Professional Documents
Culture Documents
Whiteboard 25 Jan 2024
Whiteboard 25 Jan 2024
Uploaded by
aryankmsingh220 ratings0% found this document useful (0 votes)
7 views1 pageThe document discusses various concepts related to chemical kinetics and equilibrium including:
1. Factors that affect the rate of a chemical reaction such as changing the concentrations of reactants.
2. The role of a catalyst in lowering the activation energy of a reaction and helping the reaction reach equilibrium faster.
3. The definition of chemical equilibrium as when the rates of the forward and reverse reactions are equal.
It provides examples and questions related to these concepts.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document discusses various concepts related to chemical kinetics and equilibrium including:
1. Factors that affect the rate of a chemical reaction such as changing the concentrations of reactants.
2. The role of a catalyst in lowering the activation energy of a reaction and helping the reaction reach equilibrium faster.
3. The definition of chemical equilibrium as when the rates of the forward and reverse reactions are equal.
It provides examples and questions related to these concepts.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
7 views1 pageWhiteboard 25 Jan 2024
Whiteboard 25 Jan 2024
Uploaded by
aryankmsingh22The document discusses various concepts related to chemical kinetics and equilibrium including:
1. Factors that affect the rate of a chemical reaction such as changing the concentrations of reactants.
2. The role of a catalyst in lowering the activation energy of a reaction and helping the reaction reach equilibrium faster.
3. The definition of chemical equilibrium as when the rates of the forward and reverse reactions are equal.
It provides examples and questions related to these concepts.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 1
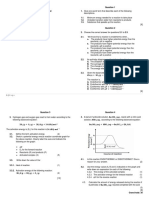
FACTORS AFFECTING RATE OF REACTION Ratio of active masses of 22g CO,, 3g H, and
1. In an elementary reaction A + 2B ’2C + D. 7g N, in a gaseous mixtureis
Ifthe concentration of Ais increased four times and (1) 22: 3:7 (2) 0.5: 3:7
B is decreased to half of its initial concentration
(3) 1:3:1 (1:3:0.5
then the rate becomes
CE0006
(1) Twice
(7. Which of the following example shows effect of
(2) Half
catalyst on reversible reaction
(3Unchanged
lt gives new reaction path with low activation
(4) One fourth of the rate
energy.
CEO001
(2)It shifts equilibrium right side.
2 The role of catalyst in a chemical reaction is :
(3) It decreases kinetic energy of activated moleaules.
(1)To help attain equilibrium ina shorter time. (4) It decreases rate of backward reaction.
(2) To lower the activation energy. CEO007
(3) To shift the equilibrium in such a way as to
8. In reversible chemical reaction equilibrium will
increase the concentration of the product
establish when
(4} Both 1& 2
CE0002
(1) Reactant is completely converted into product
(2YRate of forward and backward reaction is equal
EQUILIBRIUM AND CHEMICAL PROCESS (3) Minimum yield of product
3. X y reaction is said to be in equilibrium, (4)concentration of reactant and product is equal
when: CE0009
(1) Only 10% conversion x to y takes place. LAW OF MASS ACTION
(2)Complete conversion of x to y takes place 9. In a chemical equilibrium, the rate constant for
(3) Conversion of x to y is only 50% complete
(4Y The rate of change of x to y is just equal to the backward reaction is 7.5 x 10 and the
