Professional Documents
Culture Documents
Metals Olevle
Metals Olevle
Uploaded by
aayannisarOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Metals Olevle
Metals Olevle
Uploaded by
aayannisarCopyright:
Available Formats
2
Metals and their Proper�es
Introduction
Metals contain 1, 2 or 3 electrons in outermost shell. Metals lose valence electrons to make positive ions.
Metals are generally present in first three groups 1, 2 and 3.
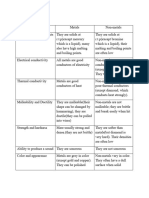
Difference in the Physical properties of metal and Non Metals
• Metals are solids at room temp with the exception of mercury and gallium which are liquids at room
temp, whereas non – metals can be solids, liquids or gases
• Luster: Metals have the quality of reflecting light from its surface and can be polished. E.g.: Gold, silver
etc. where as only diamond is lustrous, all other non - metals are non – luteus
• Malleability: metal are malleable and can be made into thin sheets called as foils. Non – metals are
brittle.
• Ductility: Metals can be drawn into wires. .
• Hardness: All metals are hard except sodium and potassium which are soft and can be cut with a
knife.
• Valency: Metals have 1 to 3 electrons in the outermost shell of their atoms. Non metals have 4, 5, 6
or 7 electrons in outermost shell
• Conduction: Metals are good conductors because they have free electrons. Non metals are insulator
except graphite
• Density: Metals have high density and are very heavy. Iridium and osmium have the highest densities
where as lithium has the lowest density.
• Melting and Boiling Point: Metals have high melting and boiling point - Tungsten has the highest
melting point where as Hg has low boiling point. Na and K have low melting points.
Chemical Reactions of metals
Reaction of Metals with Water
Potassium, Sodium, and Calcium reacts with cold water to form metal hydroxide and hydrogen
M(s) + 2H2O(l) MOH(aq) + H2(g)
Metal + Water Metal Hydroxide + Hydrogen
Reaction of metals with Steam
Magnesium, Zinc, Iron reacts with steam to form metal oxide and hydrogen
M(s) + 2H2O(g) MO(s) + H2(g)
Metal + Water Metal Oxide + Hydrogen
Iron does not react with cold water. Copper and Gold have no reaction with water and steam
Reaction of Metals with Dilute Hydrochloric Acid
Potassium, Sodium, Calcium, Magnesium, Zinc and Iron reacts with dilute hydrochloric acid to form:
M(s) + 2HCl(aq) MCl2(aq) + H2(g)
Metal + Acid Metal Chloride + Hydrogen
Lead reacts with warm hydrochloric acid slowly. Copper and Gold have no reaction with dilute
hydrochloric acid
Reaction of Metals with Oxygen
1. Metals react with oxygen to form a metallic oxide or basic oxide.
2. Active metals like (sodium Na, Potassium K) react instantaneously with oxygen to form metallic oxide.
Mubashir Sulehri [ 0322 4307040 ] O / AS / A – Level Chemistry
3
Metals and their Proper�es
Na+O2⟶Na2O
K+O2⟶K2O
3. Moderately reactive metals like (Magnesium, Aluminum, and Zinc) with oxygen after heating.
Mg+O2⟶MgO
Al+O2⟶Al2O3
4. Least reactive metals like copper Cu, Silver Ag, Gold Au do not react with oxygen.
Uses of metals
• aluminium in the manufacture of aircraft because of its low density
• aluminium in the manufacture of overhead electrical cables because of its low density and good
electrical conductivity
• aluminium in food containers because of its resistance to corrosion
• copper in electrical wiring because of its good electrical conductivity and ductility
ALLOYS
An alloy is a mixture of two or more metals fused together in molten state.
The various properties of a metal like malleability, ductility,
strength, hardness, resistance to corrosion and appearance
can be improved by mixing with other metals.
Pure metals are weak as the layers of atoms slide over each
other easily. But Alloys are harder than metals because, in
alloy of 2 metals, they have different sizes of atoms so this
disrupts the orderly layer of atoms making it difficult for atoms
to slide over.
