Professional Documents
Culture Documents
PC - Lab 2 Q1&Q2
PC - Lab 2 Q1&Q2
Uploaded by
Priyanka Nagpure0 ratings0% found this document useful (0 votes)
6 views4 pagesThe document discusses calculating the specific volume of superheated steam at 100 bar absolute and 623.15K using the van der Waals equation. It provides the constants and parameters needed and shows the iterative calculation process. It also discusses calculating the number of hydrogen cylinders a company would need to order to meet 50 days demand of hydrogenating an organic ester, given the process conditions and cylinder capacity.
Original Description:
This Excel sheet relates to how to use Vanderwaal's equation for solving the problem in Excel
Original Title
PC_LAB 2 Q1&Q2
Copyright
© © All Rights Reserved
Available Formats
XLSX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document discusses calculating the specific volume of superheated steam at 100 bar absolute and 623.15K using the van der Waals equation. It provides the constants and parameters needed and shows the iterative calculation process. It also discusses calculating the number of hydrogen cylinders a company would need to order to meet 50 days demand of hydrogenating an organic ester, given the process conditions and cylinder capacity.
Copyright:
© All Rights Reserved
Available Formats
Download as XLSX, PDF, TXT or read online from Scribd
Download as xlsx, pdf, or txt
0 ratings0% found this document useful (0 votes)
6 views4 pagesPC - Lab 2 Q1&Q2
PC - Lab 2 Q1&Q2
Uploaded by
Priyanka NagpureThe document discusses calculating the specific volume of superheated steam at 100 bar absolute and 623.15K using the van der Waals equation. It provides the constants and parameters needed and shows the iterative calculation process. It also discusses calculating the number of hydrogen cylinders a company would need to order to meet 50 days demand of hydrogenating an organic ester, given the process conditions and cylinder capacity.
Copyright:
© All Rights Reserved
Available Formats
Download as XLSX, PDF, TXT or read online from Scribd
Download as xlsx, pdf, or txt
You are on page 1of 4
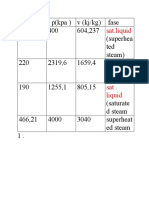
P 100 Question1:Calculate the specfic volume of super heated steam at 10
T 623.15 Pc= 221.2 Bar
R 0.083145 Tc= 623.15 K
Tc 647.3
Pc 221.2
a 5.524362
b 0.030414
Iteration Vi f(Vi) f'(Vi)
1 1 50.50319 195.818
2 0.742091 14.59088 89.32209
3 0.57874 4.04095 42.5149
4 0.483692 0.987102 22.64768
5 0.440107 0.163173 15.35008
6 0.429477 0.008601 13.74314
7 0.428851 2.896E-05 13.65065
8 0.428849 3.321E-10 13.65034
9 0.428849 -1.61E-15 13.65034
10 0.428849 1.943E-15 13.65034
e of super heated steam at 100 bar absolute and 623.15K using vanderwaals eqn .
P 70
T 303 100 kg/hr of an organic ester of formula C19H36O2 is
R 0.08 continuous process. The company purchases its hydr
Tc 33 initially at 70 bar and 303 K. If the company buys 50
Pc 12.8 cylinders it should order? For H2, T = 33 K, P = 12.8 b
a 0.229711 a = (27R2T2/64P) and b = (RT/8P) where R = 0.08 lit.a
b 0.025781 for solving the problem.
Iteration Vi f(Vi) f'(Vi)
1 1 44.1791 158.1403
2 0.720634 12.83067 71.74803
3 0.541804 3.606396 33.65331
4 0.434641 0.921389 17.26119
5 0.381262 0.175215 10.89572
6 0.36518 0.013679 9.212613
7 0.363696 0.000111 9.062689
8 0.363683 7.607E-09 9.061451
9 0.363683 3.201E-16 9.061451
10 0.363683 -1.38E-16 9.061451
f formula C19H36O2 is being hydrogenated to C19H38O ₂ by a
any purchases its hydrogen in cylinders of 10 m³ capacity
the company buys 50 days demand of H ₂ at a time, how many
H2, T = 33 K, P = 12.8 bar. Vanderwaals constants are given by
P) where R = 0.08 lit.atm/gmol.°K. Use Vanderwaals equation
You might also like
- Steam Power Board ProblemsDocument6 pagesSteam Power Board ProblemsCaguioa Mark Anthony G.100% (5)
