Professional Documents
Culture Documents
Cee Rev
Cee Rev
Uploaded by
Jorica Bren MinervaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Cee Rev
Cee Rev
Uploaded by
Jorica Bren MinervaCopyright:
Available Formats
GENERAL BIOLOGY [Ms. Jeanette Anne J.
Desamparado]
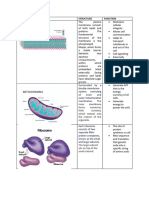
Cell Membrane the immune system as role in cell adhesion.
Why cell membrane is semi permeable? self or non-self.
Because of phospholipid bilayer
MEMBRANE PROTEIN FUNCTIONS
Six Major Functions [by proteins of the plasma membrane]
1. Transport
2. Enzymatic Activity
3. Signal Transduction
4. Cell-cell Recognition
5. Intercellular Joining
6. Attached to the cytoskeleton and extracellular matrix
(ECM).
Cholesterol, lubricant that helps bigger substances to
move. It is also present in the membrane. It maintains
The phospholipid is made out of glycerol. the fluidity and increases the stability of the membrane
Hydrophilic head, water loving (because it adapts to the with cholesterol; Membrane would easily split apart.
surrounding environment).
Hydrophobic tail, water fearing. TRANSPORT ACROSS THE CELL MEMBRANE
Deviation of transport mechanisms – how cells or
FUNCTIONS OF CELL MEMBRANE substances move in and outside of the cell membrane.
Providing a Selectively Permeable Barrier Types of Transport
Transferring Solutes Passive Transport
Transporting Macromolecules Active Transport
(GO Foods, Grow, Glow, Biomolecules)
Responding to External Signals Passive Transport
Intercellular Interaction
- high concentration of
Energy Transduction Concentration
molecules
Gradient
Fluid Mosaic Model
Describes the cell membrane as a tapestry of several
Movements of ions and other atomic or molecular
types of molecules (phospholipids, cholesterols, and
substances across the cell membrane without the
proteins) that are constantly moving. This movement
need of energy input.
helps the cell membrane maintain its role as a barrier
between the inside and the outside of the cell
Types of Passive Transport
environments.
Simple Diffusion
S.J Singer & Garth L. Nicolson (1972)
Facilitated Diffusion
Explained the structure of the plasma membrane
Osmosis
(mosaic of components).
Phospholipids, Cholesterol, Proteins, and carbohydrates
Diffuse
that gives the membrane a fluid character. Simple Diffusion [does not use transport protein]
o Random movements of particles or molecules of a
Structures Found in the Cell Membrane
solute from an area of higher concentration to an area of
Phospholipid bilayer, critical components of cell
lower concentration.
membrane. Lipid bilayer acts as a barrier to the passage
o Most basic and easy.
of molecules and ions into and out of the cell.
Carbohydrates (present in plasma membrane),
Factors Affecting Diffusion of Materials
sometimes branched chains of sugars attached either to
Size
exterior peripheral proteins (forming glycoproteins) or to
Polarity
the polar ends of phospholipid molecules in the outer
Charge
lipid layer (forming glycolipids).
As recognition, molecules are also important during
Facilitated Diffusion [uses transport protein]
embryonic development.
o Transport proteins are needed to move molecules from
Are major recognition and attaching sites for
an area of greater concentration to an area of lesser
pathogens during infection.
concentration.
Pathogens are disease causing microorganisms.
2 Types of Transport Protein
Glycolipid Glycoprotein
o Carrier Protein, embedded in the plasma or cell
[glyco – sugar] [glyco – sugar] membrane. It can change structure to fit in with what
Carbohydrates attached Carbohydrates attached molecules pass through.
to lipid. to protein. o Channel Protein, charged particles (ions) pass through
Serve as cell markers or Serve as receptors for the channel protein.
antigens recognized by chemical signals play a Osmosis
f:17 Natalie ashley b. grio [12 – stem 1 brr ] 1
GENERAL BIOLOGY [Ms. Jeanette Anne J. Desamparado]
o Water molecules move from an area of higher o Receptor-Mediated – found in pit coated with a protein
concentration to an area of lesser concentration. called clathrin (enzymes that can catalyst the LDL)
LDL – LOW DENSITY LIPOPROTEIN
ENVIRONMENT OF THE CELL
“Tonicity of a Cell” Exocytosis
o Isotonic Solution o PROCESS which cells move materials from within the
o Hypotonic Solution cell into the extracellular fluid.
o Hypertonic Solution o Transcription – one major process in cell dogma.
o Passageway outside of the cell.
