Professional Documents
Culture Documents
2.03 - Functional Groups
2.03 - Functional Groups
Uploaded by
Juan Miguel SalvadorOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
2.03 - Functional Groups
2.03 - Functional Groups
Uploaded by
Juan Miguel SalvadorCopyright:
Available Formats
CHEM113: BIOCHEMISTRY PRELIMS
02
Prof. Merly Alfafara / Second Semester
Transcriber: Kathleen Venus 23
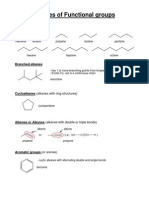
FUNCTIONAL GROUPS
SATURATED HYDROCARBON
OUTLINE - Hydrocarbon with all carbon–carbon bonds are
I. ORGANIC VS. INORGANIC COMPOUNDS single bonds
II. BONDING CHARACTERISTICS OF THE CARBON UNSATURATED HYDROCARBON
ATOM - Hydrocarbon with one or more carbon–carbon
multiple bonds (double bonds, triple bonds, or both).
ORGANIC VS. INORGANIC COMPOUNDS
ORGANIC CHEMISTRY
- Study of hydrocarbons (only carbon and hydrogen
atoms) and their various derivatives.
o natural gas
o petroleum
o plastics
o rubbers
o paper
o carbohydrates
▪ sugar
▪
▪ starch
o proteins COMMON
COMMON
o enzymes CLASS
GENERAL
EXAMPLE
NAME
SUFFIX/PREFIX
FORMULA (SYSTEMATIC
o fatty acids NAME)
(SYSTEMATIC)
o food stuff HYDROCARBONS
o drugs
Alkanes RH CH3CH3 Ethane -ane
o textiles, etc.
Ethylene
INORGANIC CHEMISTRY Alkenes RR’C==CR”R”’ H2C==CH2
(ethene)
-ene
- Study of all substances other than hydrocarbons Alkynes RC==CR’ HC==CH
Acetylene
(-yne)
and their derivatives (ethyne)
o Sulfuric acid Arenes ArHa Benzene -ene
o nitric acid
o ores and minerals HALOGEN-CONTAINING COMPOUNDS
o air Alkyl
RX CH3CH2CI
Ethyl chloride
Halide (halo-)
o baking powder halides (chloroethane)
o caustic soda Aryl
ArXa Chlorobenzene Halo-
halides
o table salt
o metal alloys OXYGEN-CONTAINING COMPOUNDS
▪ brass Ethyl alcohol
ROHa
▪ bronze Alcohols CH3CH2OH
(Ethanol)
-ol
b
Phenols ArOH Phenol -ol
BONDING CHARACTERISTICS OF
THE CARBON ATOM Ethers ROR’ Diethyl ether Ether
H3CH2COCH2CH3
- C-atom always makes total of 4 Bonds Aldehydes RCHO
Acetaldehyde
-aldehyde (-al)
- The sharing of four valance electrons requires the (ethanal)
formation of four covalent bonds which are Acetone
represented by four lines. Ketones RR’C==O -one
(2-propanone)
FUNCTIONAL GROUPS
Carboxylic Acetic acid -ic acid
- are groups of atoms in organic molecules that are Acids
RCO2H
(ethanoic acid) (-oic acid)
responsible for the characteristics, chemical
reactions of those molecules. CARBOXYLIC ACID DERIVATIVES
- Simple molecules that contain the same functional Methyl acetate
Esters RCO2R’ (methyl -ate (-oate)
group in their structure can be expected to react in ethanoate)
similar ways.
Amides RCONHR’ N-methylacetamide -amide
- More complicated chemical molecules may contain
more than one functional group within their NITROGEN-CONTAINING COMPOUNDS
structure. RNH2, RNHR’,
Amines CH3CH2NH2 Ethylamine -amine
- The names of organic molecules are systematic RNR’R”
references to the functional groups within the Nitriles RC==N H3CC=N Acetonitrile -nitrile
molecule.
Nitro
HYDROCARBONS AND HYROCARBON DERIVATIVES compounds ArNO2a Nitrobenzene Nitro-
HYDROCARBON
- Compound that contains only carbon and hydrogen
atoms.
HYDROCARBON DERIVATIVE:
Page1
- Compound that contains carbon and hydrogen and
one or more additional elements.