the rate of change of y to x in the system equilibrium constant is 1.5. The rate constant for
CE0003 the forward reaction is:
(1) 2 x103 (2) 5 x 10*
4. In the chemical reaction N, + 3H, 2NH,
at equilibrium, state whether : (3+1.12x10 (4) 9.0 x 10*
(1) Equal volumes of N, & H, are reacling CE0010
(2) Equal masses ofN, & H, are reacting 10. The equilibrium concentration of B for the
(3) The reaction has stopped
reversible reaction A B can be evaluated
4The same amount of armmonia is formed as is
decomposed into N, and H, in the same time by the expression:
CE0004
(1) K,[AI;
Active mass of 5 g CaO is :
(2) kL;A
(1) 56 (eY1 (3) 3.5 (4) 2 (4) k, k,[AJ,'
CEO005
CE0011
You might also like
- 2021 Kinetics MCQ Quiz - Worked SolnsDocument3 pages2021 Kinetics MCQ Quiz - Worked SolnsPROgamer GTNo ratings yet
- Electric Submersible Pump BasicsDocument146 pagesElectric Submersible Pump BasicsAbhoe Stank100% (6)
- Chemical EquilibriumDocument12 pagesChemical EquilibriumRaj RastogiNo ratings yet
- 1Document2 pages1rajmanesandesh6No ratings yet
- Chemical Kinetics DPP08Document3 pagesChemical Kinetics DPP08Amit KumarNo ratings yet
- Chemical KineticsDocument36 pagesChemical Kineticssinghaniamodit3No ratings yet
- Jee Mains TestDocument5 pagesJee Mains TestHaina KumariNo ratings yet
- Chemical EquilibriumDocument8 pagesChemical EquilibriumBulla AbhinavNo ratings yet
- Chemical KineticsDocument4 pagesChemical KineticsShubhankar SinhaNo ratings yet
- C09-NEET Chemical KineticsDocument21 pagesC09-NEET Chemical KineticsonehalfticketshowNo ratings yet
- Sample Paper 01 - Class 12th NEET 2024 - Chemistry - Vijay Gupta - Questions 2Document5 pagesSample Paper 01 - Class 12th NEET 2024 - Chemistry - Vijay Gupta - Questions 2adityavd179No ratings yet
- Chemical Equilibria QPDocument26 pagesChemical Equilibria QPnabihakashif444No ratings yet
- Chemistry Revision Sheet (JEE MAINS PART-II) (Day by Day) (Without Ans) Tiwari Sir 20.02.2024Document16 pagesChemistry Revision Sheet (JEE MAINS PART-II) (Day by Day) (Without Ans) Tiwari Sir 20.02.2024Priyansh jasejaNo ratings yet
- Practice Test Chapter 12 and 13Document9 pagesPractice Test Chapter 12 and 13luis arauzNo ratings yet
- 31 TT-Poll - C-31 (Chemistry) Chemical KineticsDocument5 pages31 TT-Poll - C-31 (Chemistry) Chemical KineticsNandish PatelNo ratings yet
- 2017 Y5 T4 Chem Focus - KineticsDocument4 pages2017 Y5 T4 Chem Focus - KineticsxmxmxmxmxmNo ratings yet
- Physical Test - Q - 05.07.2021Document3 pagesPhysical Test - Q - 05.07.2021joydeep17590No ratings yet
- Practice Paper 1 1Document5 pagesPractice Paper 1 1DurgadeviNo ratings yet
- Chemical Kinetics - DPP 08 - Lakshya NEET 2.0 2024Document2 pagesChemical Kinetics - DPP 08 - Lakshya NEET 2.0 2024Minaz SheikhNo ratings yet
- Equilibria SlidesDocument33 pagesEquilibria SlidesHamna MehmoodNo ratings yet
- Lakshya JEE (2024) : Chemical KineticsDocument3 pagesLakshya JEE (2024) : Chemical KineticsDev KotechaNo ratings yet
- Adobe Scan 16 Nov 2022Document16 pagesAdobe Scan 16 Nov 2022Shaik mohammed NizamuddinNo ratings yet
- Equilibrium 1 16Document15 pagesEquilibrium 1 16moholokatleho102No ratings yet
- Major Test 4 2021 FinalDocument8 pagesMajor Test 4 2021 FinalLevi JohnsonNo ratings yet
- SHEET-2-CKDocument6 pagesSHEET-2-CKpryandeepNo ratings yet
- XI-Chemistry Chapter Test-7-EquilibriumDocument5 pagesXI-Chemistry Chapter Test-7-Equilibriumcakof67215No ratings yet
- Lakshya Jee Air (2025) Chemical Kinetics: Single Correct Questions 1. 4Document3 pagesLakshya Jee Air (2025) Chemical Kinetics: Single Correct Questions 1. 4Meet ShahNo ratings yet
