Professional Documents
Culture Documents
Screenshot 2022-11-04 at 12.01.58 PM
Screenshot 2022-11-04 at 12.01.58 PM
Uploaded by
hossainatef7Copyright:
Available Formats
You might also like
- Document 0Document3 pagesDocument 0arnolbienesraicesrmNo ratings yet
- Dwnload Full Physical Chemistry Principles and Applications in Biological Sciences 5th Edition Tinoco Solutions Manual PDFDocument36 pagesDwnload Full Physical Chemistry Principles and Applications in Biological Sciences 5th Edition Tinoco Solutions Manual PDFsithprisus100% (14)
- Screenshot 2022-11-04 at 12.01.18 PMDocument1 pageScreenshot 2022-11-04 at 12.01.18 PMhossainatef7No ratings yet
- Screenshot 2023-09-15 at 18.50.35Document1 pageScreenshot 2023-09-15 at 18.50.35moppommtyNo ratings yet
- Yo MammaDocument1 pageYo Mammananyghazi12345No ratings yet
- Screenshot 2021-08-16 at 14.41.09Document1 pageScreenshot 2021-08-16 at 14.41.09Saurabh SharmaNo ratings yet
- Screenshot 2024-01-09 at 7.46.41 PMDocument1 pageScreenshot 2024-01-09 at 7.46.41 PMemiliopro2228No ratings yet
- Augie Compassionate Pet Store - Pets Store in BangaloreDocument2 pagesAugie Compassionate Pet Store - Pets Store in BangaloreAugie PetsNo ratings yet
- Screenshot 2023-05-11 at 9.46.28 PMDocument1 pageScreenshot 2023-05-11 at 9.46.28 PMJacque Anne Marie V. GalvanNo ratings yet
- More Related Content: T.L.E. Grade 7 LessonsDocument1 pageMore Related Content: T.L.E. Grade 7 LessonsandreiNo ratings yet
- If I Find A BOT Comment, The Video Ends - YouTubeDocument1 pageIf I Find A BOT Comment, The Video Ends - YouTubeMantu SawNo ratings yet
- Bill Nye The Science Guy Atoms & Molecules - YouTubeDocument1 pageBill Nye The Science Guy Atoms & Molecules - YouTubeTorri HunsenNo ratings yet
- Screenshot 2023-12-27 at 3.40.36 AMDocument1 pageScreenshot 2023-12-27 at 3.40.36 AMridumaheshkumarNo ratings yet
- Present Value 4 (And Discounted Cash Flow) - Finance & Capital Markets - Khan Academy - YouTubeDocument4 pagesPresent Value 4 (And Discounted Cash Flow) - Finance & Capital Markets - Khan Academy - YouTubeAnonymous RQQTvjNo ratings yet
- Sequences: Find NTH Term For Quadratic (An 2 + BN + C) (Grade 6) - Onmaths Gcse Maths RevisionDocument1 pageSequences: Find NTH Term For Quadratic (An 2 + BN + C) (Grade 6) - Onmaths Gcse Maths RevisionPositive DairiesNo ratings yet
- SodaPDF Converted 29L7sqXSjFUDocument1 pageSodaPDF Converted 29L7sqXSjFUSaihudin GuramehNo ratings yet
- Screenshot 2023-10-12 at 8.44.27 AMDocument1 pageScreenshot 2023-10-12 at 8.44.27 AMHayley TsangNo ratings yet
- Screenshot 2023-11-29 at 7.38.39 PMDocument1 pageScreenshot 2023-11-29 at 7.38.39 PMayoob.moh01No ratings yet
- A LEVEL MECHANICS M1 PAST PAPER 42 FEBRUARY MARCH 2018 PART 3 IN URDU HINDI - YouTubeDocument1 pageA LEVEL MECHANICS M1 PAST PAPER 42 FEBRUARY MARCH 2018 PART 3 IN URDU HINDI - YouTubeSamah HafezNo ratings yet
- Jared in The Lush Store A BMC/DEH Parody: SearchDocument1 pageJared in The Lush Store A BMC/DEH Parody: SearchConnor CoxNo ratings yet
- Demo 1Document2 pagesDemo 1Mayank NegiNo ratings yet
- NEW Cornetto Mango Tango - YouTubeDocument1 pageNEW Cornetto Mango Tango - YouTubeferraterkimdaniel25No ratings yet
- Screenshot 2023-02-08 at 7.54.35 AMDocument1 pageScreenshot 2023-02-08 at 7.54.35 AMAshanty PalafoxNo ratings yet
- Youtu Be yJ1Py5P6FMEDocument1 pageYoutu Be yJ1Py5P6FMERobbie YbanezNo ratings yet
- R Technicallythetruth Images That Burn You - YouTubeDocument1 pageR Technicallythetruth Images That Burn You - YouTubeeajkduosNo ratings yet