Isotonic Solution, the concentration of solute inside & o Occurs when a vesicle fuses with the plasma
outside of the cell is the same. membrane, allowing its contents to be released outside
Hypotonic Solution, the concentration of solute is lower of the cell.
outside the cell than inside the cell. o Removing toxins or waste products from the cell’s
It has more water outside the cell, so water moves interior.
into the cell. o Facilitating cellular communication.
Hypertonic Solution, the concentration of solute is o Facilitating cellular membrane growth, repair, signaling,
higher outside the cell than inside the cell. and migration.
Have more water inside the cell, so water moves out
of the cell. Where do these vesicles go?
- It goes to the mitochondria (generates energy).
BIOMOLECULES (ORGANIC)
Bio ~ life Molecules ~ substances
Monomers “Monosaccharides”
Building Blocks
4 Major Types of Biomolecules
o Carbohydrates (pasta, bread) “Energy” CHO
o Lipids (fatty acids, glycerol) “Fats” CHO
Provide Insulation.
o Proteins (meat, beans) “Amino Acids” CHON
Muscle Development.
o Nucleic Acids (DNA)(RNA) “Nucleotides” CHONP
C - carbon
Carbon & Hydrogen =bonding
H - hydrogen
O - oxygen
N - nitrogen
P - phosphorus
Biomolecules Elements Monomers Bonding
Found
Carbohydrate C,H,O Monosaccharides Glycosidic
s or Simple Sugar Bond
Lipids C,H,O Fatty Acids & No Specific
Active Transport [mainly C & H, but few O]
Glycerol Bonding
- Needs a lot of energy input; movements of molecules from Proteins C,H,O,N Amino Acids Peptide Bond
an area of lower concentration to an area of higher Nucleic Acid C,H,O,N,P Nucleotide Phosphodiester
Bond
concentration.
Bulk (vesicular) Transport Mechanism Carbohydrates
- Movement of macromolecules, such as proteins or o Quick energy
polysaccharides into or out of the cell which require the o Sugar, Chocolate, Bread, Pasta, Fruits, Vegetables
expenditure of energy (ATP). o Macromolecules necessary for immediate use of energy,
storage energy and cell structure.
2 Types of Bulk
Endocytosis Types
Exocytosis Polysaccharides (MANY)
Structural – guides shape Storage
Endocytosis Cellulose (plant) Glycogen (can be
o PROCESS by which cells take in substances from found in the liver)
outside of the cell by engulfing them in a vesicle. Chitin (fungi) Starch (plant)
o Helps generate energy. Peptidoglycan
o Phagocytosis – cell eating (solid) (bacteria)
o Pinocytosis – cell drinking (liquid)
f:17 Natalie ashley b. grio [12 – stem 1 brr ] 2
GENERAL BIOLOGY [Ms. Jeanette Anne J. Desamparado]
Note: Poly = Many Di = Two Mono = One An insulating layer, or sheath that forms around nerves,
Saccharides = Sugar including those in the brain and spinal cord. Made up of protein
and fatty substances. This myelin sheath allows electrical
impulses to transmit quickly and efficiently along the nerve
Disaccharides (TWO) cells. If myelin is damaged, these impulses slow down.
Major Disaccharides Types of Protein Structures
o Maltose > Glucose + Glucose o Primary structure
o Lactose > Glucose + Galactose o Secondary structure
o Sucrose > Glucose + Fructose o Tertiary structure
o Quaternary structure
Monosaccharides (ONE); BUILDING BLOCKS
o Glucose – product after photosynthesis.
o Fructose – “fruits”; sweetest natural sugar.
o Galactose – milk. Dipeptide bond
Amino Amino
DIABETES
Type I Type II
o Insulin tolerant o Auto immune disease
Process where quaternary structure converts to primary
o Hereditary o Not curable
structure: Denaturation
Glucagon Insulin
Nucleic Bonds
For higher insulin
o Nucleotides are its monomer.
Insulin
o The more C-H bonds a biomolecule has, the more
Regulates sugar
energy it has!
o Fats have the most energy because they have the most
Lipids [are hydrophobic]
C-H bonds.
o Mainly Carbon & Hydrogen.
o Fat is the best method of STORING.
Contains 3 parts
o Forms cell membranes.
5-carbon sugar
o Insulates nerve cells (Myelin)
Phosphate group
o Insulates body (maintains homeostasis)
Nitrogenous base
o Monomer: 3 fatty acids + glycerol
Lipid Metabolism – term when we get energy from lipids.
Triglycerides
Phospholipids
Steroids
Waxes
Proteins [control speed of chemical reactions; growth & repair]
o The most diverse among the biomolecules.
o Central compound of life.
o Amino acids is its monomer.
o Provides us with the building blocks for life. Energy – ability to do work.
o Also regulate most functions in a cell. Newton’s first law of Thermodynamics:
o Glycoproteins (antigens). ”Energy cannot be created or destroyed; it can only be
o Combines with DNA to form chromosomes. transformed from one form to another”
o Turns genes on and off. Once a cell has used energy to do work, it cannot be
used again by any organism.
o Antibodies (fight disease).
