Professional Documents
Culture Documents
Yr 12 Equilibrium
Yr 12 Equilibrium
Uploaded by
Ralph Rezin MooreOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Yr 12 Equilibrium
Yr 12 Equilibrium
Uploaded by
Ralph Rezin MooreCopyright:
Available Formats
Page 1 of 7
Chapter test
Chapter 1 Equilibrium
Name: _____________________
Class: _____________________
Time permitted: 50 minutes
Number of
Section Marks available Marks achieved
questions
A Multiple choice 15 15
B Short answer 5 15
Total 20 30
Grade: _____________________
Scale:
A+ 29–30 A 26–28 B 23–25 C 19–22 D 15–18 E 9–14 UG 0–8
Comments:
© Cengage Learning Australia Pty Ltd 2015 CHEAC12TT00059 www.nelsonnet.com.au
Page 2 of 7
Section A Multiple choice (15 marks)
Section A consists of 15 questions, each worth one mark. Each question has only one correct
answer. Circle the correct answer. Attempt all questions. Marks will not be deducted for
incorrect answers. You are advised to spend no more than 15 minutes on this section.
1 Which of the following is not a dynamic equilibrium?
A Water evaporating and condensing in a closed bottle of water
B Water evaporating and condensing in an open bottle of water
C A reaction where the concentration of reactants and products is
unchanging
D Two different reactions proceeding at the same rate in the same closed
container
2 Collision theory states a successful reaction can occur when:
A particles collide.
B the reactants are heated strongly.
C the particles collide with sufficient energy to break bonds.
D sufficient energy is released during the reaction.
3 A reaction can be sped up in several ways, including increasing the
temperature, concentration and surface area and introducing a catalyst.
N2(g) + 3H2(g) 2NH3(g) + energy
Which of these would not affect the reaction above?
A Temperature change
B Temperature change and pressure change
C Addition of more reactants
D None of the above
4 What is the correct equilibrium expression for the following reaction?
CaCO3(s) CaO(s) + CO2(g)
B
C [CO2]
D [CaCO3]
5 In an equilibrium reaction:
A all reactants become products.
B products do not form as they quickly return to reactants.
C the amount of reactants and products are the same.
D the rate of forward and reverse reactions are the same.
© Cengage Learning Australia Pty Ltd 2015 CHEAC12TT00059 www.nelsonnet.com.au
Page 3 of 7
6 The equilibrium expression equals [O2] for which of the following reactions?
A O2(l) O2(g) and 3O2(g) 2O3(g)
B O2(l) O2(g)
C 2H2O2(aq) 2H2O(l) + O2(g)
D O2(l) O2(g) and 2H2O2(aq) 2H2O(l) + O2(g)
7 Equilibrium can occur in:
A chemical reactions only.
B physical changes only.
C both chemical and physical changes.
D all chemical reactions.
8 Activation energy:
A determines whether a reaction can be reversible or not.
B only affects the rate of a reaction.
C is unaffected by catalysts.
D of a reverse reaction is the same as the forward reaction at equilibrium.
9 Dynamic equilibrium:
A occurs only at certain temperatures.
B is unaffected by temperature change.
C is when the concentrations of all species are the same.
D occurs when the concentrations of all species is constant.
10 Equilibrium has occurred in which of the following graphs of concentration?
A
© Cengage Learning Australia Pty Ltd 2015 CHEAC12TT00059 www.nelsonnet.com.au
Page 4 of 7
© Cengage Learning Australia Pty Ltd 2015 CHEAC12TT00059 www.nelsonnet.com.au
Page 5 of 7
11 The equilibrium expression, K, is based on the equation below. Which one of
the expressions shown does it equal?
aA + bB cC + dD
D
12 The reaction quotient, Q, equals the equilibrium quotient, K, at equilibrium.
Which of the following is also correct?
A If Q > K, then there are more products than reactants.
B If Q < K, then there are less products than reactants.
C If Q > 1, then there are more reactants than products.
D If K < 1, then there are more products than reactants.