- Learn Python - Full Course For Beginners (Tutorial) - YouTubeDocument22 pagesLearn Python - Full Course For Beginners (Tutorial) - YouTubeSyed Abdul AzeemNo ratings yet
- Bookmarks 3Document1 pageBookmarks 3panesarnarinderNo ratings yet
- Promil TVC 1993 - Philippines - YouTubeDocument1 pagePromil TVC 1993 - Philippines - YouTubeferraterkimdaniel25No ratings yet
- Limit Fungsi Aljabar: Dalil L'Hospital: HalodocDocument1 pageLimit Fungsi Aljabar: Dalil L'Hospital: Halodocx chanelNo ratings yet
- Document 0Document3 pagesDocument 0Silvio PonceNo ratings yet
- Physical Assessment Handouts - PDFDocument23 pagesPhysical Assessment Handouts - PDFEstefanía Rivas-García (Fefa)No ratings yet
- PANDORA (PANDORA) - YouTubeDocument1 pagePANDORA (PANDORA) - YouTubeDR KayaSNo ratings yet
- Peste Noire - La Sanie Des Siècles - Panégyrique de La Dégénérescence (Full Album) - YouTubeDocument1 pagePeste Noire - La Sanie Des Siècles - Panégyrique de La Dégénérescence (Full Album) - YouTubearyaNo ratings yet
- Bubbline Full Breakup - YouTubeDocument1 pageBubbline Full Breakup - YouTubeeggNo ratings yet
- Screenshot 2021-07-06 at 11.16.30 PMDocument1 pageScreenshot 2021-07-06 at 11.16.30 PMAlex VogelNo ratings yet
- Ship Playlist Because You Are Falling in Love - YouTubeDocument1 pageShip Playlist Because You Are Falling in Love - YouTubeJimena Robles CastilloNo ratings yet
- Employees Compensation Issues - YouTubeDocument1 pageEmployees Compensation Issues - YouTubepearly jane navalesNo ratings yet
- Screenshot 2023-12-21 at 6.46.34 PMDocument1 pageScreenshot 2023-12-21 at 6.46.34 PMOm NawaleNo ratings yet
- EYUUUTTYGGYGBDocument1 pageEYUUUTTYGGYGBeggNo ratings yet
- LEGO Car 033 Building Instructions - LEGO Classic 10696 - YouTubeDocument1 pageLEGO Car 033 Building Instructions - LEGO Classic 10696 - YouTubeLahith ChappaliNo ratings yet
- Get An Awning: Realtors DirectoryDocument40 pagesGet An Awning: Realtors DirectoryPennywise PublishingNo ratings yet
- Capture D'écran, Le 2024-02-01 À 11.23.05Document1 pageCapture D'écran, Le 2024-02-01 À 11.23.05lloydludjeNo ratings yet
- UndefinedDocument1 pageUndefinedPamela Venegas FranciaNo ratings yet
- (":TIME) Walk Down Brooklyn Br5dge 9 TX" (투모로우바이투게더) : (The @rst city of their US Tour! Free time in New York)Document1 page(":TIME) Walk Down Brooklyn Br5dge 9 TX" (투모로우바이투게더) : (The @rst city of their US Tour! Free time in New York)Celica AzaleaNo ratings yet
- $1,295 in 5 Days With Adult Method - Course Back Open - YouTubeDocument4 pages$1,295 in 5 Days With Adult Method - Course Back Open - YouTubesilenthqNo ratings yet
- Get An Awning: Realtors DirectoryDocument40 pagesGet An Awning: Realtors DirectoryPennywise PublishingNo ratings yet
- Youtube Com WatchDocument1 pageYoutube Com Watchczzdjq5szxNo ratings yet
- Screenshot 2023-10-12 at 8.40.29 AMDocument1 pageScreenshot 2023-10-12 at 8.40.29 AMHayley TsangNo ratings yet
- Screenshot Tester 143aeDocument1 pageScreenshot Tester 143aeseetjialeNo ratings yet
- 3 Hinged ArchDocument14 pages3 Hinged Arch김대경No ratings yet
- How To Convert TrigoDocument4 pagesHow To Convert TrigoOmar OuNo ratings yet
- Kassiba RSC 2 2015Document10 pagesKassiba RSC 2 2015Minh TrầnNo ratings yet
- VT Tiktok Com ZSFUu6v2yDocument1 pageVT Tiktok Com ZSFUu6v2yJeri-Mei CadizNo ratings yet
- PV Solar Technology For Energy Cost and Emissions Reduction in Rural AreaDocument4 pagesPV Solar Technology For Energy Cost and Emissions Reduction in Rural AreaSaiful Amri IsmailNo ratings yet