Examples of Alloys
Cu Zn Sn
Brass 60-80% 40-20%
Bronze 80% 2% 18%
• Steel (mixture of iron, little carbon and trace elements)
• Brass (copper and zinc) – tough and corrosive-resistant
• Coin metals (copper with other metals e.g. nickel) – tough, resistant and stand up to wear
Points to Remembers
Alloys can be harder and stronger than the pure metals because the different sized atoms or ions in alloys
mean the layers of atoms [ ions ] can no longer slide over each other
stainless steel used in cutlery because of its hardness and resistance to rusting
Mubashir Sulehri [ 0322 4307040 ] O / AS / A – Level Chemistry
4
Metals and their Proper�es
Uses of Alloys
Stainless steel
is an alloy of iron containing Fe, C, Ni, Cr. This is the most expensive.
Applications for:
• Cutleries
• Medical instruments for hospital operations
• Kitchen sinks
• Steel objects in chemical factories and oil refineries
Bronze
This is an alloy of copper which contains 90% of copper and 10% of tin.
Applications for:
• It is used in preparation of statues.
• In bearings, clips, electrical connectors and springs
Reactivity Series
The reactivity series is a list of metals with the mot reactive metal at the top and the least reactive metal at the
bottom. Like Reactivity series is given as [ from more reactive to less reactive ] potassium, sodium, calcium,
magnesium, aluminum, carbon, zinc, iron, hydrogen, copper, silver, gold
Reactive metals tend to form positive ions easily, by losing electrons and forming compounds. Unreactive
metals prefer to remain in uncombined form, as the element itself. The order of reactivity is determined by their
reactions with cold water, steam, dilute acids etc.
DISPLACEMENT REACTIONS
More reactive metals can displace less reactive metals from their compounds.
• E.g. Magnesium displaces copper from copper(II) chloride
Mg(s) + CuCl2(aq) MgCl2(aq) + Cu(s)
For observation, we’ll see magnesium metal coated with brown copper metal
Displacement takes place as Mg atoms transfer electrons to Cu2+ ions forming Cu atoms.
Mg(s) → Mg2+(aq) + 2e-
Cu2+(aq) + 2e- → Cu(s)
This shows that Mg is more reactive than Cu.
• E.g. The Thermit Reaction (Displacement from metal oxides)
Similarly, when iron(III) oxide and aluminium powder are heated in a crucible, with a magnesium fuse
to start the reaction. The aluminium is more reactive and takes the oxygen from the iron oxide, leaving
molten iron at the bottom of the crucible.
Fe2O3(s) + 2Al(s) Al2O3(s) + 2Fe(l)
This is called Thermit reaction large amount of heat is produced.
Reduction of Metal Oxides
Metal oxides can be reduced into metals by
• Electrolysis of molten metal oxide
• Reacting metal oxide with carbon
• Reacting metal oxide with hydrogen
The oxides of metals above zinc in the series can only be reduced to the metal by using Electrolysis. E.g. In
electrolysis of molten Al2O3, Al is obtained.
Mubashir Sulehri [ 0322 4307040 ] O / AS / A – Level Chemistry
5
Metals and their Proper�es
2Al2O3 4Al + 3O2
The oxides below aluminium can be reduced by heating with carbon or hydrogen, except zinc oxide which
cannot be reduced by the action of hydrogen
• Reaction of Metal Oxides with Carbon
The lower the position of metal in reactivity series, the easier for carbon to remove oxygen from metal oxide
by heating. At higher position, stronger heat is needed.
E.g. CuO can by reduced into Cu by C at Bunsen burner flame temperature as Cu is at the bottom of reactivity
series
2CuO(s) + C(s) 2Cu(s) + CO2(g)
For iron oxide to be reduced, it needs very high temperature.
2FeO(s) + C(s) 2Fe(s) + CO2(g)
• Reaction of Metal Oxides with Hydrogen
The lower position of metal in reactivity series, the easier for hydrogen to remove oxygen from metal oxide by
heating. At higher position, stronger heat is needed.
E.g. PbO can be reduced by H2 at Bunsen burner flame temperature
PbO(s) + H2(g) Pb(s) + H2O(l)
Decomposition of Metal Carbonates
More reactive metal carbonates like sodium, potassium carbonates do not decompose on heating. Medium
reactive metals undergo thermal decomposition to give metal oxide and CO2.
Carbonates of more reactive metals are decomposed at higher temperature, where as carbonates of less
reactive metals are decomposed at lower temperature.
