesArticleASimilatrixNo ratings yet
- Pourbaix Diagrams For Multielement Systems PDFDocument12 pagesPourbaix Diagrams For Multielement Systems PDFJuan Carlos Campos CondoriNo ratings yet
- Air TorqueDocument1 pageAir TorqueJagannathNo ratings yet
- Davissmch07 PDFDocument4 pagesDavissmch07 PDFsaiNo ratings yet
- A Mathematical Model For Equilibrium Solubility of Hydrogen Sulfide and Carbon Dioxide in Aqueous Alkanolamine SolutionsDocument8 pagesA Mathematical Model For Equilibrium Solubility of Hydrogen Sulfide and Carbon Dioxide in Aqueous Alkanolamine Solutionsiitgn007100% (1)
- Editor: It SoDocument1 pageEditor: It SoEsteban Olvera MagañaNo ratings yet
- Propiedades Del ArgonDocument11 pagesPropiedades Del ArgonJuanVelaverdeNolazcoSalasNo ratings yet
- IUPAC Subcommittee On Gas Kinetic Data Evaluation - Data Sheet VI.A1.10Document5 pagesIUPAC Subcommittee On Gas Kinetic Data Evaluation - Data Sheet VI.A1.10Vanzatorul de IluziiNo ratings yet
- A Kinetic Model of The Water Gas Shift ReactionDocument24 pagesA Kinetic Model of The Water Gas Shift ReactionClementia CatherineNo ratings yet
- A Simple CEOSDocument9 pagesA Simple CEOSnghiabactramyNo ratings yet
- Chemistry XI - Part-1Document4 pagesChemistry XI - Part-1Janki VernekarNo ratings yet
- Reaction of Co2 With Ethanolamines: Kinetics From Gas-AbsorptionDocument4 pagesReaction of Co2 With Ethanolamines: Kinetics From Gas-AbsorptionmppatilmayurNo ratings yet
- The Application of Potential - PH Diagrams To Solvent Extraction SystemsDocument14 pagesThe Application of Potential - PH Diagrams To Solvent Extraction SystemsandyNo ratings yet
- Isotope 3Document6 pagesIsotope 3Ashish Manatosh BarikNo ratings yet
- Kinetics of Ethylbenzene Hydrogenation On Ni-Al2O3 - 10Document1 pageKinetics of Ethylbenzene Hydrogenation On Ni-Al2O3 - 10Mourad kharbachNo ratings yet
- EH PH Fundamental PDFDocument5 pagesEH PH Fundamental PDFsekarNo ratings yet
- Gas Absorption With Chemical Reaction in Packed PDFDocument5 pagesGas Absorption With Chemical Reaction in Packed PDFCatherine CcasaNo ratings yet
- Pourbaix DiagramDocument4 pagesPourbaix DiagramagnyrezaNo ratings yet
- Adsorption Modeling With The Esd Equation of State: Aaron D. Soule, Cassandra A. Smith, Xiaoning Yang Carl T. LiraDocument38 pagesAdsorption Modeling With The Esd Equation of State: Aaron D. Soule, Cassandra A. Smith, Xiaoning Yang Carl T. LiraSanjeeva YedavalliNo ratings yet
- A Semiempirical Procedure To Describe The Thermodynamics of Dissolution of Non-Polar Gases in WaterDocument18 pagesA Semiempirical Procedure To Describe The Thermodynamics of Dissolution of Non-Polar Gases in WaterzibaNo ratings yet
- Pourbaix DiagramDocument8 pagesPourbaix DiagramDauji SahaNo ratings yet
- 0378 3812 2889 2980369 3 PDFDocument12 pages0378 3812 2889 2980369 3 PDFsaeedt538No ratings yet
- Electrochemical Kinetics of Corrosion and PassivityDocument36 pagesElectrochemical Kinetics of Corrosion and PassivityWan Ibrahim Al-KutaniNo ratings yet
- Low-Temperature NMR Spectra of Fluoride - Acetic Acid Hydrogen-Bonded Complexes in Aprotic Polar EnvironmentDocument10 pagesLow-Temperature NMR Spectra of Fluoride - Acetic Acid Hydrogen-Bonded Complexes in Aprotic Polar EnvironmentMuhammad JavedNo ratings yet
- A New Generalized Alpha Function For A Cubic Equation of StateDocument11 pagesA New Generalized Alpha Function For A Cubic Equation of StateJenn QuintoNo ratings yet