- Screenshot 2022-10-21 at 11.12.31 AM PDFDocument1 pageScreenshot 2022-10-21 at 11.12.31 AM PDFJoshua GreenNo ratings yet
- Youtu Be whlnzsyT9fIDocument1 pageYoutu Be whlnzsyT9fIjaoshitsbrNo ratings yet
- Screenshot 2024-05-19 at 02.18.55Document1 pageScreenshot 2024-05-19 at 02.18.55Lizzy Gaming NumberFanagramNo ratings yet
- Captura 2023-10-30 A Las 10.34.11Document1 pageCaptura 2023-10-30 A Las 10.34.11danielacassarolNo ratings yet
- Derek Prince YouTube - March2021Document2 pagesDerek Prince YouTube - March2021Giovanna VargasNo ratings yet
- Screenshot 2024-04-29 at 8.15.36 PMDocument1 pageScreenshot 2024-04-29 at 8.15.36 PMferraterkimdaniel25No ratings yet
- MCQ of RACDocument7 pagesMCQ of RACMalav Purohit100% (1)
- Boiling Point PDFDocument4 pagesBoiling Point PDFCheng Khie ChiehNo ratings yet
- JEE Chemistry Sample EbookDocument57 pagesJEE Chemistry Sample EbookmisostudyNo ratings yet
- Study ChemDocument13 pagesStudy ChemJanthina Rose AusteroNo ratings yet
- ASHRAE Psychrometric ChartDocument1 pageASHRAE Psychrometric ChartKenneth Dale San JuanNo ratings yet
- Topic 3 - Thermal Physics - IB PhysicsDocument10 pagesTopic 3 - Thermal Physics - IB PhysicsAzzahra Yeasmin SaikaNo ratings yet
- SCH 4U Molar Enthalpy and CalorimetryDocument8 pagesSCH 4U Molar Enthalpy and CalorimetryKhairatun NisaNo ratings yet
- Lesson 4 Solution ChemistryDocument34 pagesLesson 4 Solution ChemistryAaliyah Quesha LadjamatliNo ratings yet
- Scari de TemperaturaDocument25 pagesScari de Temperaturajo_rz_57No ratings yet
- Enthalpy of Formation MgODocument8 pagesEnthalpy of Formation MgOJessica Ashley HaynesNo ratings yet
- Chemical Engineering Thermodynamics: T T V S S V P T V T S P S P V TDocument22 pagesChemical Engineering Thermodynamics: T T V S S V P T V T S P S P V TSandeep CharanNo ratings yet
- Tabla y Grafica Del Vapor de Agua de ASHRAEDocument2 pagesTabla y Grafica Del Vapor de Agua de ASHRAEMiguel BejarNo ratings yet
- Thermodynamic Properties of Saturated I-ButaneDocument1 pageThermodynamic Properties of Saturated I-ButanemaikolNo ratings yet
- Paras Leah - The Boyle Model Atomic Notation Isotopes RamDocument4 pagesParas Leah - The Boyle Model Atomic Notation Isotopes Ramapi-233267698No ratings yet
- T6 - Temperature & HeatDocument5 pagesT6 - Temperature & Heatdayang ishamNo ratings yet
- Mixed Moles 5 Multiple Element ConversionsDocument21 pagesMixed Moles 5 Multiple Element Conversionsapi-483662721No ratings yet
- Estimation of Properties PDFDocument34 pagesEstimation of Properties PDFLee860531No ratings yet
- Kinetic Theory of GasesDocument32 pagesKinetic Theory of GasesBhanti100% (1)
- Unit 5 Study Guide - Sample WorkDocument3 pagesUnit 5 Study Guide - Sample WorkSarinagillNo ratings yet
- Solution Chemistry NotesDocument23 pagesSolution Chemistry NotesSanaanKhanNo ratings yet
- Avogadro's LawDocument3 pagesAvogadro's LawEn CsakNo ratings yet
- Enthalpy: H U PV H U+PV TP UDocument3 pagesEnthalpy: H U PV H U+PV TP UAnshul TeotiaNo ratings yet
- Sandler Chemical, Biochemical, and Engineering Thermodynamics Problem 7.1Document2 pagesSandler Chemical, Biochemical, and Engineering Thermodynamics Problem 7.1Egregious McAlbert0% (5)
- Public Procurement Rules 2008 BangladeshDocument211 pagesPublic Procurement Rules 2008 BangladeshOsman GoniNo ratings yet
- 4 Ponchon Savarit MethodDocument15 pages4 Ponchon Savarit MethodsirishanallakukkalaNo ratings yet
