Professional Documents
Culture Documents
Kinetics 03
Kinetics 03
Uploaded by
smytwx62jzCopyright:
Available Formats
You might also like
- Mechanism Multiple Choice QuestionsDocument4 pagesMechanism Multiple Choice QuestionsAnonymous pgjIAZoNo ratings yet
- KINETICS Practice Problems and SolutionsDocument9 pagesKINETICS Practice Problems and SolutionsnairdanipsoNo ratings yet
- Chemistry 132 NT: Nothing Great Was Ever Achieved Without EnthusiasmDocument50 pagesChemistry 132 NT: Nothing Great Was Ever Achieved Without Enthusiasmlorraine_cuaNo ratings yet
- Ntroduction To Eaction Echanisms: E U: L O: E KDocument3 pagesNtroduction To Eaction Echanisms: E U: L O: E KJannah ElmaghrabyNo ratings yet
- 5.4 Elementary Reactions StudentDocument3 pages5.4 Elementary Reactions Studenthoàng michelleNo ratings yet
- Chapter - 13 - Chemical - Kinetics (Compatibility Mode) PDFDocument40 pagesChapter - 13 - Chemical - Kinetics (Compatibility Mode) PDFLel Lailiy100% (1)
- Chemical Kinetics: Class 9.2Document4 pagesChemical Kinetics: Class 9.2Voora GowthamNo ratings yet
- Kinetika KimiaDocument35 pagesKinetika Kimiablank-56No ratings yet
- Laju Reaksi PDFDocument9 pagesLaju Reaksi PDFHafsemi RapsanjaniNo ratings yet
- Deriving Rate Laws From Reaction Mechanisms Involving Equilibrium Elementary Steps With AnswersDocument5 pagesDeriving Rate Laws From Reaction Mechanisms Involving Equilibrium Elementary Steps With AnswersSukaran SinghNo ratings yet
- 13lectureppt 101122094708 Phpapp01Document41 pages13lectureppt 101122094708 Phpapp01Salim Ahmad Salim AlirajaNo ratings yet
- Chemical Kinetics: A Study On Reaction Rate and MechanismDocument34 pagesChemical Kinetics: A Study On Reaction Rate and MechanismM Akhsanul FikriNo ratings yet
- Reaction MechanismDocument37 pagesReaction MechanismNurshuhada NordinNo ratings yet
- Chemical Kinetics: Rate of Reaction MechanismDocument60 pagesChemical Kinetics: Rate of Reaction MechanismAneudis Javier BritoNo ratings yet
- Introduction To Chemical Kinetics: CHEM 102 T. HughbanksDocument16 pagesIntroduction To Chemical Kinetics: CHEM 102 T. HughbanksKarthikNo ratings yet
- 11 Reaction KineticsDocument95 pages11 Reaction KineticsSyamil Adzman100% (1)
- Rate of ReactionsDocument51 pagesRate of ReactionsEisa IshaqzaiNo ratings yet
- Chemical Kinetics-12Document18 pagesChemical Kinetics-12Manas ChhabraNo ratings yet
- Final Exam - TA Class - Updated 12 15 2022Document70 pagesFinal Exam - TA Class - Updated 12 15 2022tran huyNo ratings yet
- Zumdahl Chemical Kinetics NotesDocument6 pagesZumdahl Chemical Kinetics NotesMalletNjonkemNo ratings yet
- Chemical KineticsDocument13 pagesChemical KineticsOM SableNo ratings yet
- Reaction MechanismsDocument4 pagesReaction MechanismsAryaa KapilNo ratings yet
- Chemical Kinetics: Chung (Peter) Chieh Professor of Chemistry University of Waterloo Waterloo, Ontario, CanadaDocument34 pagesChemical Kinetics: Chung (Peter) Chieh Professor of Chemistry University of Waterloo Waterloo, Ontario, Canadadescar84No ratings yet
- Chemical KineticsDocument2 pagesChemical KineticsMoonlit CosmicNo ratings yet
- Stem H Galvez Chemistry ModulesDocument6 pagesStem H Galvez Chemistry ModulesLorenzblazeeNo ratings yet
