Professional Documents
Culture Documents
Dokumen PDF 55
Dokumen PDF 55
Uploaded by
Farhan FarhanOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Dokumen PDF 55
Dokumen PDF 55
Uploaded by
Farhan FarhanCopyright:
Available Formats
the quantum mechanical model limits an electron’s energy to certain values.
Nucleus However, unlike Bohr’s model, the quantum mechanical model makes no
attempt to describe the electron’s path around the nucleus.
The Schrödinger wave equation is too complex to be considered here.
However, each solution to the equation is known as a wave function. And most
importantly, the wave function is related to the probability of finding the elec-
tron within a particular volume of space around the nucleus. Recall from your
study of math that an event having a high probability is more likely to occur
than one having a low probability.
What does the wave function predict about the electron’s location in an
a atom? A three-dimensional region around the nucleus called an atomic orbital
describes the electron’s probable location. You can picture an atomic orbital
as a fuzzy cloud in which the density of the cloud at a given point is propor-
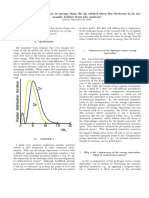
tional to the probability of finding the electron at that point. Figure 5-13a illus-
trates the probability map, or orbital, that describes the hydrogen electron in
its lowest energy state. It might be helpful to think of the probability map as
a time-exposure photograph of the electron moving around the nucleus, in
which each dot represents the electron’s location at an instant in time. Because
the dots are so numerous near the positive nucleus, they seem to form a dense
cloud that is indicative of the electron’s most probable location. However,
because the cloud has no definite boundary, it also is possible that the elec-
b tron might be found at a considerable distance from the nucleus.
Figure 5-13 Hydrogen’s Atomic Orbitals
a This electron density dia- Because the boundary of an atomic orbital is fuzzy, the orbital does not have
gram for a hydrogen atom rep- an exactly defined size. To overcome the inherent uncertainty about the elec-
resents the likelihood of finding tron’s location, chemists arbitrarily draw an orbital’s surface to contain 90%
an electron at a particular point
of the electron’s total probability distribution. In other words, the electron
in the atom. The greater the
density of dots, the greater the spends 90% of the time within the volume defined by the surface, and 10%
likelihood of finding hydrogen’s of the time somewhere outside the surface. The spherical surface shown in
electron. b The boundary of Figure 5-13b encloses 90% of the lowest-energy orbital of hydrogen.
an atom is defined as the vol- Recall that the Bohr atomic model assigns quantum numbers to electron
ume that encloses a 90% proba- orbits. In a similar manner, the quantum mechanical model assigns principal
bility of containing its electrons.
quantum numbers (n) that indicate the relative sizes and energies of atomic
n = 4 (4 sublevels)
n = 3 (3 sublevels)
n = 2 (2 sublevels)
n = 1 (1 sublevels)
Figure 5-14
Energy sublevels can be thought
of as a section of seats in a the-
ater. The rows that are higher
up and farther from the stage
contain more seats, just as
energy levels that are farther
from the nucleus contain more
sublevels.