- Chapter 14Document30 pagesChapter 14mahmoudkhal334No ratings yet
- CALORIMETRYDocument10 pagesCALORIMETRYsujalsuhaas2007No ratings yet
- Solutions Board QuestionsDocument14 pagesSolutions Board QuestionsElsa HarryNo ratings yet
- COLLIGDocument3 pagesCOLLIGYokaris JTNo ratings yet
Screenshot 2022-11-04 at 12.01.58 PM
Screenshot 2022-11-04 at 12.01.58 PM
Uploaded by
hossainatef7Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Screenshot 2022-11-04 at 12.01.58 PM
Screenshot 2022-11-04 at 12.01.58 PM
Uploaded by
hossainatef7Copyright:
Available Formats
BD
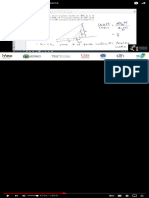
oxidation states
All From your search Electrons Ions Chemical bonds Listenable Related Watch
How to Calculate Oxidation
Numbers Introduction
Tyler DeWitt
2.4M views • 7 years ago
13:26
GCSE Chemistry - Oxidation
and Reduction - Redox…
Reactions
Cognito #39 (Higher Tier)
304K views • 3 years ago
4:54
Continuity of a function at a
point #class12 #maths…
#competitiveexams
Pranshu Kothari #function
#continuity
52 views • 23 hours ago
18:07 New
How To Calculate Oxidation
How %o Find Ox,da.,on Numbers (Rules and Examples) Numbers - Basic Introduction
Wayne Breslyn The Organic Chemistry Tutor
Subscribe 13K Share
596K subscribers 1.1M views • 5 years ago
31:15
853,205 views Dec 28, 2018 How to Balance Chemical
Using a list of simple rules you’ll learn how to Dnd the oxidation numbers for elements and Equations in 5 Easy Steps:…
compounds. For each rule there are examples and practice calculating oxidation numbers. Balancing Equations Tutorial
Wayne Breslyn
4.6M views • 6 years ago
5:01
Note: at 3:01 the oxidation number for N in N2O should be +1.
Mix - Wayne Breslyn
More practice calculating oxidation number: • Finding Oxidation... More from this channel for you
Visit http://www.breslyn.org for more chemistry help and practice.
Zbigniew Preisner Trois
--- General Rules for Determining Oxidation Numbers --- Couleurs Bleu OST 1993
Plasma Radio
1. In a neutral compound all oxidation numbers must add up to zero. 310K views • 3 years ago
41:37
2. In an ion the all oxidation numbers must add up to the charge on the ion. Good Presentation VS Bad
Presentation *
3. Free elements have an oxidation number of zero (e.g. Na, Fe, H2, O2, S8). Project IDEA
2.7M views • 5 years ago
5:13
4. Fluorine (F) = -1
How to Calculate Oxidation
5. Group 1 = +1 Group 2 = +2 Number Practice Problems
Tyler DeWitt
6.Hydrogen with Non-Metals = +1 Hydrogen with Metals (or Boron) = -1 939K views • 7 years ago
15:25
7. Oxygen = -2 (except with Fluorine or Peroxides) Oxidizing Agents and Reducing
Agents
8. Group 17(7A) = -1 Group 16 (6A) = -2 Group 15 (5A) = -3 The Organic Chemistry Tutor
260K views • 6 years ago
--- 13:39
Introduction to Oxidation States
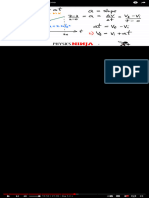
Full Explanations for compounds shown in video:
Khan Academy
442K views • 13 years ago
CaC2: -
ClO3- : • How to Dnd the O... 17:39
FeCl3: • How to Dnd the O...
Oxidation and Reduction
HCl: -
Reactions - Basic Introduction
HF: • How to Dnd the O...
The Organic Chemistry Tutor
H2O2: • How to Dnd the O...
1.1M views • 5 years ago
LiH: • How to Dnd the O... 16:05
NaCl: • How to Dnd the O...
How to Calculate the Oxidation
N2O: • How to Dnd the O...
State of Transition Metals in…
Na2O2: -
Coordination Compounds
The Complete Guide to Everything
NO: • How to Dnd the O...
34K views • 1 year ago
OF2: • How to Dnd the O... 4:58
PF5: -
How to Balance Redox
P4: • How to Dnd the O...
