Professional Documents
Culture Documents
Expansion and Contraction-2022
Expansion and Contraction-2022
Uploaded by
x82gtj6j6z0 ratings0% found this document useful (0 votes)
4 views12 pagesThe document discusses the particle theory explanation of how solids, liquids, and gases expand when heated and contract when cooled. It states that while the size of atoms remains the same, the distance between atoms increases when heated, causing the overall volume to increase. Real-world applications of expansion and contraction discussed include expansion gaps in railways and bridges to prevent buckling, and loose telephone wires in summer to prevent snapping in winter.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document discusses the particle theory explanation of how solids, liquids, and gases expand when heated and contract when cooled. It states that while the size of atoms remains the same, the distance between atoms increases when heated, causing the overall volume to increase. Real-world applications of expansion and contraction discussed include expansion gaps in railways and bridges to prevent buckling, and loose telephone wires in summer to prevent snapping in winter.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
4 views12 pagesExpansion and Contraction-2022
Expansion and Contraction-2022
Uploaded by
x82gtj6j6zThe document discusses the particle theory explanation of how solids, liquids, and gases expand when heated and contract when cooled. It states that while the size of atoms remains the same, the distance between atoms increases when heated, causing the overall volume to increase. Real-world applications of expansion and contraction discussed include expansion gaps in railways and bridges to prevent buckling, and loose telephone wires in summer to prevent snapping in winter.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 12
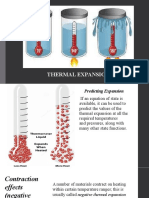
STATES OF MATTER
and the Particle Theory
Expansion and contraction
of
Solids and Liquids
Learning Objectives
• State that matter expands when it is
heated and contracts when cooled.
• Explain the expansion and contraction
of solids, liquids and gases in terms of
particle theory.
• Describe applications of expansion of
solids, liquids and gases.
How the particle theory explains this:
All three states of matter (solid, liquid and gas)
expand when heated.
The size of the atoms remains unchanged
The distance between the atoms increases.
Heat makes the atoms vibrate faster so they take up
more space.
This results in the volume/size of the matter
increasing. ( it takes up more space)
Applications in real life situations
Hot weather and railway tracks.
Buckling of track on a hot day Expansion gaps between rails.
• Train has derailed • Space allows for expansion
of track on a hot day
• No buckling of tracks
leading to accidents.
Expansion joints in bridges.
How they work….
• Bridges and sidewalks are built in segments.
• They have spaces called expansion joints
between them.
• These joints allow the concrete and steel to
expand without buckling and cracking
• The thumping sound you hear when you drive
over a bridge in a car or bus is the sound of
the tires going over the expansion joints.
Expansion & contraction of solids
When a solid is heated it gets bigger or expands.
When it cools it gets smaller or contracts.
Telephone wires are put up loose in
the summer so when winter comes
they don’t get too tight and snap.
Expansion and Contraction of Liquids
• Affect the volumes of liquids that
are used every day.
• Cars provide a good example of this.
• Cold gasoline in a car’s gas tank
expands in hot weather. If the tank is
filled to the brim, the gas may
overflow.