- JV Leyendekkers Viscosity TTG ModelDocument17 pagesJV Leyendekkers Viscosity TTG Modelthibaud.rosinNo ratings yet
- Surface Tension For Aqueous Electrolyte Solutions by The Modified Mean Spherical ApproximationDocument16 pagesSurface Tension For Aqueous Electrolyte Solutions by The Modified Mean Spherical ApproximationMonk KongNo ratings yet
- Eulllllia: A Modified NRTL Equation For The Calculation of Phase Equilibrium of Polymer SolutionsDocument15 pagesEulllllia: A Modified NRTL Equation For The Calculation of Phase Equilibrium of Polymer SolutionslauraNo ratings yet
- Vapor-Liquid Equilibria of Nonideal Solutions: Harrison C. Carlson, and Allan P. ColburnDocument10 pagesVapor-Liquid Equilibria of Nonideal Solutions: Harrison C. Carlson, and Allan P. ColburnAlfonso Dominguez GonzalezNo ratings yet
- Reator de Cal - Como CalcularDocument7 pagesReator de Cal - Como CalculareduardobajoNo ratings yet
- 00 B 495395 Ac 3 Cedca 6000000Document28 pages00 B 495395 Ac 3 Cedca 6000000SuhailyShukriNo ratings yet
- Pourbaix Diagram (Stability Diagram)Document5 pagesPourbaix Diagram (Stability Diagram)AravindNaiduNo ratings yet
- Calculations of The Exchange Current Density For Hydrogen Electrode Reactions PDFDocument6 pagesCalculations of The Exchange Current Density For Hydrogen Electrode Reactions PDFVandam65No ratings yet
- Characterization and Modification of Fillers For Paints and CoatingsDocument17 pagesCharacterization and Modification of Fillers For Paints and CoatingsDũng NguyễnNo ratings yet
- Pourbaix Diagrams: Pourbaix Diagram of IronDocument9 pagesPourbaix Diagrams: Pourbaix Diagram of IronLatif RadwanNo ratings yet
- High Pressure Phase Equilibrium of (Solvent + Salt + CO) Systems by The Extended Peng-Robinson Equation of StateDocument9 pagesHigh Pressure Phase Equilibrium of (Solvent + Salt + CO) Systems by The Extended Peng-Robinson Equation of StateJuan Sebastian LopezNo ratings yet
- Kinetics of MethanationDocument12 pagesKinetics of MethanationGabriela Campos DávilaNo ratings yet
- Chmarzynski and H. Piekarski 2: KeywordsDocument7 pagesChmarzynski and H. Piekarski 2: KeywordspravkovoilaNo ratings yet
- 0378 38122987010 7Document15 pages0378 38122987010 7Tiên PhạmNo ratings yet
- Kinetics of The Esterifieation of Palmitie Acid With Isobutyl AlcoholDocument10 pagesKinetics of The Esterifieation of Palmitie Acid With Isobutyl AlcoholKemal MohammadNo ratings yet
- Solubility of Gases in Liquids - A Critical ReviewDocument20 pagesSolubility of Gases in Liquids - A Critical ReviewEn CsakNo ratings yet
- Qian Et Al-2019-Environmental Science & Technology - Sup-1Document40 pagesQian Et Al-2019-Environmental Science & Technology - Sup-1Nazia HassanNo ratings yet
- Thermodynamic and Experimental Approach To Ceramic Materials: Gas - Solid/liquid EquilibriaDocument7 pagesThermodynamic and Experimental Approach To Ceramic Materials: Gas - Solid/liquid Equilibriafofia1955No ratings yet
- Applications: of ThermDocument7 pagesApplications: of ThermThaligari Sandeep KumarNo ratings yet
- A Thermodynamic Analysis of The Copper-Sulfur System: - 1/2 WJPXJXDocument9 pagesA Thermodynamic Analysis of The Copper-Sulfur System: - 1/2 WJPXJXmaxi roaNo ratings yet
- Balancing Chemical EquationsDocument8 pagesBalancing Chemical EquationsRAIEL ALVARONo ratings yet