Equations in Acidic Solution
NCl3: • Video
Tyler DeWitt
1.3M views • 7 years ago
15:00
Key moments View all
Best of Bach - Classical Guitar
Compilation - BWV | Siccas…
Guitars
SiccasGuitars
3.7M views • 3 years ago
1:04:29
Polar and Nonpolar Molecules
0:07 0:43 1:00 2:05 5:50 The Organic Chemistry Tutor
learn the rules for Fnd the oxidation Fgure out the try to Fnd the Fgure out the 917K views • 4 years ago
assigning oxidation… numbers for a neutra… oxidation number on… oxidation number for… oxidation number for…
numbers compound the carbon in co2 each element chlorine
The Most Beautiful Equation in
Math
Transcript
Carnegie Mellon University
Follow along using the transcript. 12M views • 6 years ago
Show transcript Practice determining oxidation
states | Chemistry | Khan…
Academy
Khan Academy
478K views • 8 years ago
Show less
Salyu - Kaifuku suru Kizu
574 Comments Sort by skenzok22
247K views • 12 years ago
Add a comment...
The Riemann Hypothesis,
Explained
Pinned by Wayne Breslyn Quanta Magazine
Wayne Breslyn 3 years ago 3.6M views • 1 year ago
Note: at 3:01 the oxidation number for N in N2O should be +1. --- Dr. B
318 Reply
9 replies
Last Steak 1 year ago
You explained it better in 7 minutes than my chemistry teacher in 2 hours
131 Reply
· 4 replies
Lillian Pham 2 years ago
This is so helpful, especially during quarantine with schools closing and online school.
Thank you so much!
410 Reply
· 7 replies
Saif Ul Islam 1 month ago
You explained it better in 7 minutes than my chemistry teacher in the 9th grade...
Thanks a million
6 Reply
· 1 reply
Jasmine Benson 1 year ago
Sitting here trying to teach myself this concept before my exam this weekend.
Quarantine college is rough, but this is so helpful. Thank you so much.
170 Reply
· 27 replies
Sara 2 weeks ago
I’m a senior in school and was always bad with chemistry, my midterm is tomorrow and
I know nothing. This taught me so much thank you now I can feel more at ease knowing
I understand the concept and the process behind getting the answer. Wish me luck and
thank you!!
Reply
· 1 reply
SLNSBPsusruti 11 months ago (edited)
Very understandable !!! Thank you so much sir ! actually I were absent for
classes while this topic is going on.. today I just kept my hands on my head ! Now
I'm totally alright so proud that I understood the topic thank you
once again sir !
3 Reply
Kim Jhun 8 months ago
This makes it so easy to understand. I was so frustrated because I could not
understand this topic . Thank you so much Mr Wayne
2 Reply
Connor Gajewski 11 months ago
Much appreciated. The video was well-explained and very time elcient, the examples
were good too.
Reply
Thea Eleftheriou 1 year ago
I love this video, every time I have a quiz, test or exam I refresh my oxidation numbers
and I'm good to go. Learnt this concept during online school so this is amazing!!
3 Reply
InaXash 7 2 years ago
if not for YouTube I'd be failing chemistry right now
373 Reply
9 replies
Alyssa V 1 year ago
thank you! you explain everything so clearly and make it super simple :)
1 Reply
Aditya Sarkar 1 year ago
Thank you soo much!! I really love your fun and simple way of teaching. It makes even
the most exhausting to learn concepts, easy and clearer.
Reply
· 1 reply
B1llyTheK1d245 1 year ago
Just wanna say, your channel has been pulling me through chemistry since my
highschool years. thank you.
Reply
· 1 reply
हर हर महादेव 1 year ago
Thank you for a wonderful teaching Sir.You teach well than my chemistry teacher .Great
job
Reply
DHRUV S NAIR 2 years ago
Amazing video !!!! Never understood the concept of oxidation numbers so well before
this .....your video helped a lot !! Thank you so much !
# Respect for Wayne Breslyn!!!
3 Reply
· 1 reply
Janhavi 1 year ago
Thank you so much, it was all so easy to understand. I know exactly what to do now if I
come across any question that tells me to Dnd the oxidation number.
1 Reply
Aman shaw 3 weeks ago (edited)
In Drst 3 minutes most of my doubts got solved
Better than watching 1 hrs long videos
1 Reply
· 2 replies
Hetty Richards 2 months ago
I like your cool composure and your clear examples, Great job!
Reply
· 1 reply
Ansh Gates 2 years ago
simply perfect!!!! i got what i came here for right in the Drst example.