132 Chapter 5 Electrons in Atoms
You might also like
- STP 767Document13 pagesSTP 767vatrushka69No ratings yet
- TEST BANK FinalDocument220 pagesTEST BANK FinalPrabhjot MundiNo ratings yet
- 5.1 Revising The Atomic ModelDocument2 pages5.1 Revising The Atomic ModelosamaNo ratings yet
- Schrodinger's Atomic ModelDocument4 pagesSchrodinger's Atomic ModeljaiNo ratings yet
- The Bohr Model and The Quantum Mechanical ModelDocument50 pagesThe Bohr Model and The Quantum Mechanical ModelKenny James CuberoNo ratings yet
- The Quantum AtomDocument10 pagesThe Quantum AtomNavneetRaiNo ratings yet
- Chemistry Mechanical ModelDocument5 pagesChemistry Mechanical ModelKc RamosNo ratings yet
- D0597179 CHEM12 C0500 SWBT Mig PDFDocument12 pagesD0597179 CHEM12 C0500 SWBT Mig PDFMr: Mohamed BesharaNo ratings yet
- Electronic Structure of MatterDocument32 pagesElectronic Structure of Matterdejesusangelito103No ratings yet
- Ho Atomic ModelDocument3 pagesHo Atomic ModelbiswektylerNo ratings yet
- Plasma OscillationDocument5 pagesPlasma OscillationMuhammad AkramNo ratings yet
- Nuclear ModelDocument10 pagesNuclear ModelnapilgabethNo ratings yet
- Electron StructureDocument80 pagesElectron StructureCacey Daiwey CalixtoNo ratings yet
- Chapter 3.4, Many-Electron Atoms: Fermi Holes and Fermi HeapsDocument14 pagesChapter 3.4, Many-Electron Atoms: Fermi Holes and Fermi HeapsBoceNo ratings yet
- AMP Class NotesDocument127 pagesAMP Class NotesHritikNo ratings yet
- Atomic OrbitalsDocument3 pagesAtomic Orbitalskida3442No ratings yet
- Como o Eltron Passa Atravs Dos NSDocument2 pagesComo o Eltron Passa Atravs Dos NSMeus filhos Evellyn e EnzoNo ratings yet
- Orbital EnergyDocument3 pagesOrbital EnergyaneNo ratings yet
- SCIENCE - Punnett SquaresDocument3 pagesSCIENCE - Punnett Squaresrabano1417No ratings yet
- Bohr Model of Hydrogen AtomDocument26 pagesBohr Model of Hydrogen AtomDERICK YINDANo ratings yet
- Electronic Structure of The Atom 2ND Quarter Prepared by Michael R. MaglaqueDocument41 pagesElectronic Structure of The Atom 2ND Quarter Prepared by Michael R. MaglaqueMichael Conan MaglaqueNo ratings yet
- Mossbauer Spectros PDFDocument8 pagesMossbauer Spectros PDFFollow SNo ratings yet
- The Original Hartree Paper From 1928Document22 pagesThe Original Hartree Paper From 1928Henrique CastroNo ratings yet
- Lec 38Document32 pagesLec 38Roxy PerezNo ratings yet
- LEARNING ACTIVITY SHEET-CHEM 1 q1 Week 6Document16 pagesLEARNING ACTIVITY SHEET-CHEM 1 q1 Week 6Jhude JosephNo ratings yet
- 3Document1 page311ABM Isamiel Grace MendozaNo ratings yet
- Lab Report-2 - 18-36652-1Document11 pagesLab Report-2 - 18-36652-1Zahid Hasan KhokaNo ratings yet
- Quantum Theory and The Electronic Structure of AtomsDocument17 pagesQuantum Theory and The Electronic Structure of AtomsSalama NaumanNo ratings yet
- Atom OrbitalDocument4 pagesAtom OrbitalRinaldi SatriaNo ratings yet
- The Electronic ConfigurationDocument13 pagesThe Electronic Configurationjoan ruby bautistaNo ratings yet
- The Wave Model of The AtomDocument8 pagesThe Wave Model of The AtomDravid AryaNo ratings yet
- The QuantumDocument2 pagesThe QuantumRohit SahuNo ratings yet
- The Quantum MechanicalDocument3 pagesThe Quantum MechanicalRohit SahuNo ratings yet
- Atom 33Document18 pagesAtom 33linkgogo69No ratings yet
- Reviewer in ChemistryDocument6 pagesReviewer in ChemistryStella SalvadorNo ratings yet
- Quantum Mechanical Model of The AtomDocument19 pagesQuantum Mechanical Model of The AtomJames Paul Micael Garganta100% (1)
- Class Lecture Atomic Model Conductor SemiconductorDocument8 pagesClass Lecture Atomic Model Conductor SemiconductorRajaul Morshad SaikotNo ratings yet