- Liebhafsky1932 PDFDocument15 pagesLiebhafsky1932 PDFK K LoachNo ratings yet
- 1954 Irving - The Calculation of Formation Curves of Metal Complexes PDFDocument7 pages1954 Irving - The Calculation of Formation Curves of Metal Complexes PDFsyth2010No ratings yet
- Physical Organic Notes 9pDocument9 pagesPhysical Organic Notes 9pprincejoes194No ratings yet
- Calculating Free Energy Changes in Continuum Solvation ModelsDocument31 pagesCalculating Free Energy Changes in Continuum Solvation Models이민우No ratings yet
- POURBAIX DIAGRAMS (AutoRecovered)Document10 pagesPOURBAIX DIAGRAMS (AutoRecovered)IslamNo ratings yet
- CH 26 ReactionsDocument52 pagesCH 26 ReactionsjiggychengNo ratings yet
- MechanismWGSonPt Lars 2008Document10 pagesMechanismWGSonPt Lars 2008leonardoNo ratings yet
- 199 VetrivelDocument12 pages199 VetrivelMiran VrhovacNo ratings yet
- WGS Article 1Document12 pagesWGS Article 1cymyNo ratings yet
- Gas Hydrates 1: Fundamentals, Characterization and ModelingFrom EverandGas Hydrates 1: Fundamentals, Characterization and ModelingDaniel BrosetaNo ratings yet
- Computational Methods in Lanthanide and Actinide ChemistryFrom EverandComputational Methods in Lanthanide and Actinide ChemistryMichael DolgNo ratings yet
- T15140 4Document1 pageT15140 4Mourad kharbachNo ratings yet
- AcknowledgementDocument1 pageAcknowledgementMourad kharbachNo ratings yet
- Reactors1 16Document3 pagesReactors1 16Mourad kharbachNo ratings yet
- Reactors1 27Document2 pagesReactors1 27Mourad kharbachNo ratings yet
- Reactors1 23Document2 pagesReactors1 23Mourad kharbachNo ratings yet
- Kinetics 2Document1 pageKinetics 2Mourad kharbachNo ratings yet
- Kinetics of Ethylbenzene Hydrogenation On Ni-Al2O3 - 10Document1 pageKinetics of Ethylbenzene Hydrogenation On Ni-Al2O3 - 10Mourad kharbachNo ratings yet
- Reactors1 2Document4 pagesReactors1 2Mourad kharbachNo ratings yet
- MDPN457 - Final Exam 2023-Spring SemesterDocument3 pagesMDPN457 - Final Exam 2023-Spring SemestermemarawanNo ratings yet
- Example For A Dry Type Thermal SimulationDocument1 pageExample For A Dry Type Thermal SimulationRejith MuraleeNo ratings yet
- 402 - Mass BalanceDocument5 pages402 - Mass BalanceSajesh S KumarNo ratings yet
- Manual 9Document6 pagesManual 9Vraja Kisori100% (1)
- Multi-Element Airfoil in Ground Effect-An Experimental and Computational StudyDocument9 pagesMulti-Element Airfoil in Ground Effect-An Experimental and Computational StudyMarcos SoarNo ratings yet
- Annular Pressure Management - OISD Well Integrity Workshop, Nov., 2013Document20 pagesAnnular Pressure Management - OISD Well Integrity Workshop, Nov., 2013azareiforoush0% (1)
- CE-3203 Hydrology: Dr. Mohammed Alauddin, DUET, GazipurDocument25 pagesCE-3203 Hydrology: Dr. Mohammed Alauddin, DUET, GazipurRafiq RehanNo ratings yet
- Projectile Motion-With Drag ModelDocument25 pagesProjectile Motion-With Drag ModelharshilNo ratings yet
- Natural Gas Dehydration With TEG 1Document20 pagesNatural Gas Dehydration With TEG 1koo_kamel4664100% (1)
- Extrusion SpheronisationDocument16 pagesExtrusion SpheronisationmattathersNo ratings yet
- Rate Law Worksheet PDFDocument3 pagesRate Law Worksheet PDFJunghoon Lee100% (1)
- Curriculum Vitae: Alex Gnanamani.KDocument4 pagesCurriculum Vitae: Alex Gnanamani.KDIJUNo ratings yet
- Plano Hidraulico PDFDocument2 pagesPlano Hidraulico PDFYefry LBNo ratings yet