White Blood Cells: Immunoglobulin
Food Chain
Red Blood Cells: Hemoglobin
o Provides structure and strength (fibers).
o Transport molecules in and out cells.
o Hemoglobin (transports O2). Sunlight → Plants → Worms → Roosters → Human
100% 80% 60% 40%
o Enzymes (speeds up rxns).
has -ase suffix
o Acts as hormones (insulin).
Helps in secondary growth development. Nucleobases:
o We have 20 amino acids. (adenine) ATP – primary energy currency of cell.
o Essential: what we eat. Has bigger storage; energy.
o Non - Essential: what we make. (guanine) GTP - guanosine triphosphate.
ATP & GTP are under purines.
Mylin Sheath
ATP (Adenosine triphosphate)
f:17 Natalie ashley b. grio [12 – stem 1 brr ] 3
GENERAL BIOLOGY [Ms. Jeanette Anne J. Desamparado]
o A single molecule ATP contains ten carbon atoms, o The set of metabolic pathways that construct molecules
sixteen hydrogen, five nitrogen, thirteen oxygen and from smaller units. These reactions require energy,
three phosphorus atoms. known also as an endergonic process.
o The secret behind the power of ATP lies in the breaking
of the chemical bond between the second and third
phosphate groups. When this happens, a large amount
of energy is released. PHOTOSYNTHESIS
o ATP is water soluble. o Comes from the 2 words: photo and synthesis.
Photo = light
ATP structure Synthesis = combine or mix
o It is a process wherein the light energy is converted to
make chemical energy (glucose).
o Photons – basic unit of light.
o Absorbs light and its wave.
o
Karl Lahmann (1898-1978)
Discovered ATP from extracts of muscles and liver.
His analysis on the ATP structure was in close The stem also
competition with the other scientists but he succeeded transports the
through hydrolysis. nutrients.
Hydrolysis
o Any chemical reaction in which a molecule of water
ruptures one or more chemical bonds.
o
Additional Information:
ATPases
o A group of enzymes that catalyze the hydrolysis of a
phosphate bond in adenosine triphosphate (ATP) to
form adenosine diphosphate (ADP).
Phosphorylation & Hydrolysis
o Pi = Inorganic Phosphate
Epidermis, in botany, outermost, protoderm-derived
Exergonic & Endergonic layer of cells covering the stem, root, leaf, flower, fruit,
o Exergonic – releases energy. and seed parts of a plant.
o Endergonic – absorbs energy.
Parts of Leaf
Metabolism [sum total of the chemical inside our body] Mesophyll Cell
o The process involving chemical reactions needed to o Found in dermal tissue
maintain life processes. Specifically, located in upper and lower dermal tissues.
Catabolism o Specialized cell that contains the chloroplast.
o Breakdowns macro particles into smaller particles. Chlorophyll – green pigments.
o Responsible for breaking complex molecules down into primary pigment used in photosynthesis.
Plants contain two main forms of chlorophyll: a & b.
smaller molecules.
Chlorophyll has a hydrocarbon tail that anchors it to
o During catabolism, energy is released from the bonds of
an integral protein in the thylakoid membrane of the
the large molecules being broken down. chloroplast. Chlorophyll is the source of the green
o color of plants and certain other autotrophs.
Chloroplast (double membrane, has inner & outer part)
site of photosynthesis.
organelle in a plant cell where photosynthesis occurs.
Parts of Chloroplast
Thylakoid
o Where light dependent reactions occur.
Granum – stacks of thylakoid.
Anabolism Grana – many stacks of thylakoid (plural term).
o Builds up macro molecules.
o From small to big molecules. Stroma
o Fluid part of the chloroplast.
f:17 Natalie ashley b. grio [12 – stem 1 brr ] 4
GENERAL BIOLOGY [Ms. Jeanette Anne J. Desamparado]
o Where light independent reactions occur. o refers to the gain of electrons. It often occurs in
Also known as “Calvin Cycle” or dark reaction. conjunction with oxidation.
o These 2 reactions are interconnected with each other. Rubisco, an enzyme that bonds carbon dioxide with
RuBP.
Stomata [ stoma: plural ] Examples [can undergo photosynthesis]
o “Breathing organ” or nose of the plant. (a) Mosses, ferns, and flowering plants.
o 2 guard cells. ○ Releases oxygen. (b) Kelp
Terminologies (c) Euglena
ADP (d) Cyanobacteria
o Stands for adenosine diphosphate, a product of the Visible Lights – among all EM waves.