13 Reply
You might also like
- Document 0Document3 pagesDocument 0arnolbienesraicesrmNo ratings yet
- Dwnload Full Physical Chemistry Principles and Applications in Biological Sciences 5th Edition Tinoco Solutions Manual PDFDocument36 pagesDwnload Full Physical Chemistry Principles and Applications in Biological Sciences 5th Edition Tinoco Solutions Manual PDFsithprisus100% (14)
- Screenshot 2022-11-04 at 12.01.18 PMDocument1 pageScreenshot 2022-11-04 at 12.01.18 PMhossainatef7No ratings yet
- Screenshot 2023-09-15 at 18.50.35Document1 pageScreenshot 2023-09-15 at 18.50.35moppommtyNo ratings yet
- Yo MammaDocument1 pageYo Mammananyghazi12345No ratings yet
- Screenshot 2021-08-16 at 14.41.09Document1 pageScreenshot 2021-08-16 at 14.41.09Saurabh SharmaNo ratings yet
- Screenshot 2024-01-09 at 7.46.41 PMDocument1 pageScreenshot 2024-01-09 at 7.46.41 PMemiliopro2228No ratings yet
- Augie Compassionate Pet Store - Pets Store in BangaloreDocument2 pagesAugie Compassionate Pet Store - Pets Store in BangaloreAugie PetsNo ratings yet
- Screenshot 2023-05-11 at 9.46.28 PMDocument1 pageScreenshot 2023-05-11 at 9.46.28 PMJacque Anne Marie V. GalvanNo ratings yet
- More Related Content: T.L.E. Grade 7 LessonsDocument1 pageMore Related Content: T.L.E. Grade 7 LessonsandreiNo ratings yet
- If I Find A BOT Comment, The Video Ends - YouTubeDocument1 pageIf I Find A BOT Comment, The Video Ends - YouTubeMantu SawNo ratings yet
- Bill Nye The Science Guy Atoms & Molecules - YouTubeDocument1 pageBill Nye The Science Guy Atoms & Molecules - YouTubeTorri HunsenNo ratings yet
- Screenshot 2023-12-27 at 3.40.36 AMDocument1 pageScreenshot 2023-12-27 at 3.40.36 AMridumaheshkumarNo ratings yet
- Present Value 4 (And Discounted Cash Flow) - Finance & Capital Markets - Khan Academy - YouTubeDocument4 pagesPresent Value 4 (And Discounted Cash Flow) - Finance & Capital Markets - Khan Academy - YouTubeAnonymous RQQTvjNo ratings yet
- Sequences: Find NTH Term For Quadratic (An 2 + BN + C) (Grade 6) - Onmaths Gcse Maths RevisionDocument1 pageSequences: Find NTH Term For Quadratic (An 2 + BN + C) (Grade 6) - Onmaths Gcse Maths RevisionPositive DairiesNo ratings yet
- SodaPDF Converted 29L7sqXSjFUDocument1 pageSodaPDF Converted 29L7sqXSjFUSaihudin GuramehNo ratings yet
- Screenshot 2023-10-12 at 8.44.27 AMDocument1 pageScreenshot 2023-10-12 at 8.44.27 AMHayley TsangNo ratings yet
- Screenshot 2023-11-29 at 7.38.39 PMDocument1 pageScreenshot 2023-11-29 at 7.38.39 PMayoob.moh01No ratings yet
- A LEVEL MECHANICS M1 PAST PAPER 42 FEBRUARY MARCH 2018 PART 3 IN URDU HINDI - YouTubeDocument1 pageA LEVEL MECHANICS M1 PAST PAPER 42 FEBRUARY MARCH 2018 PART 3 IN URDU HINDI - YouTubeSamah HafezNo ratings yet
- Jared in The Lush Store A BMC/DEH Parody: SearchDocument1 pageJared in The Lush Store A BMC/DEH Parody: SearchConnor CoxNo ratings yet
- Demo 1Document2 pagesDemo 1Mayank NegiNo ratings yet
- NEW Cornetto Mango Tango - YouTubeDocument1 pageNEW Cornetto Mango Tango - YouTubeferraterkimdaniel25No ratings yet
- Screenshot 2023-02-08 at 7.54.35 AMDocument1 pageScreenshot 2023-02-08 at 7.54.35 AMAshanty PalafoxNo ratings yet
- Youtu Be yJ1Py5P6FMEDocument1 pageYoutu Be yJ1Py5P6FMERobbie YbanezNo ratings yet
- R Technicallythetruth Images That Burn You - YouTubeDocument1 pageR Technicallythetruth Images That Burn You - YouTubeeajkduosNo ratings yet
- Learn Python - Full Course For Beginners (Tutorial) - YouTubeDocument22 pagesLearn Python - Full Course For Beginners (Tutorial) - YouTubeSyed Abdul AzeemNo ratings yet