- Chemical Equilibrium: Cato Maximilian Guldberg and His Brother-In-Law Peter Waage Developed The Law of Mass ActionDocument19 pagesChemical Equilibrium: Cato Maximilian Guldberg and His Brother-In-Law Peter Waage Developed The Law of Mass Actionmonster40lbsNo ratings yet
- Tutorial 1 SolutionsDocument20 pagesTutorial 1 Solutionsanushka shagunNo ratings yet
- D (A) DTDocument19 pagesD (A) DTSingh AnujNo ratings yet
- 16.2 Reaction Mechanism: IntermediateDocument13 pages16.2 Reaction Mechanism: IntermediateGauri ThakurNo ratings yet
- Mechanisms1Document14 pagesMechanisms1Anthony Mafuta MayilameneNo ratings yet
- ws13 3Document4 pagesws13 3Abdurahman KrisNo ratings yet
- CH 14 Kinetics Part1 WebDocument39 pagesCH 14 Kinetics Part1 Webblue educationNo ratings yet
- Kinetics PracticeDocument1 pageKinetics PracticekateliubanskyNo ratings yet
- Paper: Chemistry:: Chemistry MCQ: Chemical KineticsDocument10 pagesPaper: Chemistry:: Chemistry MCQ: Chemical KineticsShakeel AhmadNo ratings yet
- Feb2020-Chuong4-Toc Do Va Co Che Phan Ung Hoa HocDocument36 pagesFeb2020-Chuong4-Toc Do Va Co Che Phan Ung Hoa HocHồng NgọcNo ratings yet
- Kinetics: The Rates and Mechanisms of Chemical ReactionsDocument88 pagesKinetics: The Rates and Mechanisms of Chemical ReactionsKishore KishoreNo ratings yet
- CHEM 26.1 ReviewerDocument6 pagesCHEM 26.1 ReviewerClara MirabuenoNo ratings yet
- Suny Chemistryformajorsxmasterchapterreaction Mechanisms Missing FormulasDocument1 pageSuny Chemistryformajorsxmasterchapterreaction Mechanisms Missing Formulasavni jainNo ratings yet
- Chem Kinetics Ib QuestionsDocument26 pagesChem Kinetics Ib QuestionsDhruv KhuranaNo ratings yet
- Chapter No 6 - Chemical KineticsDocument45 pagesChapter No 6 - Chemical KineticsTanish SalviNo ratings yet
- DP Chemical KineticsDocument32 pagesDP Chemical KineticsAniket RayNo ratings yet
- CHM 096 Tutorial 1Document4 pagesCHM 096 Tutorial 1Muhammad ShafiqNo ratings yet
- C CC CDocument9 pagesC CC CAkhil KhannaNo ratings yet
- Chem102 Chemical Kinetics 3Document22 pagesChem102 Chemical Kinetics 3Zabo TrewNo ratings yet
- 201B Work 1 KineticsDocument9 pages201B Work 1 Kineticsahraz93No ratings yet
- 12 Chemical Kinetics - CN - STDT7Document3 pages12 Chemical Kinetics - CN - STDT7Nkemzi Elias NzetengenleNo ratings yet
- Topic 1 and 2-ChemicalKineticsDocument86 pagesTopic 1 and 2-ChemicalKineticsNOR AZAM BIN ENDOT / FSNo ratings yet
- Chapter 17 - Rate LawsDocument30 pagesChapter 17 - Rate Lawsjim tannerNo ratings yet
- Kinetic Latesst Part A 2016Document79 pagesKinetic Latesst Part A 2016cikgu_aminNo ratings yet
- Chapter 5 Chemical Kinetics - ExercisesDocument7 pagesChapter 5 Chemical Kinetics - Exercisestran huyNo ratings yet
- Handout No. in General Chemistry 2Document7 pagesHandout No. in General Chemistry 2Portgas D. AceNo ratings yet
- SCH 305Document34 pagesSCH 305Qwin NajyaNo ratings yet
- Kinetics MC CrackAPDocument7 pagesKinetics MC CrackAPhylee102594No ratings yet
- Chapter 2 - Chemical KineticsDocument92 pagesChapter 2 - Chemical KineticsJohan Daniyal100% (1)
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Critical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsFrom EverandCritical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
Kinetics 03
Kinetics 03
Uploaded by
smytwx62jzOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Kinetics 03
Kinetics 03
Uploaded by
smytwx62jzCopyright:
Available Formats
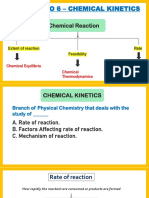
12.