Calvin Cycle that is used in the light-dependent Light Energy:
reactions. 6CO₂+6H₂O > C₆H₁₂O₆+6O₂
ATP
o Stands for adenosine triphosphate. ATP is a major 2 Reactions in Photosynthesis
energy molecule in cells. ATP and NADPH are products o Light Dependent Reaction
of the light- dependent reactions in plants. ATP is used
in reduction and regeneration of RuBP.
Autotrophs
o Are photosynthetic organisms which convert light energy
into the chemical energy they need to develop, grow,
and reproduce.
Calvin Cycle
o Name given to the set of chemical reactions of
photosynthesis that does not necessarily require light.
The Calvin cycle takes place in the stroma of the
chloroplast. It involves the fixing of carbon dioxide into
glucose using NADPH and ATP.
carbon dioxide (CO2)
o A gas naturally found in the atmosphere that is a
reactant for the Calvin Cycle. o Light Independent Reaction; Dark Reaction or Calvin
Carbon Fixation Cycle
o ATP and NADPH are used to fix CO2 into
carbohydrates. Carbon fixation takes place in the General Equation for Photosynthesis
chloroplast stroma. o Reactants, also called raw materials or input.
Light o Product, also called “output”.
o A form of electromagnetic radiation; the shorter the End product
wavelength the greater amount of energy. Light supplies o Products that are not released to the atmosphere
the energy for the light reactions of photosynthesis. because they are still needed in the process.
Light harvesting complexes (photosystems Biproduct
complexes) o Are released to the atmosphere because they are not
o A photosystem (PS) complex is a multi-protein unit in needed in the process.
the thylakoid membrane that absorbed light to serve as Coenzyme are capable to be electron carrier.
energy for reactions inside the thylakoid. Carbon Fixation, a process wherein inorganic
Light reactions (light dependent reactions) compounds are converted to organic compound.
o Are chemical reactions requiring electromagnetic energy
(light) that occur in the thylakoid membrane of the
chloroplast to convert light energy into chemical forms
ATP and NAPDH.
Lumen
o Region within the thylakoid membrane where water is
split to obtain oxygen. The oxygen diffuses out of the
cell, while the protons remain inside to build positive
electrical charge.
o Fluid area of Thylakoid.
NADPH, A high-energy electron carrier used in
reduction.
LIGHT REACTION
Oxidation refers to the loss of electrons.
3 major processes of light reaction
Oxygen (O2), a gas that is a product of the light-
o Reduction
dependent reactions
Photosynthesis Losses electrons.
o The process by which organisms convert light energy o Oxidation
into chemical energy (glucose). Gains electrons.
Reduction o Photophosphorylation
f:17 Natalie ashley b. grio [12 – stem 1 brr ] 5
GENERAL BIOLOGY [Ms. Jeanette Anne J. Desamparado]
the process where ADP is converted to ATP using Rubisco – enzyme, capable of converting inorganic to
light energy. organic (ribulose biphosphate carboxylase/oxygenase)
can be found in the atmosphere
Photosystem I & II
o Protein complex capable of absorbing light energy. RUBP is located inside stroma
First to process: Photosystem II
Discovered First: Photosystem I
Photosystem I Photosystem II Reduction
o Will gain electron from ATP and NADPH.
Capacity to absorb P700 Capacity to absorb P680
o 3 PGA will undergo reduction.
o If ATP will bind with PGA, 1-3 biphosphoglycerate will
ATP synthase
o Factory of ATP
o ADP + P = ATP
Chemiosmosis, making of gradians of hydrogen ion.
Reaction Center, where electrons rests.
Light Independent Cycle
o It is Cyclic.
o Occurs in stroma of chloroplast.
o Primary compound to start dark reaction is carbon
dioxide.
o 2 molecules that is essential: ATP and NADPH.
o Can process even without direct input of light energy.
Melvin Calvin be formed.
o Bind NADPH to PGA to produce Glyceraldehyde.
G3P = Glyceraldehyde 3 Phosphate
Regeneration
o Will start if G3P is made. [6 G3P is needed, 1 will serve
[store] as a starter to form glucose and the other 5 will
regenerate again with ATP to form RUBP.
Cellular Respiration
GLYCOLYSIS
o Location: Inside cytoplasm
o Doesn’t need oxygen (anaerobic)
o Glucose is converted into pyruvate.
o Net yield: 2 pyruvate, 2 ATP molecules, and 2 NADH.
o NADPH- co enzyme, has the ability to transfer electrons.