- UntitledDocument6 pagesUntitledyish ChaudhariNo ratings yet
- Lecture 2 EGM 241 - @2022 EditedDocument41 pagesLecture 2 EGM 241 - @2022 EditedIwell PhiriNo ratings yet
- Chemistry Questions OnlyDocument66 pagesChemistry Questions OnlyBHUVAN I (RA2111003011433)No ratings yet
- Atomic Orbital: 1 Electron PropertiesDocument15 pagesAtomic Orbital: 1 Electron PropertiesMMGNo ratings yet
- Spectroscopy, Molecular Orbitals, and Chemical Bonding: Nobel Lecture, December 12, 1966Document30 pagesSpectroscopy, Molecular Orbitals, and Chemical Bonding: Nobel Lecture, December 12, 1966GilbertoAugustoDeOliveiraBritoNo ratings yet
- General Chemistry Lecture 3Document80 pagesGeneral Chemistry Lecture 3Aaron Dela CruzNo ratings yet
- Lesson 4Document9 pagesLesson 4Rahul DevarakondaNo ratings yet
- Fine Structure Constant and The Golden SectionDocument37 pagesFine Structure Constant and The Golden SectionRaskoJovanovicNo ratings yet
- Origin and Propagation of The ELECTRIC FIELDDocument6 pagesOrigin and Propagation of The ELECTRIC FIELDAKISNo ratings yet
- Atomic and Molecular Spectroscopy Lecture 2Document29 pagesAtomic and Molecular Spectroscopy Lecture 2Hammed LawalNo ratings yet
- Velarde Quantum Mechanical Model of AtomDocument29 pagesVelarde Quantum Mechanical Model of AtomTito V. Bautista Jr.No ratings yet
- CBI 1 - Fundamentals of ChemistryDocument10 pagesCBI 1 - Fundamentals of ChemistryRianna NNo ratings yet
- P.S Week 5Document18 pagesP.S Week 5nicole MenesNo ratings yet
- The High Q Resonance Characteristics of The CEWL Electron Model May Predict Directionality of Virtual Photons and NeutrinosDocument13 pagesThe High Q Resonance Characteristics of The CEWL Electron Model May Predict Directionality of Virtual Photons and NeutrinosMephistoNo ratings yet
- Lesson 11 Quality ManagementDocument20 pagesLesson 11 Quality ManagementDennisBrionesNo ratings yet
- Aim: How Is The Electron Organized in The Atom?Document14 pagesAim: How Is The Electron Organized in The Atom?MjoyTibayNo ratings yet
- Why Doesn T The Electron Fall Into The NucleusDocument3 pagesWhy Doesn T The Electron Fall Into The NucleusVanessaLassoNo ratings yet
- General Chemistry 1: Module 4, Lesson 1: Quantum Mechanical Model of An AtomDocument4 pagesGeneral Chemistry 1: Module 4, Lesson 1: Quantum Mechanical Model of An AtomKeano GelmoNo ratings yet
- Ivy Mae ADocument1 pageIvy Mae Aapi-233333580No ratings yet
- Chapter 2.1 - Structure of AtomsDocument71 pagesChapter 2.1 - Structure of Atomsahmad yasinNo ratings yet
- Atomic Structure QuestionsDocument6 pagesAtomic Structure QuestionsSumit BeraNo ratings yet
- Chapter 4 OutlineDocument3 pagesChapter 4 Outlinedill1233No ratings yet
- Atomic Structure... (3) PPTDocument32 pagesAtomic Structure... (3) PPTVaibhav KargetiNo ratings yet
- School WorkDocument15 pagesSchool Workjdubey4258No ratings yet
- Principles of Solar Cells, LEDs and Related Devices: The Role of the PN JunctionFrom EverandPrinciples of Solar Cells, LEDs and Related Devices: The Role of the PN JunctionNo ratings yet
- Dokumen PDF 45Document1 pageDokumen PDF 45Farhan FarhanNo ratings yet
- Dokumen PDF 38Document1 pageDokumen PDF 38Farhan FarhanNo ratings yet
- Dokumen PDF 36Document1 pageDokumen PDF 36Farhan FarhanNo ratings yet
- Dokumen PDF 32Document1 pageDokumen PDF 32Farhan FarhanNo ratings yet
- Dokumen PDF 28Document1 pageDokumen PDF 28Farhan FarhanNo ratings yet
- Dokumen PDF 42Document1 pageDokumen PDF 42Farhan FarhanNo ratings yet
- Dokumen PDF 26Document1 pageDokumen PDF 26Farhan FarhanNo ratings yet