13 Calculate K for the following reaction:
CH3COOH CH3COO– + H+
[CH3COOH] = 0.90 mol L–1
[CH3COO–] = 0.015 mol L–1
[H+] = 0.001 mol L–1
A K = 1.80 × 105
B K = 1.66 × 105
C K = 1.67 × 105
D K = 1.70 × 105
14 What is the correct expression of K for the following reaction?
CH3COOH CH3COO– + H+
© Cengage Learning Australia Pty Ltd 2015 CHEAC12TT00059 www.nelsonnet.com.au
Page 6 of 7
D
15 What is the correct expression(s) for the following reaction?
H2O + H2O H3O+ + OH–
A K = [H3O+]2
B K = [H3O+]2, K = [OH–]2 and K = [H3O+][ OH–]
C
D K = [H3O+][ OH–]
Section B Short answer (15 marks)
Section B consists of five questions. Write your answers in the spaces provided. You are
advised to spend 20 minutes on this section.
1 For the following equilibrium reactions, write out the expression and calculate
the constant stating which side of the reaction is favoured.
a 2SO2(g) + O2(g) 2SO3(g), [SO2] = 1 mol L–1, [O2] = 3 mol L–1, [SO3] =
2.5 mol L–1
b N2(g) + 3H2(g) 2NH3(g), [N2] = 0.4 mol L–1, [H2] = 1.5 mol L–1, [NH3] =
0.03 mol L–1
c 3O2(g) 2O3(g), [O2] = 2 mol L–1, [O3] = 0.5 mol L–1
(2 + 2 + 2 = 6 marks)
Use the following information and illustration to answer Questions 2–5.
Two flasks containing equal amounts of gases in each (hydrogen in one and iodine in the
other) and joined by a glass tube with a valve. The temperature is 330K.
© Cengage Learning Australia Pty Ltd 2015 CHEAC12TT00059 www.nelsonnet.com.au
Page 7 of 7
2 When the valve is opened, the gases mix and reach equilibrium. Describe the
colours of the gases and what observations will be observed.
(3 marks)
3 Write a balanced chemical equation and an equilibrium expression for the
reaction.
(3 marks)
4 How will the equilibrium amount of H2 (g) be affected by the addition of more
I2 (g)?
(1 mark)
5 How will the equilibrium amount of H2 (g) be affected by an increase in
temperature when the enthalpy change for the reaction is +25 kJ mol–1?
(2 marks)
© Cengage Learning Australia Pty Ltd 2015 CHEAC12TT00059 www.nelsonnet.com.au
You might also like
- Methods OT Lee Textbook NOT COMPRESSED (HIGHER QUALITY)Document265 pagesMethods OT Lee Textbook NOT COMPRESSED (HIGHER QUALITY)Ralph Rezin MooreNo ratings yet
- Quiz 13Document5 pagesQuiz 13Hằng Thanh0% (1)
- 2012 Gce A Level h2 p1 p2 p3 Qns AnsDocument62 pages2012 Gce A Level h2 p1 p2 p3 Qns AnsJoel Chia100% (2)
- HESI A2 Math Practice Tests: HESI A2 Nursing Entrance Exam Math Study GuideFrom EverandHESI A2 Math Practice Tests: HESI A2 Nursing Entrance Exam Math Study GuideNo ratings yet
- Haw Catalogue EbookDocument290 pagesHaw Catalogue Ebookneeraj pandeyNo ratings yet
- Marketing Mix: ProductDocument20 pagesMarketing Mix: ProductBao HoNo ratings yet
- Jc1 h2 2012 Promos (Paper 1)Document8 pagesJc1 h2 2012 Promos (Paper 1)Jimmy TanNo ratings yet
- 1st GENERAL CHEMISTRY 2 2nd QuarterDocument2 pages1st GENERAL CHEMISTRY 2 2nd QuarterSid Eleazar R. Gaffud100% (2)
- Chemistry: Year 12 Assessment Block Semester 1Document23 pagesChemistry: Year 12 Assessment Block Semester 1nichollsl24No ratings yet