You might also like
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- 2-14 Determination of The Dissociation Constant of Weak AcidsDocument3 pages2-14 Determination of The Dissociation Constant of Weak Acidsdbroncos78087100% (6)
- Module 1 - Organic ChemistryDocument12 pagesModule 1 - Organic ChemistrySelena MoonNo ratings yet
- Organic Chemistry: (Hydrocarbons and Functional Groups)Document38 pagesOrganic Chemistry: (Hydrocarbons and Functional Groups)MB LoterteNo ratings yet
- (-ENE) (-YNE) (-OL) (-OXY) (Benzene) : ChlorobenzeneDocument2 pages(-ENE) (-YNE) (-OL) (-OXY) (Benzene) : ChlorobenzeneyasiraNo ratings yet
- Introduction To Organic ChemistryDocument31 pagesIntroduction To Organic Chemistryauni ramizahNo ratings yet
- Chem113lec Week 3.1Document3 pagesChem113lec Week 3.1Darryl orcaNo ratings yet
- Organic Chem Part 1 - NomenclatureDocument10 pagesOrganic Chem Part 1 - Nomenclatureyamkela060.comNo ratings yet
- Haken 1987Document8 pagesHaken 1987Dewala KutaNo ratings yet
- Basics of Organic ChemistryDocument9 pagesBasics of Organic ChemistryGovind Mani BhattNo ratings yet
- Orgarnic Chemistry Functional Group TestDocument9 pagesOrgarnic Chemistry Functional Group TestShourya veer singhNo ratings yet
- An Example of Addition Reaction: Saturated Hydrocarbon Synthesis of Alkanes Hydrogenation of AlkenesDocument21 pagesAn Example of Addition Reaction: Saturated Hydrocarbon Synthesis of Alkanes Hydrogenation of AlkenesMuhammad FirdausNo ratings yet
- 12chem Nomenclature Worksheet AnswersDocument9 pages12chem Nomenclature Worksheet AnswersAya AbdelsanadNo ratings yet
- Organic: ChemistryDocument16 pagesOrganic: ChemistryroythomascNo ratings yet
- LG 1.2 Properties of Hydrocarbons and Functional GroupsDocument10 pagesLG 1.2 Properties of Hydrocarbons and Functional GroupswangmorisNo ratings yet
- Basic IUPAC Nomenclature of Organic CompoundsDocument15 pagesBasic IUPAC Nomenclature of Organic CompoundsApril Joyce Raymundo100% (1)
- Analysis of Concept Chemistry For Senior High School Grade 12 Subject Matter: Carbon Compound and Its DerivativeDocument3 pagesAnalysis of Concept Chemistry For Senior High School Grade 12 Subject Matter: Carbon Compound and Its DerivativeAhmad RamadanaNo ratings yet
- PDF Document 3Document14 pagesPDF Document 3Akshat GuptaNo ratings yet
- Chemistry Data BookletDocument7 pagesChemistry Data Bookletbob turnerNo ratings yet
- Unit 1 Organic Chemistry PDFDocument39 pagesUnit 1 Organic Chemistry PDFFarzanNo ratings yet
- CHEM1090 Final - Module 2Document10 pagesCHEM1090 Final - Module 2Dani R.No ratings yet
- C1 Ioc 1Document24 pagesC1 Ioc 1Archana SharmaNo ratings yet
- General Chemistry I M2W4Document5 pagesGeneral Chemistry I M2W4Warley JabelNo ratings yet
- Chemistry DataDocument7 pagesChemistry DataJ LevinsNo ratings yet
- Nomenclature of Functional Groups, Aldehydes & Ketones, Carboxylic Acids, Derivatives of Carboxylic Acids, EstersDocument11 pagesNomenclature of Functional Groups, Aldehydes & Ketones, Carboxylic Acids, Derivatives of Carboxylic Acids, EstersH to O ChemistryNo ratings yet
- Alkane and AlkeneDocument40 pagesAlkane and AlkenePawankumar Gupta91% (11)
- FHSC1124 Tutorial Ebook QDocument72 pagesFHSC1124 Tutorial Ebook QTeo CinnyNo ratings yet
- Chapter 2. Introduction To Organic Chemistry: 2.1 Functional Group and Homologous SeriesDocument8 pagesChapter 2. Introduction To Organic Chemistry: 2.1 Functional Group and Homologous SeriesDavid PhilipNo ratings yet
- Science 112 STUDY GUIDE 3Document6 pagesScience 112 STUDY GUIDE 3Dominador RomuloNo ratings yet
- Hydrocarbons: Ella EH EH ÉDocument19 pagesHydrocarbons: Ella EH EH ÉIsabella LopezNo ratings yet