3 Major Process (Cycle of Light Independent Reaction)
Intermediate Step:
Carbon Fixation
o Pyruvate transported by active transport into
Reduction
mitochondria, particularly mitochondrial matrix
Regeneration
o The two pyruvates are converted into 2 acetyl CoA
Raw Materials: CO2 & NADPH o Carbon dioxide is released and 2 NADH is produced.
C02 + RUBP – [Inside of Stroma]
(1,5- ribulose biphosphate)\ KREBS CYCLE
o Location: Mitochondrial matrix
Carbon fixation o Considered as aerobic, doesn’t directly consume oxygen
o First step in the cycle of light independent reaction. but some process needs oxygen.
o Conversion of inorganic to organic compound. o Carbon dioxide is released.
o Converts CO2 (inorganic, from atmosphere) to organic o Outputs: 2 ATP, 6 NADH and 2 FADH
with the help of rubisco. o FADH is also a co-enzyme and will assist in transferring
electrons to make even more ATP.
ELECTRON TRANSPORT CHAIN AND
CHEMIOSMOSIS
o Location: Inside inner mitochondrial membrane
o Aerobic
o Electrons are transferred from NADH and FADH to
protein complex and electron carriers, the electrons are
used to generate proton gradient, as protons are
f:17 Natalie ashley b. grio [12 – stem 1 brr ] 6
GENERAL BIOLOGY [Ms. Jeanette Anne J. Desamparado]
pumped across to cross into inter membrane space. All Citric acid is responsible to help ETC to make many
these protons pumped out of inter membrane space electrons.
generates chemical and electrical gradient
o In chemiosmosis, the protons travel down their
electrochemical gradient through a portion of the ATP
synthase, powering it to make ATP.
Oxygen is the final acceptor of electrons, when oxygen
combines to hydrogen, you get H2O. ELECTRON TRANSPORT CHAIN
o Crucial step it is responsible for the making of many
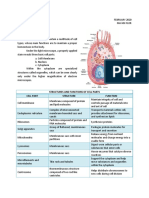
CELLULAR RESPIRATION electrons
o Occurs in mitochondrion 2 Process:
Cristae - folded Oxidative phosphorylation
Matrix - fluid structure Making of ATP by using oxygen gas.
o Exergonic Chemiosmosis
GLYCOLISIS Makes (long) linkage of hydrogen ion and electrons.
o Cytosol is its location.
o 1st step of cellular respiration. o Final e- acceptor: Oxygen, then it will be converted to
o Decides what pathways the molecules will follow. H2O (sweat).
o Also called EMP (Embden Meyerhof Parnas Pathway) 1ST STAGE: 2 ATP
2ND STAGE: 2 ATP > 4 ATP = 4 ATP
10 NADH x 3 ATP = 30 ATO
or Glycolytic Pathway. 3RD STAGE: 34 ATP
2 FADH x 2 ATP = 4 ATP
o “Splitting of sugar” TOTAL: 38 ATP
o C₆H₁₂O₆+6O₂ > 6H₂O +6CO₂+ATP
= 34 ATP
2 organ system that helps in cellular respiration
Respiratory and digestive
o If split: will yield 2 pyruvates ADDITIONAL INPUTS
o Glucose is a 6 carbon, It will undergo glycolysis to spilt
and it will yield (2) 3 carbon pyruvate.
o 2 ATP is yielded through redox.
o 2 NADH is yielded through phosphorylation.
NADP is for photosynthesis
NADH is for cellular respiration
Has two scenarios:
o Aerobic (+O2) presence of oxygen gas
o After oxygen gas joins, it will proceed to Krebs Cycle
and ETC to complete the process.
o Anaerobic (-O2) no presence of oxygen gas
o If there is no presence of oxygen gas, it will undergo
lactic acid fermentation (humans, specifically in the
muscles).
2 ATP
o Alcoholic fermentation (yeast and other organisms)
2 ATP
KREBS CYCLE
o Also known as TCA (Tricarboxylic Acid) or Citric Acid
Cycle.
o Mitochondrial matrix
o Discovered by Hans Kreb
o Reactants - CO2 and 2 pyruvates (pyruvic acid).
o Products - 4CO2, 2FADH, 2ATP, 6NADH
2FADH, 2ATP, 6NADH will proceed to third stage.
While, the CO2 will go out to the atmosphere
Where citric acid come from:
3 carbon also known as pyruvate (from glycolysis).
Since it made 2 molecules of 3carbon, 2 will also
undergo Krebs Cycle.