- Topic 3, Lesson 1Document30 pagesTopic 3, Lesson 1George chaupi NyondoNo ratings yet
- TPDocument10 pagesTPAhmed Abo RashedNo ratings yet
- Technical Service Bulletin: Procedure For Measuring Silt Density Index (SDI)Document3 pagesTechnical Service Bulletin: Procedure For Measuring Silt Density Index (SDI)JC PinoNo ratings yet
- Casing DesignDocument28 pagesCasing DesignHeris SitompulNo ratings yet
- Classification & Types of BoilerDocument13 pagesClassification & Types of BoilerGnana Subramanian Arumugam100% (1)
- Background: 1 Slugging Caused by PiggingDocument33 pagesBackground: 1 Slugging Caused by PiggingGary Jones100% (1)
- Aquatech FEWADocument6 pagesAquatech FEWAMohamed ZaghloulNo ratings yet
- WKM HP Butterfly BrochureDocument28 pagesWKM HP Butterfly BrochureAarthi PadmanabhanNo ratings yet
- Fin Pid GnalDocument113 pagesFin Pid GnalVijay GuptaNo ratings yet
- Solucionario de Treybal PDF de Treybal PDF Estan Resueltos Los Ejercicios DelDocument24 pagesSolucionario de Treybal PDF de Treybal PDF Estan Resueltos Los Ejercicios DelCarlos Baca SanchezNo ratings yet
- Instructor's Notes: This Problem Is Similar To Example 12-2. The Solution Is WorkedDocument10 pagesInstructor's Notes: This Problem Is Similar To Example 12-2. The Solution Is WorkedAljebre MohmedNo ratings yet
- Plant ManualDocument181 pagesPlant Manualscorpio1878100% (1)
- Syngas Valve Train Techno-Commercial Quote & Schematic: Mash MakesDocument9 pagesSyngas Valve Train Techno-Commercial Quote & Schematic: Mash MakesShamshuddin TanekhanNo ratings yet
- Extrusion ProcessDocument11 pagesExtrusion ProcessHimanshu PatelNo ratings yet
- Building ServicesDocument32 pagesBuilding ServicesNavil lichaNo ratings yet
- Comparison of Types of EvaporatorDocument3 pagesComparison of Types of EvaporatorLon カンイェテ100% (1)
Kinetics of Ethylbenzene Hydrogenation On Ni-Al2O3 - 8
Kinetics of Ethylbenzene Hydrogenation On Ni-Al2O3 - 8
Uploaded by
Mourad kharbachOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Kinetics of Ethylbenzene Hydrogenation On Ni-Al2O3 - 8
Kinetics of Ethylbenzene Hydrogenation On Ni-Al2O3 - 8
Uploaded by
Mourad kharbachCopyright:
Available Formats
278 S. Smeds et al.
/ Applied Catalysis A: General 125 (1995) 271 291
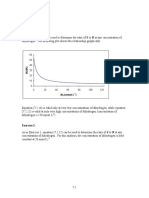
The model (2) can reproduce the temperature and partial pressure dependencies
of the reaction studied (Figs. 2-4).
3.2. Kinetic modelling and data fitting
Since hydrogenation of ethylbenzene involves destruction of the aromatic ring
as in benzene hydrogenation, we will refer to previous studies reported on benzene
hydrogenation on other metals as well. Recently Chou and Vannice [ 13] presented
a kinetic model for gas-phase benzene and toluene hydrogenation on palladium,
featuring sequential addition of adsorbed hydrogen atoms to benzene molecules
adsorbed on another type of sites, as well as concurrent formation of hydrogen-
deficient surface species. Their results suggest weak hydrogen and benzene adsorp-
tion, the adsorbed species being mobile on the surface. The calculated values of
the rate constants of the six rate determining surface addition steps were almost
equal. This would imply that adsorbed cyclohexene as such cannot possibly be an
intermediate surface species, since the hydrogenation rate of cyclohexene is several
orders of magnitude higher than has been reported for benzene [ 17 ].