- Chemical Kinetics TestDocument5 pagesChemical Kinetics Testrajneesh kumarNo ratings yet
- Chemical Kinetics JEE MAINS 2022 - 966591 - 2022 - 08 - 19 - 16 - 17Document8 pagesChemical Kinetics JEE MAINS 2022 - 966591 - 2022 - 08 - 19 - 16 - 17AbhinavNo ratings yet
- 15-01-2021 Chemical & Isomerism (Autosaved)Document41 pages15-01-2021 Chemical & Isomerism (Autosaved)cric.indian.womenNo ratings yet
- XII - Revision Sheet - 2 - ChemistryDocument3 pagesXII - Revision Sheet - 2 - ChemistryVipin VNo ratings yet
- West Bengal University of TechnologyDocument4 pagesWest Bengal University of Technologysayan_dasNo ratings yet
- CRE-1 - Mid Sem 5Document2 pagesCRE-1 - Mid Sem 5Aaditya TyagiNo ratings yet
- Chemical Kinetics - Practice SheetDocument17 pagesChemical Kinetics - Practice Sheetnepogi1509No ratings yet
- Chemical Kinetics _ DPP 06 (of Lecture 10) __ Lakshya NEET 2025 (1)Document3 pagesChemical Kinetics _ DPP 06 (of Lecture 10) __ Lakshya NEET 2025 (1)ashutoshgaur995No ratings yet
- MCQs For Class XII ChemistryDocument29 pagesMCQs For Class XII Chemistryjkc collegeNo ratings yet
- (MP PET 1999) (NCERT 1989) : Chemical KineticsDocument3 pages(MP PET 1999) (NCERT 1989) : Chemical KineticsAbhishek RavirajNo ratings yet
- MCQ 1st Internal CH101 2017Document3 pagesMCQ 1st Internal CH101 2017Ankan MukherjeeNo ratings yet
- 01-Chemical Equilibirium-Theory-Final-EDocument6 pages01-Chemical Equilibirium-Theory-Final-EOutsourcing SocietyNo ratings yet
- ERRORLESS CHEMISTRY Edujournal - in - Pages-293-339 PDFDocument47 pagesERRORLESS CHEMISTRY Edujournal - in - Pages-293-339 PDFtasnimNo ratings yet
- Chemical Kinetics FinalDocument7 pagesChemical Kinetics Finalaxiliya6No ratings yet
- Practice Paper 2 Topic 16 and 17 1p5wjq1Document6 pagesPractice Paper 2 Topic 16 and 17 1p5wjq1sharm111069No ratings yet
- XII Chemistry Chapter Test 4 Chemical KineticsDocument4 pagesXII Chemistry Chapter Test 4 Chemical KineticsVishwaaNo ratings yet
- Wa0000.Document2 pagesWa0000.Bonga percyNo ratings yet
- Question On Chemical Kinetics-MA 2022Document12 pagesQuestion On Chemical Kinetics-MA 2022Sangay ChodenNo ratings yet
- CHEM311 211 Major2 SolvedDocument9 pagesCHEM311 211 Major2 SolvedhussainNo ratings yet
- Final Exam CRIM ADGEDocument9 pagesFinal Exam CRIM ADGEBernard Agcanas FelipeNo ratings yet
- This Page Is Intentionally Left BlankDocument30 pagesThis Page Is Intentionally Left Blankdivakars100% (1)
- Alkane Alkene QuestionsDocument10 pagesAlkane Alkene QuestionsormattNo ratings yet
- Equilibrium Question Set 1.: IB Questionbank Chemistry 1Document14 pagesEquilibrium Question Set 1.: IB Questionbank Chemistry 1Eduardo JimenezNo ratings yet
- Generalchemistry 2midtermDocument3 pagesGeneralchemistry 2midtermhjlouis2004No ratings yet
- Chemical Kinetics MCQDocument6 pagesChemical Kinetics MCQnewtonenergy17No ratings yet
- Chemical KineticsDocument3 pagesChemical KineticsakritiNo ratings yet
- Unit 4 Chemical Kinetics AssignmentDocument4 pagesUnit 4 Chemical Kinetics AssignmentHansika GidwaniNo ratings yet
- Equilibrium Review SL: Answers in Back!Document8 pagesEquilibrium Review SL: Answers in Back!taengooNo ratings yet
- Eq. Module QDocument6 pagesEq. Module QNeeharika ShrivastavaNo ratings yet
- Answer All Question: Sk027 / Chapter 3: Reaction Kinetics / Exercise 1Document5 pagesAnswer All Question: Sk027 / Chapter 3: Reaction Kinetics / Exercise 1kjjkimkmkNo ratings yet