5 REACTION MECHANISMS
Kinetics gives us information about steps involved in a
reaction (reaction mechanism)
Example: NO2(g) + CO(g) NO(g) + CO2(g)
Experimentally: Rate = k [NO2]2
Proposed mechanism
K1
NO2(g) + NO2(g) NO3(g) + NO(g) (1st elementary step)
slow
K2
NO3(g) + CO(g) NO2(g) + CO2(g) (2nd elementary step)
fast
____________________________________
NO2(g) + CO(g) NO(g) + CO2(g) (overall reaction)
NO3(g) is an intermediate, mechanism is composed of
elementary steps.
Intermediate: a species that is neither a reactant nor a product but is
formed and consumed during the reaction sequence.
Elementary step: a reaction whose rate law can be written from its
molecularity (coefficients in balanced equation).
Slow-step: it is the rate-determining step (RDS).
Requirements for a proper mechanism:
1. The sum of the elementary steps must give the overall balanced
equation for the reaction.
2. The mechanism must agree with the experimentally determined rate
law.
Example: 2NO2(g) + F2(g) 2NO2 F(g)
Experimentally: Rate = k [NO2] [F2]
Suggested mechanism
k1
NO2(g) + F2(g) NO2 F(g) + F (slow)
k2
F(g) + NO2(g) NO2 F(g) (fast)
Is this an acceptable mechanism?
Answer: Check the two requirements for a proper mechanism:
1. The sum of elementary steps gives the overall reaction:
2NO2(g) + F2(g) 2NO2 F(g) (overall reaction) ✓
2. Rate law from slow step == experimental rate law ✓
∴ Yes, this is an acceptable mechanism.
Example: Write the rate law for the elementary reactions:
A) O3 + NO O2 + NO2 (bimolecular reaction: two reactants)
B) CH3NC CH3CN (unimolecular reaction: one reactant)
Answer: Since the two given reactions are elementary, the rate raw can
be written from their molecularity.
A) O3 + NO O2 + NO2 Rate = k [O3] [NO]
B) CH3NC CH3CN Rate = k [CH3NC]
Example: A proposed mechanism for a reaction is:
C4H9Br C4H9+ + Br - (slow)
C4H9+ + H2O C4H9OH2+ (fast)
C4H9OH2+ + H2O C4H9OH + H3O+ (fast)
A) Write rate law for this mechanism.
B) Write overall reaction.
C) What are the intermediates?