Due to our procedure of calculating initial rates it was reasonable to use only the
hydrogenation part of the model of Chou and Vannice [ 13] for data fitting. The
rate equation can be written as follows:
r=k6
(
1 ~-( ~ ° 5 ] ~ , 1
v + K B P B ( l + El + r2~- Y3 -{- r4-~- Ys)
)
where B denotes the aromatic molecule and Yl...Y¢~ are functions of the rate and
adsorption constants, however too complex to be reproduced here [ 13]. The data
fitting results are shown in Fig. 5 and the calculated parameter values are listed in
Table 2. Fig. 5 shows that the overall description of the model is acceptable, Also
no clear deviations from a random distribution of the residuals as functions of the
independent variables ( T, PH, PB) could be seen. The adsorption parameter values
in Table 2 suggest weak hydrogen adsorption ( AHH -- - 20 kJ tool ~) and slightly
mobile adsorbed species (ASH = -- 110 J mol - 1 K - 1, A S E ,~ _ 140 J mol - ~K 1).
For the surface rate constants a decreasing trend with the degree of hydrogen
addition was obtained, the rate constant of the first step being two orders of mag-
nitude greater than that of the last step. This is in contradiction with the view of
ethylcyclohexadiene and ethylcyclohexene as surface intermediates, since higher
rate constants would then be expected for the later addition steps.
The kinetic modelling of this work takes into account both competitive and non-
competitive adsorption of the two reactants, dissociative and non-dissociative
adsorption of hydrogen, as well as the possibility of several slow surface reaction
steps. According to isotopic transient studies [5 ] hydrogen is added pairwise, either
in the form of atoms or molecules. Adsorption steps were assumed to he in pseudo-
equilibria, desorption of the reaction product was presumed to be fast and irrevers-
You might also like
- Principle To The Aquatic Chemistry 2nd Edition Morel HeringDocument18 pagesPrinciple To The Aquatic Chemistry 2nd Edition Morel Heringcsh8911290% (1)
- ArticleDocument12 pagesArticleASimilatrixNo ratings yet
- Pourbaix Diagrams For Multielement Systems PDFDocument12 pagesPourbaix Diagrams For Multielement Systems PDFJuan Carlos Campos CondoriNo ratings yet
- Air TorqueDocument1 pageAir TorqueJagannathNo ratings yet
- Davissmch07 PDFDocument4 pagesDavissmch07 PDFsaiNo ratings yet
- A Mathematical Model For Equilibrium Solubility of Hydrogen Sulfide and Carbon Dioxide in Aqueous Alkanolamine SolutionsDocument8 pagesA Mathematical Model For Equilibrium Solubility of Hydrogen Sulfide and Carbon Dioxide in Aqueous Alkanolamine Solutionsiitgn007100% (1)
- Editor: It SoDocument1 pageEditor: It SoEsteban Olvera MagañaNo ratings yet
- Propiedades Del ArgonDocument11 pagesPropiedades Del ArgonJuanVelaverdeNolazcoSalasNo ratings yet
- IUPAC Subcommittee On Gas Kinetic Data Evaluation - Data Sheet VI.A1.10Document5 pagesIUPAC Subcommittee On Gas Kinetic Data Evaluation - Data Sheet VI.A1.10Vanzatorul de IluziiNo ratings yet
- A Kinetic Model of The Water Gas Shift ReactionDocument24 pagesA Kinetic Model of The Water Gas Shift ReactionClementia CatherineNo ratings yet
- A Simple CEOSDocument9 pagesA Simple CEOSnghiabactramyNo ratings yet
- Chemistry XI - Part-1Document4 pagesChemistry XI - Part-1Janki VernekarNo ratings yet
- Reaction of Co2 With Ethanolamines: Kinetics From Gas-AbsorptionDocument4 pagesReaction of Co2 With Ethanolamines: Kinetics From Gas-AbsorptionmppatilmayurNo ratings yet