- Chemical Equilibrium - DPP 01 (Of Lec 02) - Arjuna JEE 2024Document2 pagesChemical Equilibrium - DPP 01 (Of Lec 02) - Arjuna JEE 2024nrashmi743No ratings yet
- Chemical Kinetics _ DPP 07 (of Lecture 11) __ Lakshya NEET 2025Document3 pagesChemical Kinetics _ DPP 07 (of Lecture 11) __ Lakshya NEET 2025ashutoshgaur995No ratings yet
- Mock Test-2 Revision ExamDocument4 pagesMock Test-2 Revision Examariasinghhh07No ratings yet
- Lifecycle Cost ExampleDocument17 pagesLifecycle Cost ExampleAbhishek SaxenaNo ratings yet
- NGCP Draft DeterminationDocument122 pagesNGCP Draft DeterminationJake KwanNo ratings yet
- Strategies For Eliminating DecarburizationDocument3 pagesStrategies For Eliminating Decarburizationmp87_ingNo ratings yet
- Ace Main Catalogue en 04 2009 PDFDocument164 pagesAce Main Catalogue en 04 2009 PDFKrishna TiruvedulaNo ratings yet
- Pm1 Install Motor List: C2 SB C1Document26 pagesPm1 Install Motor List: C2 SB C1Mohd A IshakNo ratings yet
- UNIT CORP 10-K (Annual Reports) 2009-02-24Document264 pagesUNIT CORP 10-K (Annual Reports) 2009-02-24http://secwatch.comNo ratings yet
- OEE Industry StandardDocument30 pagesOEE Industry StandardGerardo Martin100% (2)
- MIE1514 Assignment 3 - Q2 CJDocument2 pagesMIE1514 Assignment 3 - Q2 CJJiaNo ratings yet
- Unit 1 Assignment Power DistributionDocument2 pagesUnit 1 Assignment Power Distributionapi-296582578No ratings yet
- Energy Efficiency Law RA 112851Document17 pagesEnergy Efficiency Law RA 112851Michael VinluanNo ratings yet
- Certificate Oil and Gas MGMT Collateral and SyllabusDocument4 pagesCertificate Oil and Gas MGMT Collateral and SyllabusNaz Rin ArdyNo ratings yet
- Indian Cement IndustryDocument27 pagesIndian Cement IndustrySubhamay Biswas100% (1)
- Profitability Analysis of Chilime Hydropower CompanyDocument10 pagesProfitability Analysis of Chilime Hydropower CompanySangita Ghimire50% (2)
- Ethical Obligations of Green BusinessDocument22 pagesEthical Obligations of Green BusinessMuhammad SalmanNo ratings yet
- Nav Ratna CompaniesDocument11 pagesNav Ratna CompaniesFabeena RoselineNo ratings yet
- Kinds of ParagraphDocument15 pagesKinds of ParagraphroisNo ratings yet
- Home Power Magazine Issue 031Document116 pagesHome Power Magazine Issue 031Bogdan Dragan100% (1)
- Power Safety CodeDocument23 pagesPower Safety CodeEngr Imtiaz Hussain GilaniNo ratings yet
- AHU Start-Up FormDocument4 pagesAHU Start-Up FormAhmed SofaNo ratings yet
- ATP catalogoOR Ita Def WEBDocument15 pagesATP catalogoOR Ita Def WEBSebastian QuespeNo ratings yet
- Robodon Doro I Calculation SpreadsheetDocument2 pagesRobodon Doro I Calculation SpreadsheetTg TarroNo ratings yet
- Company PresentationDocument2 pagesCompany PresentationLorena TudorascuNo ratings yet
- Vinaybhai - Docx New CVDocument5 pagesVinaybhai - Docx New CVDonNo ratings yet
- QuintaPRO Flue BrochureDocument28 pagesQuintaPRO Flue Brochurepdragos9No ratings yet
- Date Open High Low Close: Janfebmar13Document26 pagesDate Open High Low Close: Janfebmar13Badri NarayananNo ratings yet
- Dry Granulation: Made by GerteisDocument14 pagesDry Granulation: Made by GerteisBinh NguyenNo ratings yet
- Atlas Copco Compressor Filters Replacements - Filters Parts Factory - Great Price - High Performance - Airmax FiltrationDocument3 pagesAtlas Copco Compressor Filters Replacements - Filters Parts Factory - Great Price - High Performance - Airmax FiltrationseyedAli TabatabaeeNo ratings yet
- Sharekhan Morning Tiger (Pre Market Insight) 06 June 2022Document7 pagesSharekhan Morning Tiger (Pre Market Insight) 06 June 2022Kishor PrajapatiNo ratings yet