E.g. decomposition of PbCO3 takes place at higher temperature
PbCO3(s) PbO(s) + CO2(g)
whereas CuCO3 decomposes at low temperature. It can be decomposed by Bunsen burner flame
CuCO3(s) CuO(s) + CO2(g)
Mubashir Sulehri [ 0322 4307040 ] O / AS / A – Level Chemistry
6
Metals and their Proper�es
Mubashir Sulehri [ 0322 4307040 ] O / AS / A – Level Chemistry
7
Metals and their Proper�es
Mubashir Sulehri [ 0322 4307040 ] O / AS / A – Level Chemistry
8
Metals and their Proper�es
Mubashir Sulehri [ 0322 4307040 ] O / AS / A – Level Chemistry
9
Metals and their Proper�es
10
11
Mubashir Sulehri [ 0322 4307040 ] O / AS / A – Level Chemistry
You might also like
- Metals IGCSE NotesDocument27 pagesMetals IGCSE NotesMisbah Kamran100% (1)
- DmwatersDocument70 pagesDmwatersSamay Desai100% (1)
- Mainly in Group I, Group II, and The Transition Block - Those The Staircase LineDocument14 pagesMainly in Group I, Group II, and The Transition Block - Those The Staircase LineOrderPlace AccountNo ratings yet
- Class 10 Metals and Non Metals NotesDocument12 pagesClass 10 Metals and Non Metals NotesShreyash VishwakarmaNo ratings yet
- Metals and Non Metals - NotesDocument13 pagesMetals and Non Metals - NotesmittalshivamNo ratings yet
- CSEC Chem Metals Chemistry of Gardening EtcDocument25 pagesCSEC Chem Metals Chemistry of Gardening Etcdela2100% (2)
- Metals, Non Metals, and MetallurgyDocument13 pagesMetals, Non Metals, and MetallurgyM. Amebari NongsiejNo ratings yet
- Metals NotesDocument19 pagesMetals NotesELIJAH MUPETANo ratings yet
- Metal and NonmetalDocument26 pagesMetal and NonmetalSudhanshu Sekhar PandaNo ratings yet
- MetalsDocument12 pagesMetalsdela2No ratings yet
- Metal Notes #1Document7 pagesMetal Notes #1swcaptain2008No ratings yet
- Metals and Non MetalsDocument10 pagesMetals and Non MetalsKaran MahajanNo ratings yet
- Metals PreliminaryDocument26 pagesMetals PreliminaryIra Katriel NunagNo ratings yet
- Metals and Non Metals - Shobhit NirwanDocument17 pagesMetals and Non Metals - Shobhit NirwanBhaskar 8287No ratings yet
- DownloadedDocument12 pagesDownloadedAniket shuklaNo ratings yet
- Chapter 3 - Metals and Non MetalsDocument17 pagesChapter 3 - Metals and Non Metalskush96122No ratings yet
- Grade 12 Chemistry Booklet-1Document55 pagesGrade 12 Chemistry Booklet-1MATITO MATITONo ratings yet
- 3 NOV Class 10 Metals and Non-Metals ChemDocument40 pages3 NOV Class 10 Metals and Non-Metals Chemgourav kaliaNo ratings yet
- Metals and Non MetalsDocument57 pagesMetals and Non MetalsLOLBOINo ratings yet
- Metals NotesDocument4 pagesMetals NotesDiyaNo ratings yet
- C13 Properties of Metals PC SlidesDocument39 pagesC13 Properties of Metals PC SlidesBasil ChinNo ratings yet
- Metals and Non-MetalsDocument9 pagesMetals and Non-Metalsmonkey.luffy.kenNo ratings yet
- Metals and Non MetalsDocument9 pagesMetals and Non Metalsvishwath donepudiNo ratings yet
- Metals and Non Metals NotesDocument3 pagesMetals and Non Metals NotesVUDATHU SHASHIK MEHERNo ratings yet
- 3 NOV Class 10 Metals and Non-Metals ChemDocument40 pages3 NOV Class 10 Metals and Non-Metals Chemgourav kaliaNo ratings yet
- Metals - PropertiesDocument66 pagesMetals - Propertiesrheanna.bartonNo ratings yet
- Metals and Non-MetalsDocument23 pagesMetals and Non-MetalsAnonymous ufMAGXcskMNo ratings yet
- Metals and Non MetalsDocument15 pagesMetals and Non Metals2erwr100% (2)
- Metals and Non Metals N 1Document8 pagesMetals and Non Metals N 1rincyNo ratings yet