Once the pyruvate binds with oxygen, it will be Acetyl
CoA. One carbon will also be added, and it will create
Oxoacetate [to form citric acid].
f:17 Natalie ashley b. grio [12 – stem 1 brr ] 7
GENERAL BIOLOGY [Ms. Jeanette Anne J. Desamparado]
f:17 Natalie ashley b. grio [12 – stem 1 brr ] 8
GENERAL BIOLOGY [Ms. Jeanette Anne J. Desamparado]
Function of the Cell Membrane:
Cell membrane separates the components of a cell from
its environment—surrounds the cell
“Gatekeeper” of the cell—regulates the flow of materials
into and out of cell—selectively permeable
Cell membrane helps cells maintain homeostasis—
stable internal balance
Concentration – the amount of solute in a solution.
Solute – the dissolved substance in a solution.
Solution – a mixture in which two or more substances are
mixed evenly.
Concentration gradient - the gradual difference in the
concentration of solutes in a solution between two regions.
f:17 Natalie ashley b. grio [12 – stem 1 brr ] 9
GENERAL BIOLOGY [Ms. Jeanette Anne J. Desamparado]
f:17 Natalie ashley b. grio [12 – stem 1 brr ] 10
GENERAL BIOLOGY [Ms. Jeanette Anne J. Desamparado]
f:17 Natalie ashley b. grio [12 – stem 1 brr ] 11
You might also like
- General Biology 1 2nd Quarter NotesDocument6 pagesGeneral Biology 1 2nd Quarter NotesFiona Althea JangalayNo ratings yet
- Preliminary Biology NotesDocument36 pagesPreliminary Biology NotesSaad KashifNo ratings yet
- The Cell StructureDocument7 pagesThe Cell StructureAbcedef Wyn Grey AbrasadoNo ratings yet
- Cell Structures and Their FunctionsDocument7 pagesCell Structures and Their FunctionsClarence MallariNo ratings yet
- GenBio - 1st QuarterDocument13 pagesGenBio - 1st QuarterCc TvNo ratings yet
- Transport Mechanism HandoutsDocument2 pagesTransport Mechanism HandoutsMaicaMerylle FranciscoNo ratings yet
- MamuDocument9 pagesMamukalloliNo ratings yet
- Human HistologyDocument128 pagesHuman HistologyBreane Denece LicayanNo ratings yet
- Chapter 3 Cell Structures & Their Functions: Certain Molecules To Enter/exit The Cell)Document5 pagesChapter 3 Cell Structures & Their Functions: Certain Molecules To Enter/exit The Cell)Gellyace A.No ratings yet
- Cell BiologyDocument3 pagesCell BiologyjamjamjamNo ratings yet
- CELLDocument7 pagesCELLzairazapanta001No ratings yet
- Week 3Document3 pagesWeek 3naomimarielleNo ratings yet
- Bio Chapter 3Document41 pagesBio Chapter 3Mohd IyadRaissa HijazNo ratings yet
- Cells: The Little Chamber in Plants and Animals: Eb 2005-Introductory BiologyDocument50 pagesCells: The Little Chamber in Plants and Animals: Eb 2005-Introductory BiologyPatricia Jayshree Samuel JacobNo ratings yet
- Week 2 The CellsDocument7 pagesWeek 2 The Cells2F - MACAO, KHALILANo ratings yet
- Histology Dr. Noelyn Bernal Cytoplasm-September 7,2018: Page - PEREZDocument13 pagesHistology Dr. Noelyn Bernal Cytoplasm-September 7,2018: Page - PEREZA18- Jessa Mae DayagNo ratings yet
- Part I: Cells: Overview of The Cellular Basis of LifeDocument5 pagesPart I: Cells: Overview of The Cellular Basis of LifeAldrin OjalesNo ratings yet
- CellDocument10 pagesCelljuju on the beatNo ratings yet
- The Structural Components of The Cell Membrane and Its Functions, With Transport Mechanisms.Document17 pagesThe Structural Components of The Cell Membrane and Its Functions, With Transport Mechanisms.Vieyah Angela VicenteNo ratings yet
- Assignment For Membrane Structure: Ane Austin Lynn N. Rebancos Bsed-Science 3ADocument9 pagesAssignment For Membrane Structure: Ane Austin Lynn N. Rebancos Bsed-Science 3Ajane austin lynn rebancosNo ratings yet
- Cell StructureDocument3 pagesCell StructureKYLA LORENZ BALUMANo ratings yet
- TOPIC 3 7 PDocument7 pagesTOPIC 3 7 PPatricia Jean RodriguezNo ratings yet
- MicroDocument5 pagesMicrotahashahzad9901No ratings yet