Scientific Explanation
You might also like
- Science 9: Heat: Alya, Kaisah, Fadhlin, FathiahDocument10 pagesScience 9: Heat: Alya, Kaisah, Fadhlin, FathiahalyaNo ratings yet
- Effects of Thermal Energy - NotesDocument7 pagesEffects of Thermal Energy - NotesChrise RajNo ratings yet
- Thermal Expansion: InstructionsDocument3 pagesThermal Expansion: InstructionsHussain RezaNo ratings yet
- 4s Thermal ExpansionDocument9 pages4s Thermal ExpansionŔosè Naćùťé PŕèéshaNo ratings yet
- Thermal Expansion of Solid Bodies by Youssef FINIANOSDocument15 pagesThermal Expansion of Solid Bodies by Youssef FINIANOSYoussefNo ratings yet
- Expansion SolidDocument31 pagesExpansion SolidWulandariNo ratings yet
- 13 Thermal ExpansionDocument1 page13 Thermal ExpansionOwais Siddiqui IX-M-ANo ratings yet
- Thermal Expansion and Phase Diagram PDFDocument22 pagesThermal Expansion and Phase Diagram PDFIvy Pearl TabagNo ratings yet
- Thermal ExpansionDocument10 pagesThermal ExpansionRonald GayetaNo ratings yet
- Thermal ExpansionDocument4 pagesThermal ExpansionEjaz YusuffNo ratings yet
- The Nature of MatterDocument29 pagesThe Nature of MatterPaul AckermannNo ratings yet
- Thermal ExpansionDocument2 pagesThermal ExpansionlovemoreworrylessNo ratings yet
- Physics Learn Malawi Form 4Document148 pagesPhysics Learn Malawi Form 4Thoko Simbeye100% (1)
- Chapter 10Document7 pagesChapter 10ranaaNo ratings yet
- PHYSICS 2:thermal ExpansionDocument25 pagesPHYSICS 2:thermal ExpansionAndreana Amor GulayNo ratings yet
- Module 3 AnswersDocument3 pagesModule 3 AnswersJamesBuensalidoDellavaNo ratings yet
- Topic: Heat Subtopic: Expansion of Solids, Liquids and Gases.Document9 pagesTopic: Heat Subtopic: Expansion of Solids, Liquids and Gases.mark lwangaNo ratings yet
- Thermal Properties of MatterDocument33 pagesThermal Properties of Mattermimoakal24No ratings yet
- The Nature of HeatDocument101 pagesThe Nature of HeatfaezzuNo ratings yet
- Chapter 10-Thermal ExpansionDocument17 pagesChapter 10-Thermal Expansionuccs1100% (2)
- (2.2) A - Thermal ExpansionDocument4 pages(2.2) A - Thermal Expansionzahra1No ratings yet
- What Is Thermal Expansion?Document2 pagesWhat Is Thermal Expansion?Just GabNo ratings yet
- Conduction Convection RadiationDocument15 pagesConduction Convection Radiationsalmanismart123No ratings yet
- 1 3242 HeatDocument4 pages1 3242 HeatFunk SoulNo ratings yet
- Applications of Thermal ExpansionDocument3 pagesApplications of Thermal ExpansionMuhammad Mosa100% (1)
- Thermal Properties of MatterDocument2 pagesThermal Properties of MatterSalman Virani0% (1)
- Kinetic Theory and GasesDocument3 pagesKinetic Theory and GasesTiARA SerrantNo ratings yet
- 0653 Term 1 NotesDocument15 pages0653 Term 1 NotesphanikrishnaakellaNo ratings yet
- CHemistry IGCSE NOTESDocument10 pagesCHemistry IGCSE NOTESjonj1ntonmasterNo ratings yet
- Kinetic Theory of Matter 2Document7 pagesKinetic Theory of Matter 2ctguni23No ratings yet
- Igcse 42 ThermalenergyDocument43 pagesIgcse 42 ThermalenergyHany ElGezawy75% (4)
- Superficial ExpansionDocument10 pagesSuperficial ExpansionLakshayaNo ratings yet
- Kinetic Theory 3Document5 pagesKinetic Theory 3Ansh GaoneadryNo ratings yet
- Apperception A Space Left: Why Is Between The Railway Line Where One Piece Joins The Next?Document31 pagesApperception A Space Left: Why Is Between The Railway Line Where One Piece Joins The Next?Dona MustikaNo ratings yet