- Revision Notes On Materials Metals and Non-MetalsDocument9 pagesRevision Notes On Materials Metals and Non-MetalsHoang HaNo ratings yet
- Shiksha Group Education: Chemical Properties of MetalsDocument18 pagesShiksha Group Education: Chemical Properties of MetalsHarshit RajputNo ratings yet
- Success in ScienceDocument60 pagesSuccess in ScienceopilemutaleNo ratings yet
- Metals and Non MetalsDocument28 pagesMetals and Non MetalsALEENANo ratings yet
- Padhle 10th - Metal & Non-Metals Lecture SlidesDocument25 pagesPadhle 10th - Metal & Non-Metals Lecture SlidesBitan DasNo ratings yet
- Metals and Non-MetalsDocument23 pagesMetals and Non-MetalsPetrichorNo ratings yet
- The Periodic Table of ElementsDocument41 pagesThe Periodic Table of ElementsPawan GoswamiNo ratings yet
- AssignmentDocument13 pagesAssignmentfarusadiq.77No ratings yet
- Metals and Non MetalsDocument9 pagesMetals and Non MetalsKrishna SharmaNo ratings yet
- METALSDocument12 pagesMETALSjpkaomeNo ratings yet
- Science: (Chemistry)Document22 pagesScience: (Chemistry)Isaiah JohnsonNo ratings yet
- HB NotesDocument7 pagesHB Noteskeki kekiNo ratings yet
- Properties of MetalsDocument17 pagesProperties of MetalsDavies MasumbaNo ratings yet
- Chapter - 8 MetalDocument12 pagesChapter - 8 Metalamit_idea1No ratings yet
- Class X Science NotesDocument222 pagesClass X Science NotesPhani Kumar AllamrajuNo ratings yet
- 10 Science Notes 03 Metals and Non Metals 1Document10 pages10 Science Notes 03 Metals and Non Metals 1Rishu KaulNo ratings yet
- Chapter 4Document16 pagesChapter 4Bhavya JangidNo ratings yet
- Metals Non-MetalsDocument18 pagesMetals Non-MetalsJayaKumar SNo ratings yet
- Metals Extraction 2Document9 pagesMetals Extraction 2sujana hossainNo ratings yet
- Metals and Non-Metals Notes - RemovedDocument15 pagesMetals and Non-Metals Notes - RemovedCyber Atharv100% (1)
- Metals and Non-Metals NotesDocument18 pagesMetals and Non-Metals NotesMustafa Khan100% (1)
- Metals and The Reactiviy Series-NotesDocument4 pagesMetals and The Reactiviy Series-NotesAhmad Shafiq ZiaNo ratings yet
- MetalDocument14 pagesMetalsusanNo ratings yet
- Metals and Non-Metals NotesDocument18 pagesMetals and Non-Metals NotesAzeem IqbalNo ratings yet
- Metals, Alloys & Extraction of MetalsDocument22 pagesMetals, Alloys & Extraction of MetalsbuffuigokuNo ratings yet
- Uydz Uw WV USKa N61 MM JC 4Document6 pagesUydz Uw WV USKa N61 MM JC 4varshatagade126No ratings yet
- Metals and Non Metals Class 10Document8 pagesMetals and Non Metals Class 10Gowtham LNo ratings yet
- Metals and Their Properties - Physical and ChemicalDocument5 pagesMetals and Their Properties - Physical and Chemicalcourtz911No ratings yet
- b4b13229 14 Metals and Their ExtractionDocument21 pagesb4b13229 14 Metals and Their ExtractionMuhammad UzairNo ratings yet
- Metals and Non MetalsDocument9 pagesMetals and Non Metalsbhumika motiyaniNo ratings yet
- The Reactivity Series of MetalsDocument27 pagesThe Reactivity Series of Metals118latelyNo ratings yet
- Why Do Metals Rust? An Easy Read Chemistry Book for Kids | Children's Chemistry BooksFrom EverandWhy Do Metals Rust? An Easy Read Chemistry Book for Kids | Children's Chemistry BooksNo ratings yet
- How To Prepare Potash Alum From AluminiumDocument8 pagesHow To Prepare Potash Alum From AluminiumRed HawkNo ratings yet
- Aluminum Industry Primer V2Document20 pagesAluminum Industry Primer V2lknjcidh98fnkci8No ratings yet