- UntitledDocument6 pagesUntitled49 - Kaycee JoaquinNo ratings yet
- Molecular Biology and Diagnostic Intro To CytogeneticsDocument6 pagesMolecular Biology and Diagnostic Intro To Cytogeneticselijah montefalcoNo ratings yet
- 2.cell Membrane Physiology and TransportDocument88 pages2.cell Membrane Physiology and Transport234820No ratings yet
- Biom1070 L4 2022Document31 pagesBiom1070 L4 2022Kevin ZhangNo ratings yet
- 01 Topic-1 (The Cell) (1-15)Document16 pages01 Topic-1 (The Cell) (1-15)Ijaz ZoologistNo ratings yet
- 4 Concept Membrane StructureDocument9 pages4 Concept Membrane StructurekalloliNo ratings yet
- C) Glycolipids Make Up About 5%Document9 pagesC) Glycolipids Make Up About 5%Jerwin TullaoNo ratings yet
- CELLMOLDocument3 pagesCELLMOLJhon Rey LagosNo ratings yet
- 3rd DayDocument10 pages3rd DayJane DomingoNo ratings yet
- CELL 1 2022 Materials Part 1Document27 pagesCELL 1 2022 Materials Part 1Mutahir AliNo ratings yet
- Activity No. 3: Structure of A Cell and MitosisDocument9 pagesActivity No. 3: Structure of A Cell and MitosisEricka GenoveNo ratings yet
- Histology AssignmentDocument6 pagesHistology AssignmentBryan LebasnonNo ratings yet
- Transportation of Substances in Living OrganismsDocument45 pagesTransportation of Substances in Living Organismswhatevern3108No ratings yet
- 4) Cell MembraneDocument17 pages4) Cell Membranescobi doNo ratings yet
- Cell - Histology Trans Part 1&2Document6 pagesCell - Histology Trans Part 1&2Mark AbrazaldoNo ratings yet
- Cells and TissuesDocument8 pagesCells and TissuesA R F I J U LNo ratings yet
- Cell 1Document48 pagesCell 1Mihai ChirniciniiNo ratings yet
- 3 - Cell Structures and Their FunctionsDocument12 pages3 - Cell Structures and Their FunctionsPrince TeodocioNo ratings yet
- Cell OrganelleDocument9 pagesCell OrganelleGwencytech DomingoNo ratings yet
- Activity Ii The Cellular Level of OrganizationDocument7 pagesActivity Ii The Cellular Level of OrganizationRjosheNo ratings yet
- Pidlaoan, Coleen - Cell Structure PracticeDocument5 pagesPidlaoan, Coleen - Cell Structure PracticeColeenNo ratings yet
- Cell Membrane StructureDocument17 pagesCell Membrane StructurearunNo ratings yet
- PHYSIO-T01-S01-Cell Organization and Membrane PhysiologyDocument8 pagesPHYSIO-T01-S01-Cell Organization and Membrane Physiologyhazelloraenne.conarcoNo ratings yet
- CellDocument36 pagesCellwnslthmangulad05No ratings yet
- CompartmentalizationDocument7 pagesCompartmentalizationnamitamohanty992No ratings yet
- Gen BioDocument9 pagesGen BioThryxia Coreen AbelgasNo ratings yet
- CellsDocument6 pagesCellsaryam aliNo ratings yet
- The Structural Components of The Cell MembraneDocument5 pagesThe Structural Components of The Cell MembraneJanine CambaNo ratings yet
- Cell Structure and Processes Practice Worksheet: Name: Pidlaoan, Coleen LDocument5 pagesCell Structure and Processes Practice Worksheet: Name: Pidlaoan, Coleen LColeenNo ratings yet
- Biochem PrelimsDocument25 pagesBiochem Prelimslcpanaligan4175antNo ratings yet
- Movement of Substances Across The Plasma Membrane: Compiled & Prepared by Dsalleh (2021)Document25 pagesMovement of Substances Across The Plasma Membrane: Compiled & Prepared by Dsalleh (2021)Dewi SallehNo ratings yet
- HISTO LEC 2 - The CystoplasmDocument7 pagesHISTO LEC 2 - The CystoplasmjkthmsvtchNo ratings yet
- Microana Prelim ReviewerDocument17 pagesMicroana Prelim ReviewerjerlerositaNo ratings yet
- Membranes: Apago PDF EnhancerDocument19 pagesMembranes: Apago PDF EnhancerHokky AlexanderNo ratings yet
- II. Chapter 2 (Cytoplasm)Document14 pagesII. Chapter 2 (Cytoplasm)rosalesjirehrNo ratings yet
- As-Level Biology Notes: By: Bianca HimawanDocument65 pagesAs-Level Biology Notes: By: Bianca HimawanLauren ChikwehwaNo ratings yet