Answer:
A) Slow step is RDS (1st step) Rate = k [C4H9Br]
B) C4H9Br + 2H2O Br - + C4H9OH + H3O+
C) C4H9+ and C4H9OH2+
You might also like
- Mechanism Multiple Choice QuestionsDocument4 pagesMechanism Multiple Choice QuestionsAnonymous pgjIAZoNo ratings yet
- KINETICS Practice Problems and SolutionsDocument9 pagesKINETICS Practice Problems and SolutionsnairdanipsoNo ratings yet
- Chemistry 132 NT: Nothing Great Was Ever Achieved Without EnthusiasmDocument50 pagesChemistry 132 NT: Nothing Great Was Ever Achieved Without Enthusiasmlorraine_cuaNo ratings yet
- Ntroduction To Eaction Echanisms: E U: L O: E KDocument3 pagesNtroduction To Eaction Echanisms: E U: L O: E KJannah ElmaghrabyNo ratings yet
- 5.4 Elementary Reactions StudentDocument3 pages5.4 Elementary Reactions Studenthoàng michelleNo ratings yet
- Chapter - 13 - Chemical - Kinetics (Compatibility Mode) PDFDocument40 pagesChapter - 13 - Chemical - Kinetics (Compatibility Mode) PDFLel Lailiy100% (1)
- Chemical Kinetics: Class 9.2Document4 pagesChemical Kinetics: Class 9.2Voora GowthamNo ratings yet
- Kinetika KimiaDocument35 pagesKinetika Kimiablank-56No ratings yet
- Laju Reaksi PDFDocument9 pagesLaju Reaksi PDFHafsemi RapsanjaniNo ratings yet
- Deriving Rate Laws From Reaction Mechanisms Involving Equilibrium Elementary Steps With AnswersDocument5 pagesDeriving Rate Laws From Reaction Mechanisms Involving Equilibrium Elementary Steps With AnswersSukaran SinghNo ratings yet
- 13lectureppt 101122094708 Phpapp01Document41 pages13lectureppt 101122094708 Phpapp01Salim Ahmad Salim AlirajaNo ratings yet
- Chemical Kinetics: A Study On Reaction Rate and MechanismDocument34 pagesChemical Kinetics: A Study On Reaction Rate and MechanismM Akhsanul FikriNo ratings yet
- Reaction MechanismDocument37 pagesReaction MechanismNurshuhada NordinNo ratings yet
- Chemical Kinetics: Rate of Reaction MechanismDocument60 pagesChemical Kinetics: Rate of Reaction MechanismAneudis Javier BritoNo ratings yet
- Introduction To Chemical Kinetics: CHEM 102 T. HughbanksDocument16 pagesIntroduction To Chemical Kinetics: CHEM 102 T. HughbanksKarthikNo ratings yet
- 11 Reaction KineticsDocument95 pages11 Reaction KineticsSyamil Adzman100% (1)
- Rate of ReactionsDocument51 pagesRate of ReactionsEisa IshaqzaiNo ratings yet
- Chemical Kinetics-12Document18 pagesChemical Kinetics-12Manas ChhabraNo ratings yet
- Final Exam - TA Class - Updated 12 15 2022Document70 pagesFinal Exam - TA Class - Updated 12 15 2022tran huyNo ratings yet
- Zumdahl Chemical Kinetics NotesDocument6 pagesZumdahl Chemical Kinetics NotesMalletNjonkemNo ratings yet
- Chemical KineticsDocument13 pagesChemical KineticsOM SableNo ratings yet
- Reaction MechanismsDocument4 pagesReaction MechanismsAryaa KapilNo ratings yet
- Chemical Kinetics: Chung (Peter) Chieh Professor of Chemistry University of Waterloo Waterloo, Ontario, CanadaDocument34 pagesChemical Kinetics: Chung (Peter) Chieh Professor of Chemistry University of Waterloo Waterloo, Ontario, Canadadescar84No ratings yet
- Chemical KineticsDocument2 pagesChemical KineticsMoonlit CosmicNo ratings yet
- Stem H Galvez Chemistry ModulesDocument6 pagesStem H Galvez Chemistry ModulesLorenzblazeeNo ratings yet