- Bookmarks 3Document1 pageBookmarks 3panesarnarinderNo ratings yet
- Promil TVC 1993 - Philippines - YouTubeDocument1 pagePromil TVC 1993 - Philippines - YouTubeferraterkimdaniel25No ratings yet
- Limit Fungsi Aljabar: Dalil L'Hospital: HalodocDocument1 pageLimit Fungsi Aljabar: Dalil L'Hospital: Halodocx chanelNo ratings yet
- Document 0Document3 pagesDocument 0Silvio PonceNo ratings yet
- Physical Assessment Handouts - PDFDocument23 pagesPhysical Assessment Handouts - PDFEstefanía Rivas-García (Fefa)No ratings yet
- PANDORA (PANDORA) - YouTubeDocument1 pagePANDORA (PANDORA) - YouTubeDR KayaSNo ratings yet
- Peste Noire - La Sanie Des Siècles - Panégyrique de La Dégénérescence (Full Album) - YouTubeDocument1 pagePeste Noire - La Sanie Des Siècles - Panégyrique de La Dégénérescence (Full Album) - YouTubearyaNo ratings yet
- Bubbline Full Breakup - YouTubeDocument1 pageBubbline Full Breakup - YouTubeeggNo ratings yet
- Screenshot 2021-07-06 at 11.16.30 PMDocument1 pageScreenshot 2021-07-06 at 11.16.30 PMAlex VogelNo ratings yet
- Ship Playlist Because You Are Falling in Love - YouTubeDocument1 pageShip Playlist Because You Are Falling in Love - YouTubeJimena Robles CastilloNo ratings yet
- Employees Compensation Issues - YouTubeDocument1 pageEmployees Compensation Issues - YouTubepearly jane navalesNo ratings yet
- Screenshot 2023-12-21 at 6.46.34 PMDocument1 pageScreenshot 2023-12-21 at 6.46.34 PMOm NawaleNo ratings yet
- EYUUUTTYGGYGBDocument1 pageEYUUUTTYGGYGBeggNo ratings yet
- LEGO Car 033 Building Instructions - LEGO Classic 10696 - YouTubeDocument1 pageLEGO Car 033 Building Instructions - LEGO Classic 10696 - YouTubeLahith ChappaliNo ratings yet
- Get An Awning: Realtors DirectoryDocument40 pagesGet An Awning: Realtors DirectoryPennywise PublishingNo ratings yet
- Capture D'écran, Le 2024-02-01 À 11.23.05Document1 pageCapture D'écran, Le 2024-02-01 À 11.23.05lloydludjeNo ratings yet
- UndefinedDocument1 pageUndefinedPamela Venegas FranciaNo ratings yet
- (":TIME) Walk Down Brooklyn Br5dge 9 TX" (투모로우바이투게더) : (The @rst city of their US Tour! Free time in New York)Document1 page(":TIME) Walk Down Brooklyn Br5dge 9 TX" (투모로우바이투게더) : (The @rst city of their US Tour! Free time in New York)Celica AzaleaNo ratings yet
- $1,295 in 5 Days With Adult Method - Course Back Open - YouTubeDocument4 pages$1,295 in 5 Days With Adult Method - Course Back Open - YouTubesilenthqNo ratings yet
- Get An Awning: Realtors DirectoryDocument40 pagesGet An Awning: Realtors DirectoryPennywise PublishingNo ratings yet
- Youtube Com WatchDocument1 pageYoutube Com Watchczzdjq5szxNo ratings yet
- Screenshot 2023-10-12 at 8.40.29 AMDocument1 pageScreenshot 2023-10-12 at 8.40.29 AMHayley TsangNo ratings yet
- Screenshot Tester 143aeDocument1 pageScreenshot Tester 143aeseetjialeNo ratings yet
- 3 Hinged ArchDocument14 pages3 Hinged Arch김대경No ratings yet
- How To Convert TrigoDocument4 pagesHow To Convert TrigoOmar OuNo ratings yet
- Kassiba RSC 2 2015Document10 pagesKassiba RSC 2 2015Minh TrầnNo ratings yet
- VT Tiktok Com ZSFUu6v2yDocument1 pageVT Tiktok Com ZSFUu6v2yJeri-Mei CadizNo ratings yet
- PV Solar Technology For Energy Cost and Emissions Reduction in Rural AreaDocument4 pagesPV Solar Technology For Energy Cost and Emissions Reduction in Rural AreaSaiful Amri IsmailNo ratings yet
- Screenshot 2022-10-21 at 11.12.31 AM PDFDocument1 pageScreenshot 2022-10-21 at 11.12.31 AM PDFJoshua GreenNo ratings yet
- Youtu Be whlnzsyT9fIDocument1 pageYoutu Be whlnzsyT9fIjaoshitsbrNo ratings yet