- Dokumen PDF 46Document1 pageDokumen PDF 46Farhan FarhanNo ratings yet
- Dokumen PDF 9Document1 pageDokumen PDF 9Farhan FarhanNo ratings yet
- Dokumen PDF 17Document1 pageDokumen PDF 17Farhan FarhanNo ratings yet
- Dokumen PDF 16Document1 pageDokumen PDF 16Farhan FarhanNo ratings yet
- Dokumen PDF 58Document1 pageDokumen PDF 58Farhan FarhanNo ratings yet
- Dokumen PDF 12Document1 pageDokumen PDF 12Farhan FarhanNo ratings yet
- Dokumen PDF 2Document1 pageDokumen PDF 2Farhan FarhanNo ratings yet
- Dokumen PDF 3Document1 pageDokumen PDF 3Farhan FarhanNo ratings yet
- Dokumen PDF 4Document1 pageDokumen PDF 4Farhan FarhanNo ratings yet
- CLS Aipmt 16 17 XI Che Study Package 3 SET 2 Chapter 11Document21 pagesCLS Aipmt 16 17 XI Che Study Package 3 SET 2 Chapter 11kalloli100% (1)
- Is 4432.1988 PDFDocument15 pagesIs 4432.1988 PDFNav TalukdarNo ratings yet
- Lesson 3. The Relationship of Percent Composition and Chemical FormulaDocument4 pagesLesson 3. The Relationship of Percent Composition and Chemical FormulaRandel MontielNo ratings yet
- Factors Affecting Reaction RateDocument29 pagesFactors Affecting Reaction RateIna Chiu100% (1)
- Dynamic Forces On Curve Surfaces Due To The Impingement of Liquid JetsDocument2 pagesDynamic Forces On Curve Surfaces Due To The Impingement of Liquid JetsRafael Nazareno RivadeneiraNo ratings yet
- P 2524101Document8 pagesP 2524101Sant JorgeNo ratings yet
- PE-O-ME-TRE-001-00 Annex Piping Classes PDFDocument207 pagesPE-O-ME-TRE-001-00 Annex Piping Classes PDFmarin cristianNo ratings yet
- Review Jurnal (Kelompok 2)Document7 pagesReview Jurnal (Kelompok 2)silviaNo ratings yet
- Problems: Concept QuestionsDocument1 pageProblems: Concept QuestionsSebaz MejiaNo ratings yet
- Otdr ReportDocument1 pageOtdr ReportzeroxiclanNo ratings yet
- Histamine and Antihistaminic Agents: Pharmaceutical Chemistry 2Document45 pagesHistamine and Antihistaminic Agents: Pharmaceutical Chemistry 2د. أبجًملNo ratings yet
- Technology Profile EphedrineDocument3 pagesTechnology Profile EphedrineKaran VetNo ratings yet
- Astm D3907-03Document6 pagesAstm D3907-03Лапшин ИгорьNo ratings yet
- Chapter ObjectivesDocument30 pagesChapter ObjectivesdhaktodesatyajitNo ratings yet
- PH8251-Material Science QN Bank ValliammaiDocument10 pagesPH8251-Material Science QN Bank ValliammaiThaya GanapathyNo ratings yet
- Observation of Unknown SpectrumDocument3 pagesObservation of Unknown SpectrumOmar HusseinNo ratings yet
- Parsol 1789Document20 pagesParsol 1789Dr.Vinod SrivastavaNo ratings yet
- 13.1 STRUCTURE BONDING Copy 2Document8 pages13.1 STRUCTURE BONDING Copy 2Arman JavedNo ratings yet
- CholineDocument9 pagesCholinedanila madalinNo ratings yet
- AI Unit-IIIDocument75 pagesAI Unit-IIIchakrimvnNo ratings yet
- T6 Heat Treatment - An Overview - ScienceDirect TopicsDocument16 pagesT6 Heat Treatment - An Overview - ScienceDirect TopicstatsNo ratings yet
- Iit JeeDocument4 pagesIit JeeAvinash BillaNo ratings yet
- Raw Material Analytical Report of Ciprofloxacin HCLDocument1 pageRaw Material Analytical Report of Ciprofloxacin HCLbejoykarim2022No ratings yet
- Work Energy Theorem Lab Report 3Document7 pagesWork Energy Theorem Lab Report 3ali razaNo ratings yet
- Magnelis Book ENDocument60 pagesMagnelis Book ENSuvro ChakrabortyNo ratings yet
- DLL September 4 7Document3 pagesDLL September 4 7DENVER MOSCOSONo ratings yet
- MP Thermodynamics 1 Principle 0 Thermal Equation of StateDocument25 pagesMP Thermodynamics 1 Principle 0 Thermal Equation of Statenimeni8No ratings yet
- Eng Chem Lecture NotesDocument2 pagesEng Chem Lecture NotesJunell TadinaNo ratings yet