- Material and Energy Balance of Urea Reactor and Stripper Saipem ProcessDocument20 pagesMaterial and Energy Balance of Urea Reactor and Stripper Saipem ProcessBalas43100% (1)
- Gas Turbine CalculationDocument30 pagesGas Turbine Calculationfaisalnadim100% (6)
- PPD - Module IDocument9 pagesPPD - Module IJohn Babe CapiliNo ratings yet
- Svnas 8e Ism Chapter 04Document62 pagesSvnas 8e Ism Chapter 04김성수No ratings yet
- Chemical Process CalculationsDocument23 pagesChemical Process CalculationsRishabh Patil100% (1)
- A Robert Evaporator Is Used To Concentrate A 10Document8 pagesA Robert Evaporator Is Used To Concentrate A 10Scrappy WellNo ratings yet
- Question 1 (15 Marks)Document5 pagesQuestion 1 (15 Marks)Farouk BassaNo ratings yet
- Energy Balance:: ReactionDocument7 pagesEnergy Balance:: ReactionHarsh ShahNo ratings yet
- Masuk Keluar Komponen Kg/kmol Arus 6 Arus 6 Kmol/jam Kg/jam Kmol/jam Kg/jamDocument34 pagesMasuk Keluar Komponen Kg/kmol Arus 6 Arus 6 Kmol/jam Kg/jam Kmol/jam Kg/jamYuni SartikaNo ratings yet
- Batch Reactor CPC FinalDocument56 pagesBatch Reactor CPC FinalRUTUJA PINGALENo ratings yet
- Natural Gas Homework2Document42 pagesNatural Gas Homework2Khanz KhanNo ratings yet
- Double Pipe Heat Exchanger Analysis (Example 5.1) : Water WaterDocument3 pagesDouble Pipe Heat Exchanger Analysis (Example 5.1) : Water WaterBagusRekaNo ratings yet
- Hole Size DP Length DP Od DP Id DC Length DC Od DC Id Density of Mud VP (QT) 1 1 2 3 AreaDocument9 pagesHole Size DP Length DP Od DP Id DC Length DC Od DC Id Density of Mud VP (QT) 1 1 2 3 AreamehmetNo ratings yet
- Mass BalanceDocument20 pagesMass BalanceBhaskar BethiNo ratings yet
- Kellogg Synthesis Reactor PropertiesDocument9 pagesKellogg Synthesis Reactor PropertiesMainul HaqueNo ratings yet
- PH Diagram: Diagram On The Right SideDocument13 pagesPH Diagram: Diagram On The Right SideAllyzon Mejia100% (1)
- Hexamine 1Document66 pagesHexamine 1Pradhita Ramdani HNo ratings yet
- CHNG 3802 Heat Transfer Tutorial Answers Weeks 1-4Document9 pagesCHNG 3802 Heat Transfer Tutorial Answers Weeks 1-4IshanSaneNo ratings yet
- Final Itterations: P Sat MMHG P Sat MMHG T K From Antoine Equation GivenvideolectureDocument4 pagesFinal Itterations: P Sat MMHG P Sat MMHG T K From Antoine Equation GivenvideolectureMARYAM shahNo ratings yet
- Properties of Liquids +++Document5 pagesProperties of Liquids +++vuongNo ratings yet
- Calculos: P FLORES M SULCA Tebullición (°C) Tvapor (°C) D InternoDocument2 pagesCalculos: P FLORES M SULCA Tebullición (°C) Tvapor (°C) D InternoESTEFANY RAMIREZ PALOMINONo ratings yet
- CPED Self StudyDocument3 pagesCPED Self StudyAnonymous NayakNo ratings yet
- CH 09Document22 pagesCH 09hirenpatel_universalNo ratings yet
- Solving Basic Problems Using Spreadsheets RIVERAL CHOYSON S.Document5 pagesSolving Basic Problems Using Spreadsheets RIVERAL CHOYSON S.CHOYSON RIVERALNo ratings yet
- Modeling An Atmospheric Crude Tower: - 25.17x10 Kcal/hr - 27.80x10 Kcal/hrDocument98 pagesModeling An Atmospheric Crude Tower: - 25.17x10 Kcal/hr - 27.80x10 Kcal/hrMijail PerezNo ratings yet
- Appendix B PrintsDocument27 pagesAppendix B PrintsRahmandaniDwiCahyaNo ratings yet
- Zeroth Law: Ifaandbandbandcarein Thermal Equil, Then A and C Are in Thermal EquilDocument52 pagesZeroth Law: Ifaandbandbandcarein Thermal Equil, Then A and C Are in Thermal Equilkamal El NasharNo ratings yet
- HydraulicDocument10 pagesHydraulicjema.jampit.cocNo ratings yet
- Analysis of Waste Heat Recovery Boiler Efficiency Evaluation of Boiler - Direct Method - Indirect MethodDocument7 pagesAnalysis of Waste Heat Recovery Boiler Efficiency Evaluation of Boiler - Direct Method - Indirect MethodHasan Ahmed100% (1)
- OkqdkdwfDocument9 pagesOkqdkdwfNoname GamingzNo ratings yet
- CH 12Document30 pagesCH 12hirenpatel_universal0% (3)