- Sheet HydroformingDocument7 pagesSheet HydroformingwalidnasriNo ratings yet
- TMS 2002Document3 pagesTMS 2002Daniel StuparekNo ratings yet
- ElectrochemistryDocument2 pagesElectrochemistryfiqaNo ratings yet
- As 2503.1-2006 Refractories and Refractory Materials - Chemical Analysis Silica RefractoriesDocument7 pagesAs 2503.1-2006 Refractories and Refractory Materials - Chemical Analysis Silica RefractoriesSAI Global - APACNo ratings yet
- Aluminium and Aluminium Production Training Courses - Innoval TechnologyDocument4 pagesAluminium and Aluminium Production Training Courses - Innoval TechnologyArmanul NasarNo ratings yet
- Mil-P-23377 Rev JDocument18 pagesMil-P-23377 Rev JM H Bed100% (1)
- Phosphates and Phosphoric Acid in Everyday LifeDocument13 pagesPhosphates and Phosphoric Acid in Everyday LifeGeorge Van BommelNo ratings yet
- Concepts of Acids and Bases-Theory & ExerciseDocument53 pagesConcepts of Acids and Bases-Theory & ExerciseRaju SinghNo ratings yet
- Icse Class 8 Chemistry Sample Paper Set 2Document3 pagesIcse Class 8 Chemistry Sample Paper Set 2Prashant DhotreNo ratings yet
- Neet-Jee MetallurgyDocument14 pagesNeet-Jee MetallurgySudheerkhan MuhammedNo ratings yet
- Yeka Subcity Chemistry Model ExamDocument4 pagesYeka Subcity Chemistry Model ExamKerod Mohamed100% (3)
- Cambridge IGCSE: Chemistry 0620/12Document16 pagesCambridge IGCSE: Chemistry 0620/12Tamer AhmedNo ratings yet
- Main Group Oganometallics: Shriver and Atkins, Chapter 15Document24 pagesMain Group Oganometallics: Shriver and Atkins, Chapter 15José Augusto VillarNo ratings yet
- CITATION Mac10 /L 1033Document2 pagesCITATION Mac10 /L 1033kundayi shavaNo ratings yet
- 000000027828 - 202004222153266xxx계 알루미늄 합금의 경질 아노다이징 피막 형성 특성 연구Document41 pages000000027828 - 202004222153266xxx계 알루미늄 합금의 경질 아노다이징 피막 형성 특성 연구임학진No ratings yet
- Gcse Chemistry: Metals & The Reactivity SeriesDocument28 pagesGcse Chemistry: Metals & The Reactivity SeriesShashibhushan AshokNo ratings yet
- Acce 01 0007Document4 pagesAcce 01 0007Ram RajwadeNo ratings yet
- MercuryDocument4 pagesMercuryJessalyn ValeNo ratings yet
- Ironing Pressing FinishingDocument5 pagesIroning Pressing FinishingAman DeepNo ratings yet
- Werkstoffdatenblatt: Cw614N (Cuzn39Pb3 - 2.0401)Document1 pageWerkstoffdatenblatt: Cw614N (Cuzn39Pb3 - 2.0401)tapanNo ratings yet
- A Review: Mesoporous Santa Barbara Amorphous-15, Types, Synthesis and Its Applications Towards Biorefinery ProductionDocument8 pagesA Review: Mesoporous Santa Barbara Amorphous-15, Types, Synthesis and Its Applications Towards Biorefinery ProductionbenahmedNo ratings yet
- Gen Chem - Finals ReviewerDocument9 pagesGen Chem - Finals ReviewerMariel Dela Cruz TeanilaNo ratings yet
- ENCHO - Project Risk Management and Forecasting Assignment 1 V1Document12 pagesENCHO - Project Risk Management and Forecasting Assignment 1 V1Akum obenNo ratings yet
- What Are The Metals With Low Thermal ConductivityDocument11 pagesWhat Are The Metals With Low Thermal ConductivityjackNo ratings yet
- AS5780D-Specification For Aero and Aero-Derived Gas Turbine Engine LubricantsDocument18 pagesAS5780D-Specification For Aero and Aero-Derived Gas Turbine Engine LubricantsJicheng PiaoNo ratings yet
- Cera System Catalogue 2011Document36 pagesCera System Catalogue 2011prihartono_diasNo ratings yet
- 4.final Project ReportDocument52 pages4.final Project ReportShubham KansalNo ratings yet