- 2 Feb 2022Document35 pages2 Feb 2022Hannah NNo ratings yet
- General Organic and Biological Chemistry 2nd Edition Janice Gorzynski Smith Test Bank 1Document36 pagesGeneral Organic and Biological Chemistry 2nd Edition Janice Gorzynski Smith Test Bank 1amynash23052000xne100% (31)
- 122 Midterm 1 W15 Version 1 KEYDocument4 pages122 Midterm 1 W15 Version 1 KEYDrew LynchNo ratings yet
- August 1998 PDFDocument23 pagesAugust 1998 PDFATNo ratings yet
- Unit - 4 Chemical KineticsDocument16 pagesUnit - 4 Chemical KineticsDereje mathewosNo ratings yet
- Asdfghjkl GENERALCHEMDocument7 pagesAsdfghjkl GENERALCHEMfai hinchingNo ratings yet
- E6 IFY Chemistry 2 Exam - PaperDocument7 pagesE6 IFY Chemistry 2 Exam - PaperEdward MuiruriNo ratings yet
- Cambridge International AS & A Level: Chemistry 9701/11Document16 pagesCambridge International AS & A Level: Chemistry 9701/11Fupeng MouNo ratings yet
- Equilibrium Practice TestDocument11 pagesEquilibrium Practice TestAbeer MajdiNo ratings yet
- Chemistry 12 JANUARY 2001: Course Code CHDocument25 pagesChemistry 12 JANUARY 2001: Course Code CHCát TriệuNo ratings yet
- Equilibrium Multiple ChoiceDocument9 pagesEquilibrium Multiple ChoicefendiNo ratings yet
- Micki Zheng: Chapter 15 Worksheet 1 (Equilibrium)Document3 pagesMicki Zheng: Chapter 15 Worksheet 1 (Equilibrium)Happy PandaNo ratings yet
- Topic 7. Equilibrium HL PP Pack, MarkschemeDocument17 pagesTopic 7. Equilibrium HL PP Pack, MarkschemeAylin KasaNo ratings yet
- Chemistry 12: Provincial ExaminationDocument25 pagesChemistry 12: Provincial ExaminationCát TriệuNo ratings yet
- APEF Jan02Document4 pagesAPEF Jan02pei ClaudiaNo ratings yet
- U10 Packet 2022 Rates - EquilDocument9 pagesU10 Packet 2022 Rates - EquiliramtahiraNo ratings yet
- Exam 2 ChemistryDocument7 pagesExam 2 ChemistryEvelynNo ratings yet
- 6CH04 01 Que 20130612Document24 pages6CH04 01 Que 20130612Fuzzbuzz95No ratings yet
- 2019 Giraween Chemistry Trial SolutionsDocument27 pages2019 Giraween Chemistry Trial SolutionsJane YooNo ratings yet
- ExamDocument10 pagesExamEllen MarksNo ratings yet
- Chemistry Questions 0052Document9 pagesChemistry Questions 0052Ayii AlfredNo ratings yet
- Review Test Submission: Quiz 10 & Quiz 11 - Chemical Reaction & Reaction RateDocument5 pagesReview Test Submission: Quiz 10 & Quiz 11 - Chemical Reaction & Reaction RateNguyễn ThiệnNo ratings yet
- 1.1 Reaction Rate 22-23Document92 pages1.1 Reaction Rate 22-23m-423224No ratings yet
- CHEM2 Long Quiz 2Document4 pagesCHEM2 Long Quiz 2Maria Leonora PaltaoNo ratings yet
- Paper 1 QNDocument10 pagesPaper 1 QNchuasioklengNo ratings yet
- WCH02 01 Que 20150602Document24 pagesWCH02 01 Que 20150602ALorenso SAmNo ratings yet
- Chemistry: Trial ExaminationDocument46 pagesChemistry: Trial ExaminationYuanfeng WeiNo ratings yet
- CHEM 1212 202002 Exam 1 Form A KeyDocument7 pagesCHEM 1212 202002 Exam 1 Form A KeyHamza AhmedNo ratings yet
- EQUILIBRIUM - MCQ WorksheetDocument17 pagesEQUILIBRIUM - MCQ WorksheetAster LeeNo ratings yet