- Heat Exchanger Design - Lopez IsaacDocument26 pagesHeat Exchanger Design - Lopez Isaacisaac davidNo ratings yet
- Isobaric ProcessDocument24 pagesIsobaric ProcessWhindy Bagawisan CasugaNo ratings yet
- Experiment Thermo NewDocument9 pagesExperiment Thermo NewafnanhananyNo ratings yet
- Heat Exchanger Excel 22Document7 pagesHeat Exchanger Excel 22pandillero mafiosoNo ratings yet
- Waste Heat BoilerDocument16 pagesWaste Heat Boilerdevilturn70No ratings yet
- Introduction To Chemical Engineering Thermodynamics 8Th Edition Smith Solutions Manual Full Chapter PDFDocument36 pagesIntroduction To Chemical Engineering Thermodynamics 8Th Edition Smith Solutions Manual Full Chapter PDFmelissa.putnam771100% (17)
- Introduction To Chemical Engineering Thermodynamics 8th Edition Smith Solutions Manual 1Document84 pagesIntroduction To Chemical Engineering Thermodynamics 8th Edition Smith Solutions Manual 1yvonne100% (51)
- Laboratory 1 - Heat Exchanger DesignDocument6 pagesLaboratory 1 - Heat Exchanger Designapi-352575896No ratings yet
- Reactor Type: Reactor With Specified Constant Temperature of 150 CDocument8 pagesReactor Type: Reactor With Specified Constant Temperature of 150 CJay MaradiyaNo ratings yet
- Example 5 RegererativeDocument3 pagesExample 5 Regererativeرفل مهديNo ratings yet
- Deacetylenizer EndDocument6 pagesDeacetylenizer EndZohaib AliNo ratings yet
- (Saturated Water Tabel A-5) : x2 s2 - SF h2 HF + x2.hfg h2 HF + x2 - (HG - HF) 0.6731899259 1788.49169Document31 pages(Saturated Water Tabel A-5) : x2 s2 - SF h2 HF + x2.hfg h2 HF + x2 - (HG - HF) 0.6731899259 1788.49169Ravian CahyoNo ratings yet
- Chapter 8 - Tut-2Document24 pagesChapter 8 - Tut-2Raghav ChhaparwalNo ratings yet
- (Unit 4) Me 366 Solutions Manual (28 - 05 - 2021)Document7 pages(Unit 4) Me 366 Solutions Manual (28 - 05 - 2021)somenewguyonthewebNo ratings yet
- BKF 2453 Chemical Reaction EngineeringDocument6 pagesBKF 2453 Chemical Reaction EngineeringThurgah VshinyNo ratings yet
- Observed Data Shell: Nominal Size 6: Max MaxDocument19 pagesObserved Data Shell: Nominal Size 6: Max MaxTanvir AhmedNo ratings yet
- Caso de EsptudioDocument12 pagesCaso de EsptudioBenigno ChambillaNo ratings yet
- He 9Document29 pagesHe 9annisa plNo ratings yet
- HW 5 SolnDocument7 pagesHW 5 SolnNik Hafiy HafiziNo ratings yet
- Question #1 (13 Marks) : A KX MG P P PDocument10 pagesQuestion #1 (13 Marks) : A KX MG P P PFelix DawnNo ratings yet
- Chapter 03Document8 pagesChapter 03Gianne Karl AlmarinesNo ratings yet
- 14 Boiling LiquidsDocument5 pages14 Boiling LiquidsAnonymous z4Fe39jNo ratings yet
- Zentiva DataDocument19 pagesZentiva DataJay Sheth9No ratings yet
- Kurva Kesetimbangan Campuran Etanol-Air Kurva Kesetimbangan Campuran Etanol-AirDocument43 pagesKurva Kesetimbangan Campuran Etanol-Air Kurva Kesetimbangan Campuran Etanol-AirBASNo ratings yet
- Transport Processes & Separation Process Principles (Includes Unit Operations) Christie John Geankoplis Fourth EditionDocument7 pagesTransport Processes & Separation Process Principles (Includes Unit Operations) Christie John Geankoplis Fourth EditionBishoy MagdiNo ratings yet
- Perhitungan Dan Disain HeaterDocument19 pagesPerhitungan Dan Disain HeatersehonoNo ratings yet
- MCG 2131 Exam 08Document6 pagesMCG 2131 Exam 08子豪王No ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- DS CPDocument18 pagesDS CPPriyanka NagpureNo ratings yet
- CP (PC)Document9 pagesCP (PC)Priyanka NagpureNo ratings yet
- Extraction of Ethanol From CelluloseDocument20 pagesExtraction of Ethanol From CellulosePriyanka NagpureNo ratings yet
- PC (Seminar) 2Document25 pagesPC (Seminar) 2Priyanka NagpureNo ratings yet
- Excel Sheet PC CPDocument15 pagesExcel Sheet PC CPPriyanka NagpureNo ratings yet
- PC Lab-1Document3 pagesPC Lab-1Priyanka NagpureNo ratings yet