- Uganda Martrys' National Major Seminary Alokolum-Gulu Affiliated To Urbaniana Pontifical University-RomeDocument8 pagesUganda Martrys' National Major Seminary Alokolum-Gulu Affiliated To Urbaniana Pontifical University-RomeAbedism JosephNo ratings yet
- YP Guide SampleletterDocument10 pagesYP Guide SampleletterIllery PahugotNo ratings yet
- Key Answer Org DevDocument15 pagesKey Answer Org DevSheennah Lee LimNo ratings yet
- Resume - Jessica Minch WebsiteDocument3 pagesResume - Jessica Minch Websiteapi-594661640No ratings yet
- Bryant and Smith 2001 - Refining The Architecture of AggressionDocument30 pagesBryant and Smith 2001 - Refining The Architecture of AggressionIstván BeregiNo ratings yet
- InheritanceDocument59 pagesInheritanceTiju ThomasNo ratings yet
- 16 How To LamentDocument3 pages16 How To Lamentjudy.i.galidoNo ratings yet
- The Impact of Organizational Justice On Employee's Job Satisfaction: The Malaysian Companies PerspectivesDocument8 pagesThe Impact of Organizational Justice On Employee's Job Satisfaction: The Malaysian Companies PerspectivesNaim NordinNo ratings yet
- The Feasibility of Malunggay Leaves (Moringa Oleifera) and Chili Fruit (Capsicum Frutescens) As PesticideDocument7 pagesThe Feasibility of Malunggay Leaves (Moringa Oleifera) and Chili Fruit (Capsicum Frutescens) As Pesticidepearlkaye agravanteNo ratings yet
- Some Words Against The Staff of Face BookDocument50 pagesSome Words Against The Staff of Face BookGiorgi Leon KavtarażeNo ratings yet
- 71 Things Your Child Needs To Know Before KindergartenDocument3 pages71 Things Your Child Needs To Know Before KindergartenCong ThanhNo ratings yet
- Oracle Rac Pratice On Virtual BoxDocument94 pagesOracle Rac Pratice On Virtual BoxpandsinNo ratings yet
- Accutron Service Manual Series 218 PDFDocument52 pagesAccutron Service Manual Series 218 PDFtunimao100% (1)
- Multigenre Research Project CriteriaDocument1 pageMultigenre Research Project Criteriaapi-246632095No ratings yet
- Business Statistics: Fourth Canadian EditionDocument32 pagesBusiness Statistics: Fourth Canadian EditionomarNo ratings yet
- Uts Final OutputDocument5 pagesUts Final OutputPaulineNo ratings yet
- HistopathologyDocument1 pageHistopathologyHershei Vonne BaccayNo ratings yet
- September 2005 Limpkin Call Oklawaha Valley Audubon SocietyDocument4 pagesSeptember 2005 Limpkin Call Oklawaha Valley Audubon SocietyOklawaha Valley Audubon SocietyNo ratings yet
- Solar Generator (LB-P500-A) : Product DetailsDocument2 pagesSolar Generator (LB-P500-A) : Product Detailsbogdan danescuNo ratings yet
- Math 7 Post TestDocument2 pagesMath 7 Post TestDonabel Carios100% (3)
- Dear Sirs and Madams Cover LetterDocument4 pagesDear Sirs and Madams Cover Letterc2yyr2c3100% (1)
- Cisco Nexus B22 Fabric Extender For Flex SystemDocument2 pagesCisco Nexus B22 Fabric Extender For Flex SystemRemNo ratings yet
- 099CYUT5512017 001卵礫石Document212 pages099CYUT5512017 001卵礫石ruizhou04 wuNo ratings yet
- HRC - Forms - Employee Application - Rev2019 NewDocument3 pagesHRC - Forms - Employee Application - Rev2019 NewGanang CucuNo ratings yet
- Modbus Messaging On Tcp/ipDocument49 pagesModbus Messaging On Tcp/ipvamovengaNo ratings yet
- CBSE Class 11 Chemistry Worksheet - ThermodynamicsDocument1 pageCBSE Class 11 Chemistry Worksheet - ThermodynamicsDivyaprakash PatelNo ratings yet
- Respuestas Ingles Modul 6Document16 pagesRespuestas Ingles Modul 6Ximena Silva CelyNo ratings yet
- IQN - Diploma in SCM Guidelines - Nov 2019Document43 pagesIQN - Diploma in SCM Guidelines - Nov 2019AlexanderDeFreitasNo ratings yet
- Yct Neetjee Main Physics Chapter-Wise Solved Papers Volume-IIDocument800 pagesYct Neetjee Main Physics Chapter-Wise Solved Papers Volume-IIcoaching material100% (2)
- Water Unit 1-NotesDocument35 pagesWater Unit 1-NotesEng Venance MasanjaNo ratings yet