- Physical Chemistry MCQ Topic Quiz Lesson ElementDocument4 pagesPhysical Chemistry MCQ Topic Quiz Lesson ElementRazawu JosephNo ratings yet
- Answer: B: Selected/modified From Brown Et Al: Chemistry The Central Science, 10e, 12e, 13e TestbanksDocument6 pagesAnswer: B: Selected/modified From Brown Et Al: Chemistry The Central Science, 10e, 12e, 13e Testbanksفاطمة كليبNo ratings yet
- Ap10 Chemistry Form B q6Document9 pagesAp10 Chemistry Form B q6jessieNo ratings yet
- Exam I Review QuestionsDocument9 pagesExam I Review QuestionsRylan SmolikNo ratings yet
- Which Statement Is True About Chemical Reactions at Equilibrium?Document9 pagesWhich Statement Is True About Chemical Reactions at Equilibrium?Abdusalam IdirisNo ratings yet
- Chemistry: Written ExaminationDocument52 pagesChemistry: Written ExaminationluctonNo ratings yet
- Practice Exam 2.2-1Document7 pagesPractice Exam 2.2-1jamalNo ratings yet
- REVIEWER 3rd ADVANCED CHEM MAJOR EXAMDocument2 pagesREVIEWER 3rd ADVANCED CHEM MAJOR EXAMDanielle BihasaNo ratings yet
- Exam 5 6 7Document29 pagesExam 5 6 7Nubar MammadovaNo ratings yet
- Chemistry: Year 12 Assessment Block Semester 1Document23 pagesChemistry: Year 12 Assessment Block Semester 1nichollsl24No ratings yet
- Chemistry LDocument4 pagesChemistry L?ジェーNo ratings yet
- 00 Chemistry 2 UDocument48 pages00 Chemistry 2 UHarkaraj KangNo ratings yet
- 2 Quizizz 2019 ptVIIIe DocDocument10 pages2 Quizizz 2019 ptVIIIe DocKM Tsang Ka ManNo ratings yet
- Full Download Test Bank For General Organic and Biological Chemistry Structures of Life 6Th Edition Karen C Timberlake PDFDocument55 pagesFull Download Test Bank For General Organic and Biological Chemistry Structures of Life 6Th Edition Karen C Timberlake PDFwilliam.dean276100% (17)
- Gen-Chem 2 - Worksheet-New-Template - Quarter 4-Week-2-Days-1-to-4Document7 pagesGen-Chem 2 - Worksheet-New-Template - Quarter 4-Week-2-Days-1-to-4CJ RhodesNo ratings yet
- National Senior Certificate: Grade 12Document21 pagesNational Senior Certificate: Grade 12MfanafuthiNo ratings yet
- Please Note:: Submitting Multiple Images or Naming Your PDF Incorrectly Will Slow Down Your Application ProcessDocument18 pagesPlease Note:: Submitting Multiple Images or Naming Your PDF Incorrectly Will Slow Down Your Application Processjerzie cheethamNo ratings yet
- Aa + BB: Chapter 13 - Chemical EquilibriumDocument3 pagesAa + BB: Chapter 13 - Chemical EquilibriumAlex TingNo ratings yet
- Ap11 Chemistry Form B q3Document9 pagesAp11 Chemistry Form B q3albertosdbartNo ratings yet
- CHEM2122Document33 pagesCHEM2122Avianna ValenciaNo ratings yet
- 20.1 - Equilibrium I - Multiple Choice QP 1Document15 pages20.1 - Equilibrium I - Multiple Choice QP 1Manasha GuruprasathNo ratings yet
- Pe 2Document13 pagesPe 2smetzNo ratings yet
- Pre Progression ExamDocument19 pagesPre Progression Examhibajama72No ratings yet
- Topic 7-17 Practice Questions Key 1 2Document8 pagesTopic 7-17 Practice Questions Key 1 2Isaline GurneNo ratings yet