- Screenshot 2024-05-19 at 02.18.55Document1 pageScreenshot 2024-05-19 at 02.18.55Lizzy Gaming NumberFanagramNo ratings yet
- Captura 2023-10-30 A Las 10.34.11Document1 pageCaptura 2023-10-30 A Las 10.34.11danielacassarolNo ratings yet
- Derek Prince YouTube - March2021Document2 pagesDerek Prince YouTube - March2021Giovanna VargasNo ratings yet
- Screenshot 2024-04-29 at 8.15.36 PMDocument1 pageScreenshot 2024-04-29 at 8.15.36 PMferraterkimdaniel25No ratings yet
- MCQ of RACDocument7 pagesMCQ of RACMalav Purohit100% (1)
- Boiling Point PDFDocument4 pagesBoiling Point PDFCheng Khie ChiehNo ratings yet
- JEE Chemistry Sample EbookDocument57 pagesJEE Chemistry Sample EbookmisostudyNo ratings yet
- Study ChemDocument13 pagesStudy ChemJanthina Rose AusteroNo ratings yet
- ASHRAE Psychrometric ChartDocument1 pageASHRAE Psychrometric ChartKenneth Dale San JuanNo ratings yet
- Topic 3 - Thermal Physics - IB PhysicsDocument10 pagesTopic 3 - Thermal Physics - IB PhysicsAzzahra Yeasmin SaikaNo ratings yet
- SCH 4U Molar Enthalpy and CalorimetryDocument8 pagesSCH 4U Molar Enthalpy and CalorimetryKhairatun NisaNo ratings yet
- Lesson 4 Solution ChemistryDocument34 pagesLesson 4 Solution ChemistryAaliyah Quesha LadjamatliNo ratings yet
- Scari de TemperaturaDocument25 pagesScari de Temperaturajo_rz_57No ratings yet
- Enthalpy of Formation MgODocument8 pagesEnthalpy of Formation MgOJessica Ashley HaynesNo ratings yet
- Chemical Engineering Thermodynamics: T T V S S V P T V T S P S P V TDocument22 pagesChemical Engineering Thermodynamics: T T V S S V P T V T S P S P V TSandeep CharanNo ratings yet
- Tabla y Grafica Del Vapor de Agua de ASHRAEDocument2 pagesTabla y Grafica Del Vapor de Agua de ASHRAEMiguel BejarNo ratings yet
- Thermodynamic Properties of Saturated I-ButaneDocument1 pageThermodynamic Properties of Saturated I-ButanemaikolNo ratings yet
- Paras Leah - The Boyle Model Atomic Notation Isotopes RamDocument4 pagesParas Leah - The Boyle Model Atomic Notation Isotopes Ramapi-233267698No ratings yet
- T6 - Temperature & HeatDocument5 pagesT6 - Temperature & Heatdayang ishamNo ratings yet
- Mixed Moles 5 Multiple Element ConversionsDocument21 pagesMixed Moles 5 Multiple Element Conversionsapi-483662721No ratings yet
- Estimation of Properties PDFDocument34 pagesEstimation of Properties PDFLee860531No ratings yet
- Kinetic Theory of GasesDocument32 pagesKinetic Theory of GasesBhanti100% (1)
- Unit 5 Study Guide - Sample WorkDocument3 pagesUnit 5 Study Guide - Sample WorkSarinagillNo ratings yet
- Solution Chemistry NotesDocument23 pagesSolution Chemistry NotesSanaanKhanNo ratings yet
- Avogadro's LawDocument3 pagesAvogadro's LawEn CsakNo ratings yet
- Enthalpy: H U PV H U+PV TP UDocument3 pagesEnthalpy: H U PV H U+PV TP UAnshul TeotiaNo ratings yet
- Sandler Chemical, Biochemical, and Engineering Thermodynamics Problem 7.1Document2 pagesSandler Chemical, Biochemical, and Engineering Thermodynamics Problem 7.1Egregious McAlbert0% (5)
- Public Procurement Rules 2008 BangladeshDocument211 pagesPublic Procurement Rules 2008 BangladeshOsman GoniNo ratings yet
- 4 Ponchon Savarit MethodDocument15 pages4 Ponchon Savarit MethodsirishanallakukkalaNo ratings yet
- Chapter 14Document30 pagesChapter 14mahmoudkhal334No ratings yet
- CALORIMETRYDocument10 pagesCALORIMETRYsujalsuhaas2007No ratings yet
- Solutions Board QuestionsDocument14 pagesSolutions Board QuestionsElsa HarryNo ratings yet
- COLLIGDocument3 pagesCOLLIGYokaris JTNo ratings yet