- The Application of Potential - PH Diagrams To Solvent Extraction SystemsDocument14 pagesThe Application of Potential - PH Diagrams To Solvent Extraction SystemsandyNo ratings yet
- Isotope 3Document6 pagesIsotope 3Ashish Manatosh BarikNo ratings yet
- Kinetics of Ethylbenzene Hydrogenation On Ni-Al2O3 - 10Document1 pageKinetics of Ethylbenzene Hydrogenation On Ni-Al2O3 - 10Mourad kharbachNo ratings yet
- EH PH Fundamental PDFDocument5 pagesEH PH Fundamental PDFsekarNo ratings yet
- Gas Absorption With Chemical Reaction in Packed PDFDocument5 pagesGas Absorption With Chemical Reaction in Packed PDFCatherine CcasaNo ratings yet
- Pourbaix DiagramDocument4 pagesPourbaix DiagramagnyrezaNo ratings yet
- Adsorption Modeling With The Esd Equation of State: Aaron D. Soule, Cassandra A. Smith, Xiaoning Yang Carl T. LiraDocument38 pagesAdsorption Modeling With The Esd Equation of State: Aaron D. Soule, Cassandra A. Smith, Xiaoning Yang Carl T. LiraSanjeeva YedavalliNo ratings yet
- A Semiempirical Procedure To Describe The Thermodynamics of Dissolution of Non-Polar Gases in WaterDocument18 pagesA Semiempirical Procedure To Describe The Thermodynamics of Dissolution of Non-Polar Gases in WaterzibaNo ratings yet
- Pourbaix DiagramDocument8 pagesPourbaix DiagramDauji SahaNo ratings yet
- 0378 3812 2889 2980369 3 PDFDocument12 pages0378 3812 2889 2980369 3 PDFsaeedt538No ratings yet
- Electrochemical Kinetics of Corrosion and PassivityDocument36 pagesElectrochemical Kinetics of Corrosion and PassivityWan Ibrahim Al-KutaniNo ratings yet
- Low-Temperature NMR Spectra of Fluoride - Acetic Acid Hydrogen-Bonded Complexes in Aprotic Polar EnvironmentDocument10 pagesLow-Temperature NMR Spectra of Fluoride - Acetic Acid Hydrogen-Bonded Complexes in Aprotic Polar EnvironmentMuhammad JavedNo ratings yet
- A New Generalized Alpha Function For A Cubic Equation of StateDocument11 pagesA New Generalized Alpha Function For A Cubic Equation of StateJenn QuintoNo ratings yet
- JV Leyendekkers Viscosity TTG ModelDocument17 pagesJV Leyendekkers Viscosity TTG Modelthibaud.rosinNo ratings yet
- Surface Tension For Aqueous Electrolyte Solutions by The Modified Mean Spherical ApproximationDocument16 pagesSurface Tension For Aqueous Electrolyte Solutions by The Modified Mean Spherical ApproximationMonk KongNo ratings yet
- Eulllllia: A Modified NRTL Equation For The Calculation of Phase Equilibrium of Polymer SolutionsDocument15 pagesEulllllia: A Modified NRTL Equation For The Calculation of Phase Equilibrium of Polymer SolutionslauraNo ratings yet
- Vapor-Liquid Equilibria of Nonideal Solutions: Harrison C. Carlson, and Allan P. ColburnDocument10 pagesVapor-Liquid Equilibria of Nonideal Solutions: Harrison C. Carlson, and Allan P. ColburnAlfonso Dominguez GonzalezNo ratings yet
- Reator de Cal - Como CalcularDocument7 pagesReator de Cal - Como CalculareduardobajoNo ratings yet
- 00 B 495395 Ac 3 Cedca 6000000Document28 pages00 B 495395 Ac 3 Cedca 6000000SuhailyShukriNo ratings yet
- Pourbaix Diagram (Stability Diagram)Document5 pagesPourbaix Diagram (Stability Diagram)AravindNaiduNo ratings yet
- Calculations of The Exchange Current Density For Hydrogen Electrode Reactions PDFDocument6 pagesCalculations of The Exchange Current Density For Hydrogen Electrode Reactions PDFVandam65No ratings yet
- Characterization and Modification of Fillers For Paints and CoatingsDocument17 pagesCharacterization and Modification of Fillers For Paints and CoatingsDũng NguyễnNo ratings yet