- College Organic Chemistry Semester II: Practice Questions with Detailed ExplanationsFrom EverandCollege Organic Chemistry Semester II: Practice Questions with Detailed ExplanationsNo ratings yet
- Chapter 6 Sample ProportionsDocument23 pagesChapter 6 Sample ProportionsRalph Rezin MooreNo ratings yet
- 2 Ex 2B - The Exponential FunctionDocument11 pages2 Ex 2B - The Exponential FunctionRalph Rezin MooreNo ratings yet
- 26 Ex 6A Area Under A GraphDocument20 pages26 Ex 6A Area Under A GraphRalph Rezin MooreNo ratings yet
- 25 Ex 5I Families of FunctionDocument10 pages25 Ex 5I Families of FunctionRalph Rezin MooreNo ratings yet
- 28 - Ex 6C Other AntiderivativesDocument18 pages28 - Ex 6C Other AntiderivativesRalph Rezin MooreNo ratings yet
- Chapter 7 Calculus of Trig FNCDocument34 pagesChapter 7 Calculus of Trig FNCRalph Rezin MooreNo ratings yet
- 04 RedoxDocument31 pages04 RedoxRalph Rezin MooreNo ratings yet
- 37 - Ex 8C Discrete Random VariablesDocument16 pages37 - Ex 8C Discrete Random VariablesRalph Rezin MooreNo ratings yet
- 27 - Ex 6B Review of AntiderivativeDocument23 pages27 - Ex 6B Review of AntiderivativeRalph Rezin MooreNo ratings yet
- Biology Unit 2 Notes LucyDocument39 pagesBiology Unit 2 Notes LucyRalph Rezin MooreNo ratings yet
- 65 - Ex 12D Confidence IntervalsDocument23 pages65 - Ex 12D Confidence IntervalsRalph Rezin MooreNo ratings yet
- Toaz - Info Davies The Trompowsky 2nd Edition PRDocument145 pagesToaz - Info Davies The Trompowsky 2nd Edition PRRalph Rezin MooreNo ratings yet
- Pathogens and Their DiseasesDocument29 pagesPathogens and Their DiseasesRalph Rezin MooreNo ratings yet
- 62 - Ex 12A Populations and SamplesDocument30 pages62 - Ex 12A Populations and SamplesRalph Rezin MooreNo ratings yet
- A Guide To Do Well in Human BiologyDocument10 pagesA Guide To Do Well in Human BiologyRalph Rezin MooreNo ratings yet
- 18 Ex 5B Rates of ChangeDocument16 pages18 Ex 5B Rates of ChangeRalph Rezin MooreNo ratings yet
- Biology Y11 ATAR Syllabus MSCDocument27 pagesBiology Y11 ATAR Syllabus MSCRalph Rezin MooreNo ratings yet
- Biology WA ATAR Units 3 & 4 Student Book With 1 X 26 Month NelsonNetBook Access CodeDocument547 pagesBiology WA ATAR Units 3 & 4 Student Book With 1 X 26 Month NelsonNetBook Access CodeRalph Rezin MooreNo ratings yet
- Methods OT Lee Revision Series SOLUTIONSDocument114 pagesMethods OT Lee Revision Series SOLUTIONSRalph Rezin MooreNo ratings yet
- 2017 Exam SolnsDocument30 pages2017 Exam SolnsRalph Rezin MooreNo ratings yet
- Chapter 12Document26 pagesChapter 12Ralph Rezin MooreNo ratings yet
- 2.physical - Properties of MatterDocument17 pages2.physical - Properties of MatterRalph Rezin MooreNo ratings yet
- 2019 Human Biology Unit 1Document41 pages2019 Human Biology Unit 1Ralph Rezin MooreNo ratings yet
- 6 Ex 4A - The Derivative ReviewDocument7 pages6 Ex 4A - The Derivative ReviewRalph Rezin MooreNo ratings yet
- 8 Ex 4C Differentiating X N When N Is NegativeDocument12 pages8 Ex 4C Differentiating X N When N Is NegativeRalph Rezin MooreNo ratings yet
- 23 Ex 5G Absolute Maximum and MinimumDocument8 pages23 Ex 5G Absolute Maximum and MinimumRalph Rezin MooreNo ratings yet