- Chemical Equilibrium: Cato Maximilian Guldberg and His Brother-In-Law Peter Waage Developed The Law of Mass ActionDocument19 pagesChemical Equilibrium: Cato Maximilian Guldberg and His Brother-In-Law Peter Waage Developed The Law of Mass Actionmonster40lbsNo ratings yet
- Tutorial 1 SolutionsDocument20 pagesTutorial 1 Solutionsanushka shagunNo ratings yet
- D (A) DTDocument19 pagesD (A) DTSingh AnujNo ratings yet
- 16.2 Reaction Mechanism: IntermediateDocument13 pages16.2 Reaction Mechanism: IntermediateGauri ThakurNo ratings yet
- Mechanisms1Document14 pagesMechanisms1Anthony Mafuta MayilameneNo ratings yet
- ws13 3Document4 pagesws13 3Abdurahman KrisNo ratings yet
- CH 14 Kinetics Part1 WebDocument39 pagesCH 14 Kinetics Part1 Webblue educationNo ratings yet
- Kinetics PracticeDocument1 pageKinetics PracticekateliubanskyNo ratings yet
- Paper: Chemistry:: Chemistry MCQ: Chemical KineticsDocument10 pagesPaper: Chemistry:: Chemistry MCQ: Chemical KineticsShakeel AhmadNo ratings yet
- Feb2020-Chuong4-Toc Do Va Co Che Phan Ung Hoa HocDocument36 pagesFeb2020-Chuong4-Toc Do Va Co Che Phan Ung Hoa HocHồng NgọcNo ratings yet
- Kinetics: The Rates and Mechanisms of Chemical ReactionsDocument88 pagesKinetics: The Rates and Mechanisms of Chemical ReactionsKishore KishoreNo ratings yet
- CHEM 26.1 ReviewerDocument6 pagesCHEM 26.1 ReviewerClara MirabuenoNo ratings yet
- Suny Chemistryformajorsxmasterchapterreaction Mechanisms Missing FormulasDocument1 pageSuny Chemistryformajorsxmasterchapterreaction Mechanisms Missing Formulasavni jainNo ratings yet
- Chem Kinetics Ib QuestionsDocument26 pagesChem Kinetics Ib QuestionsDhruv KhuranaNo ratings yet
- Chapter No 6 - Chemical KineticsDocument45 pagesChapter No 6 - Chemical KineticsTanish SalviNo ratings yet
- DP Chemical KineticsDocument32 pagesDP Chemical KineticsAniket RayNo ratings yet
- CHM 096 Tutorial 1Document4 pagesCHM 096 Tutorial 1Muhammad ShafiqNo ratings yet
- C CC CDocument9 pagesC CC CAkhil KhannaNo ratings yet
- Chem102 Chemical Kinetics 3Document22 pagesChem102 Chemical Kinetics 3Zabo TrewNo ratings yet
- 201B Work 1 KineticsDocument9 pages201B Work 1 Kineticsahraz93No ratings yet
- 12 Chemical Kinetics - CN - STDT7Document3 pages12 Chemical Kinetics - CN - STDT7Nkemzi Elias NzetengenleNo ratings yet
- Topic 1 and 2-ChemicalKineticsDocument86 pagesTopic 1 and 2-ChemicalKineticsNOR AZAM BIN ENDOT / FSNo ratings yet
- Chapter 17 - Rate LawsDocument30 pagesChapter 17 - Rate Lawsjim tannerNo ratings yet
- Kinetic Latesst Part A 2016Document79 pagesKinetic Latesst Part A 2016cikgu_aminNo ratings yet
- Chapter 5 Chemical Kinetics - ExercisesDocument7 pagesChapter 5 Chemical Kinetics - Exercisestran huyNo ratings yet
- Handout No. in General Chemistry 2Document7 pagesHandout No. in General Chemistry 2Portgas D. AceNo ratings yet
- SCH 305Document34 pagesSCH 305Qwin NajyaNo ratings yet
- Kinetics MC CrackAPDocument7 pagesKinetics MC CrackAPhylee102594No ratings yet
- Chapter 2 - Chemical KineticsDocument92 pagesChapter 2 - Chemical KineticsJohan Daniyal100% (1)
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Critical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsFrom EverandCritical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)