- Pourbaix Diagrams: Pourbaix Diagram of IronDocument9 pagesPourbaix Diagrams: Pourbaix Diagram of IronLatif RadwanNo ratings yet
- High Pressure Phase Equilibrium of (Solvent + Salt + CO) Systems by The Extended Peng-Robinson Equation of StateDocument9 pagesHigh Pressure Phase Equilibrium of (Solvent + Salt + CO) Systems by The Extended Peng-Robinson Equation of StateJuan Sebastian LopezNo ratings yet
- Kinetics of MethanationDocument12 pagesKinetics of MethanationGabriela Campos DávilaNo ratings yet
- Chmarzynski and H. Piekarski 2: KeywordsDocument7 pagesChmarzynski and H. Piekarski 2: KeywordspravkovoilaNo ratings yet
- 0378 38122987010 7Document15 pages0378 38122987010 7Tiên PhạmNo ratings yet
- Kinetics of The Esterifieation of Palmitie Acid With Isobutyl AlcoholDocument10 pagesKinetics of The Esterifieation of Palmitie Acid With Isobutyl AlcoholKemal MohammadNo ratings yet
- Solubility of Gases in Liquids - A Critical ReviewDocument20 pagesSolubility of Gases in Liquids - A Critical ReviewEn CsakNo ratings yet
- Qian Et Al-2019-Environmental Science & Technology - Sup-1Document40 pagesQian Et Al-2019-Environmental Science & Technology - Sup-1Nazia HassanNo ratings yet
- Thermodynamic and Experimental Approach To Ceramic Materials: Gas - Solid/liquid EquilibriaDocument7 pagesThermodynamic and Experimental Approach To Ceramic Materials: Gas - Solid/liquid Equilibriafofia1955No ratings yet
- Applications: of ThermDocument7 pagesApplications: of ThermThaligari Sandeep KumarNo ratings yet
- A Thermodynamic Analysis of The Copper-Sulfur System: - 1/2 WJPXJXDocument9 pagesA Thermodynamic Analysis of The Copper-Sulfur System: - 1/2 WJPXJXmaxi roaNo ratings yet
- Balancing Chemical EquationsDocument8 pagesBalancing Chemical EquationsRAIEL ALVARONo ratings yet
- Liebhafsky1932 PDFDocument15 pagesLiebhafsky1932 PDFK K LoachNo ratings yet
- 1954 Irving - The Calculation of Formation Curves of Metal Complexes PDFDocument7 pages1954 Irving - The Calculation of Formation Curves of Metal Complexes PDFsyth2010No ratings yet
- Physical Organic Notes 9pDocument9 pagesPhysical Organic Notes 9pprincejoes194No ratings yet
- Calculating Free Energy Changes in Continuum Solvation ModelsDocument31 pagesCalculating Free Energy Changes in Continuum Solvation Models이민우No ratings yet
- POURBAIX DIAGRAMS (AutoRecovered)Document10 pagesPOURBAIX DIAGRAMS (AutoRecovered)IslamNo ratings yet
- CH 26 ReactionsDocument52 pagesCH 26 ReactionsjiggychengNo ratings yet
- MechanismWGSonPt Lars 2008Document10 pagesMechanismWGSonPt Lars 2008leonardoNo ratings yet
- 199 VetrivelDocument12 pages199 VetrivelMiran VrhovacNo ratings yet
- WGS Article 1Document12 pagesWGS Article 1cymyNo ratings yet
- Gas Hydrates 1: Fundamentals, Characterization and ModelingFrom EverandGas Hydrates 1: Fundamentals, Characterization and ModelingDaniel BrosetaNo ratings yet
- Computational Methods in Lanthanide and Actinide ChemistryFrom EverandComputational Methods in Lanthanide and Actinide ChemistryMichael DolgNo ratings yet
- T15140 4Document1 pageT15140 4Mourad kharbachNo ratings yet
- AcknowledgementDocument1 pageAcknowledgementMourad kharbachNo ratings yet
- Reactors1 16Document3 pagesReactors1 16Mourad kharbachNo ratings yet
- Reactors1 27Document2 pagesReactors1 27Mourad kharbachNo ratings yet
- Reactors1 23Document2 pagesReactors1 23Mourad kharbachNo ratings yet
- Kinetics 2Document1 pageKinetics 2Mourad kharbachNo ratings yet