- Chapter 10Document26 pagesChapter 10Ralph Rezin MooreNo ratings yet
- Chapter 1Document17 pagesChapter 1Ralph Rezin MooreNo ratings yet
- Chapter 7Document17 pagesChapter 7Ralph Rezin MooreNo ratings yet
- m42 21 08nov21Document2 pagesm42 21 08nov21husen translatorNo ratings yet
- Worksheet CaptionDocument6 pagesWorksheet CaptionViona sephia putri67% (3)
- Eastgate PDF BrochureDocument9 pagesEastgate PDF BrochurealincostinNo ratings yet
- Afn 2 PDFDocument5 pagesAfn 2 PDFLovely Ann ReyesNo ratings yet
- Letter VirajDocument1 pageLetter VirajPratyushAgarwalNo ratings yet
- Audit ComplianceDocument10 pagesAudit ComplianceAl-Amin SarkarNo ratings yet
- Parker Valvula Direcional D3W PDFDocument20 pagesParker Valvula Direcional D3W PDFChagas OliveiraNo ratings yet
- 2023 Lupong Tagapamayapa Incentives Awards (Ltia) Assessment FormDocument4 pages2023 Lupong Tagapamayapa Incentives Awards (Ltia) Assessment FormNicole Chupungco100% (1)
- 10-1-1969 The Siege of Ben HetDocument64 pages10-1-1969 The Siege of Ben HetRobert Vale100% (1)
- Ravi LE ROCHUS - ResumeDocument1 pageRavi LE ROCHUS - ResumeRavi Le RochusNo ratings yet
- WiFi Troubleshooting CheatSheetDocument21 pagesWiFi Troubleshooting CheatSheetVee Pal100% (1)
- Customer LoyaltyDocument10 pagesCustomer LoyaltyCarlos PacaviraNo ratings yet
- T5ax6f 5CR7Document1 pageT5ax6f 5CR7HannOtto Store0% (1)
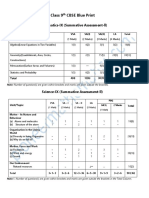
- Class 9th CBSE Blue PrintDocument2 pagesClass 9th CBSE Blue PrintYash BawiskarNo ratings yet
- Personal Branding Workbook - Ext - 2nd EdDocument23 pagesPersonal Branding Workbook - Ext - 2nd EdMiguelito JeromeNo ratings yet
- How To Complete StudentVisa Online Application InternationalDocument37 pagesHow To Complete StudentVisa Online Application InternationalAdeyinka AkinlawonNo ratings yet
- Ensemble LearningDocument7 pagesEnsemble LearningJavier Garcia RajoyNo ratings yet
- Akashic Brotherhood 4 PrintDocument33 pagesAkashic Brotherhood 4 Printmdamascus75643No ratings yet
- Ibpm 4Document64 pagesIbpm 4Miguel Angel HernandezNo ratings yet
- Winkler Test For Dissolved OxygenDocument3 pagesWinkler Test For Dissolved OxygenDOMINICNo ratings yet
- Arts Activity Sheet: Quarter 2 - Week 1-2Document8 pagesArts Activity Sheet: Quarter 2 - Week 1-2Charrie Mae MalloNo ratings yet
- PF100/PF150 Manual Postformers: Process For Postforming A Waterfall (90°) EdgeDocument1 pagePF100/PF150 Manual Postformers: Process For Postforming A Waterfall (90°) EdgeAngel CazaresNo ratings yet
- SANTOS VsDocument12 pagesSANTOS VsKristel YeenNo ratings yet
- Formula Sheet Pre-MidDocument4 pagesFormula Sheet Pre-MidUzair KhanNo ratings yet
- Democratic Life Skill 1Document1 pageDemocratic Life Skill 1andinurzamzamNo ratings yet
- Build RC Rect SectionDocument3 pagesBuild RC Rect SectionOmar NajmNo ratings yet
- SF15 XCM DS RL 29 80,100Document1 pageSF15 XCM DS RL 29 80,100Vagner PaludoNo ratings yet
- PO Lifecycle in SAPDocument76 pagesPO Lifecycle in SAPadwankarparagNo ratings yet