- Test ContentDocument45 pagesTest ContentAtifa OmerNo ratings yet
- Week 6 and 7 Thermal TransferDocument25 pagesWeek 6 and 7 Thermal TransferdrishtiNo ratings yet
- Thermal Expansion of MatterDocument10 pagesThermal Expansion of MatterAkaNayep ApNo ratings yet
- Thermal Physics-Moving Particles and Expansion-2Document6 pagesThermal Physics-Moving Particles and Expansion-2manujsurangaNo ratings yet
- Solid Liquid Gas: Absorb HeatDocument60 pagesSolid Liquid Gas: Absorb HeatSyazwana ElleasNo ratings yet
- GCSE 1a1 HeatTransferDocument64 pagesGCSE 1a1 HeatTransfernitika chawlaNo ratings yet
- Fizik Thermal ExpansionDocument19 pagesFizik Thermal ExpansionMirahmad FadzlyNo ratings yet
- Fluidity of Molten MetalDocument18 pagesFluidity of Molten Metalbittu kumarNo ratings yet
- Physics 3 FIS 0124/0334: Temperature and HeatDocument22 pagesPhysics 3 FIS 0124/0334: Temperature and HeatShahul14No ratings yet
- Muschen BroekDocument24 pagesMuschen BroekArdy25No ratings yet
- Wa0003Document3 pagesWa0003manujsurangaNo ratings yet
- Assignment 1Document17 pagesAssignment 1SIVARAJ KUMARNo ratings yet
- 50+ Mechanical Engineering Interview QuestionsDocument14 pages50+ Mechanical Engineering Interview QuestionsHammad SajjadNo ratings yet
- Thermal ExpansionDocument18 pagesThermal ExpansionElleNo ratings yet
- Three States of MatterDocument23 pagesThree States of MatterTaha Tahir New acountNo ratings yet
- Physics Investigatory ProjectDocument10 pagesPhysics Investigatory Projectsamprithi mallajosyulaNo ratings yet
- 1.state ChangesDocument7 pages1.state ChangesTadiwa MawereNo ratings yet
- Principle of Expansion and Contraction of MatterDocument9 pagesPrinciple of Expansion and Contraction of MatterJessie YanYeeNo ratings yet
- Thermal PhysicsDocument15 pagesThermal PhysicsFredroNo ratings yet
- Conduction Notes PDFDocument5 pagesConduction Notes PDFgodaxonNo ratings yet
- Particulate Nature of MatterDocument39 pagesParticulate Nature of MatterruqwNo ratings yet
- Heat Exchanger For PMS S-3 and CoCDocument36 pagesHeat Exchanger For PMS S-3 and CoCAhmed BilalNo ratings yet
- Principle of Expansion and Contraction of Matter in Our Daily LifeDocument4 pagesPrinciple of Expansion and Contraction of Matter in Our Daily Lifesathiyashri veerapathiranNo ratings yet
- Img 3010Document1 pageImg 3010x82gtj6j6zNo ratings yet
- Screenshot 2023-08-30 at 5.52.17 PMDocument1 pageScreenshot 2023-08-30 at 5.52.17 PMx82gtj6j6zNo ratings yet
- 7ge Air PressureDocument1 page7ge Air Pressurex82gtj6j6zNo ratings yet
- Img - 3002 2Document6 pagesImg - 3002 2x82gtj6j6zNo ratings yet
- Screenshot 2024-03-03 at 8.44.35 PMDocument1 pageScreenshot 2024-03-03 at 8.44.35 PMx82gtj6j6zNo ratings yet
- Planet Tours - RubricDocument1 pagePlanet Tours - Rubricx82gtj6j6zNo ratings yet
- Screenshot 2024-01-29 at 8.39.41 PMDocument1 pageScreenshot 2024-01-29 at 8.39.41 PMx82gtj6j6zNo ratings yet
- Screenshot 2024-03-03 at 8.44.10 PMDocument1 pageScreenshot 2024-03-03 at 8.44.10 PMx82gtj6j6zNo ratings yet
- Exam Revision Worksheets.Document2 pagesExam Revision Worksheets.x82gtj6j6zNo ratings yet
- Acid RainDocument8 pagesAcid Rainx82gtj6j6zNo ratings yet
- Year VIIII-Exam Syllabus 2023-2024Document12 pagesYear VIIII-Exam Syllabus 2023-2024x82gtj6j6zNo ratings yet