- Kinetics of Ethylbenzene Hydrogenation On Ni-Al2O3 - 10Document1 pageKinetics of Ethylbenzene Hydrogenation On Ni-Al2O3 - 10Mourad kharbachNo ratings yet
- Reactors1 2Document4 pagesReactors1 2Mourad kharbachNo ratings yet
- MDPN457 - Final Exam 2023-Spring SemesterDocument3 pagesMDPN457 - Final Exam 2023-Spring SemestermemarawanNo ratings yet
- Example For A Dry Type Thermal SimulationDocument1 pageExample For A Dry Type Thermal SimulationRejith MuraleeNo ratings yet
- 402 - Mass BalanceDocument5 pages402 - Mass BalanceSajesh S KumarNo ratings yet
- Manual 9Document6 pagesManual 9Vraja Kisori100% (1)
- Multi-Element Airfoil in Ground Effect-An Experimental and Computational StudyDocument9 pagesMulti-Element Airfoil in Ground Effect-An Experimental and Computational StudyMarcos SoarNo ratings yet
- Annular Pressure Management - OISD Well Integrity Workshop, Nov., 2013Document20 pagesAnnular Pressure Management - OISD Well Integrity Workshop, Nov., 2013azareiforoush0% (1)
- CE-3203 Hydrology: Dr. Mohammed Alauddin, DUET, GazipurDocument25 pagesCE-3203 Hydrology: Dr. Mohammed Alauddin, DUET, GazipurRafiq RehanNo ratings yet
- Projectile Motion-With Drag ModelDocument25 pagesProjectile Motion-With Drag ModelharshilNo ratings yet
- Natural Gas Dehydration With TEG 1Document20 pagesNatural Gas Dehydration With TEG 1koo_kamel4664100% (1)
- Extrusion SpheronisationDocument16 pagesExtrusion SpheronisationmattathersNo ratings yet
- Rate Law Worksheet PDFDocument3 pagesRate Law Worksheet PDFJunghoon Lee100% (1)
- Curriculum Vitae: Alex Gnanamani.KDocument4 pagesCurriculum Vitae: Alex Gnanamani.KDIJUNo ratings yet
- Plano Hidraulico PDFDocument2 pagesPlano Hidraulico PDFYefry LBNo ratings yet
- Topic 3, Lesson 1Document30 pagesTopic 3, Lesson 1George chaupi NyondoNo ratings yet
- TPDocument10 pagesTPAhmed Abo RashedNo ratings yet
- Technical Service Bulletin: Procedure For Measuring Silt Density Index (SDI)Document3 pagesTechnical Service Bulletin: Procedure For Measuring Silt Density Index (SDI)JC PinoNo ratings yet
- Casing DesignDocument28 pagesCasing DesignHeris SitompulNo ratings yet
- Classification & Types of BoilerDocument13 pagesClassification & Types of BoilerGnana Subramanian Arumugam100% (1)
- Background: 1 Slugging Caused by PiggingDocument33 pagesBackground: 1 Slugging Caused by PiggingGary Jones100% (1)
- Aquatech FEWADocument6 pagesAquatech FEWAMohamed ZaghloulNo ratings yet
- WKM HP Butterfly BrochureDocument28 pagesWKM HP Butterfly BrochureAarthi PadmanabhanNo ratings yet
- Fin Pid GnalDocument113 pagesFin Pid GnalVijay GuptaNo ratings yet
- Solucionario de Treybal PDF de Treybal PDF Estan Resueltos Los Ejercicios DelDocument24 pagesSolucionario de Treybal PDF de Treybal PDF Estan Resueltos Los Ejercicios DelCarlos Baca SanchezNo ratings yet
- Instructor's Notes: This Problem Is Similar To Example 12-2. The Solution Is WorkedDocument10 pagesInstructor's Notes: This Problem Is Similar To Example 12-2. The Solution Is WorkedAljebre MohmedNo ratings yet
- Plant ManualDocument181 pagesPlant Manualscorpio1878100% (1)
- Syngas Valve Train Techno-Commercial Quote & Schematic: Mash MakesDocument9 pagesSyngas Valve Train Techno-Commercial Quote & Schematic: Mash MakesShamshuddin TanekhanNo ratings yet
- Extrusion ProcessDocument11 pagesExtrusion ProcessHimanshu PatelNo ratings yet
- Building ServicesDocument32 pagesBuilding ServicesNavil lichaNo ratings yet
- Comparison of Types of EvaporatorDocument3 pagesComparison of Types of EvaporatorLon カンイェテ100% (1)