- HydrocarbonDocument1 pageHydrocarbonemeka.nn.ceNo ratings yet
- The Elementary Chemistry: of CombustionDocument2 pagesThe Elementary Chemistry: of CombustionMEHBOOB SHAIKHNo ratings yet
- Functional GroupsDocument3 pagesFunctional GroupsManofMiracles89No ratings yet
- Organic Chemistry 2Document3 pagesOrganic Chemistry 266rdsmh2mwNo ratings yet
- 2023 H1 Hydrocarbons Revision (Student)Document14 pages2023 H1 Hydrocarbons Revision (Student)2022 BALAKRISHNAN ADHITHINo ratings yet
- CBSE-XI Chemistry - Chap-9 (Hydrocarbons) 2Document14 pagesCBSE-XI Chemistry - Chap-9 (Hydrocarbons) 2Harish SharmaNo ratings yet
- Organic GOC Short RevisionDocument7 pagesOrganic GOC Short Revisionmohitjain0076No ratings yet
- Hydrocarbons, Alcohols, Phenols - Written Report - SolidumDocument13 pagesHydrocarbons, Alcohols, Phenols - Written Report - SolidumAva Mae SolidumNo ratings yet
- Functional GroupDocument20 pagesFunctional GroupCatherine R. FelipeNo ratings yet
- Edexcel Chemistry Data Sheet AlevelDocument6 pagesEdexcel Chemistry Data Sheet AlevelManoj oliNo ratings yet
- Class 10 Chemistry Chapter Hydrocarbons NotesDocument28 pagesClass 10 Chemistry Chapter Hydrocarbons Notesnaveedhafiz78612No ratings yet
- IUPACDocument110 pagesIUPACDiana MoraNo ratings yet
- Xii OrganicDocument25 pagesXii OrganicArindam GoswamiNo ratings yet
- 5all Organic TestDocument1 page5all Organic TestJaya SinghNo ratings yet
- Fire InvestigationDocument16 pagesFire InvestigationBexs BegixsNo ratings yet
- 3.1 Introduction To Organic Chemistry: Bonding in Organic CompoundsDocument20 pages3.1 Introduction To Organic Chemistry: Bonding in Organic CompoundsFadhla Fadhilatul Mariyatis SolihahNo ratings yet
- Ald and Ket Part 1Document3 pagesAld and Ket Part 1Aryan GuptaNo ratings yet
- BiomoleculesDocument5 pagesBiomoleculesDhanasriram ChintuNo ratings yet
- 4102609923604691Document538 pages4102609923604691Vishal MNo ratings yet
- Chemistry Notes PT 2Document37 pagesChemistry Notes PT 2Leng RyanNo ratings yet
- Reviewer in Science: Three KindsDocument2 pagesReviewer in Science: Three KindsMarianne Rose TenorioNo ratings yet
- Aakash Modules 03Document219 pagesAakash Modules 03Sameer chaudharyNo ratings yet
- More On Nomenclature. Compounds Other Than Hydrocarbons%: IupacDocument21 pagesMore On Nomenclature. Compounds Other Than Hydrocarbons%: Iupacmail2quraishi3084No ratings yet
- (15448) Course Planning 00 00ja Course Planning eDocument19 pages(15448) Course Planning 00 00ja Course Planning eHarish PatidarNo ratings yet
- Ib PPT 10 SL PDFDocument84 pagesIb PPT 10 SL PDFzarna nirmal rawalNo ratings yet
- Aldehydes, Ketones and Carboxylic Acids: Module - 7Document29 pagesAldehydes, Ketones and Carboxylic Acids: Module - 7TeachingTrainingCoaching KnowledgeSharingSessionNo ratings yet
- Classes and Nomenclature of Organic CompoundsDocument40 pagesClasses and Nomenclature of Organic CompoundsstephenadeyemiiNo ratings yet
- Full Organic Chemistry Flow Charts and Brief TheoryDocument214 pagesFull Organic Chemistry Flow Charts and Brief Theorykoradasirisha2007No ratings yet
- Organic - Chemistry - Part - 01 ...Document1 pageOrganic - Chemistry - Part - 01 ...science1020yhNo ratings yet
- Nomenclature Part 1Document7 pagesNomenclature Part 1gcxnhmzNo ratings yet
- LkanesDocument94 pagesLkanesNizarNo ratings yet
- Aluminium Alloy 6063 NB PDFDocument2 pagesAluminium Alloy 6063 NB PDFBrijendra Mani PandeyNo ratings yet
- Department of Electrical/Elcetronic EngineeringDocument29 pagesDepartment of Electrical/Elcetronic EngineeringFere GodspowerNo ratings yet
- Oxygen Stripping in Deaerator Feed Water: Condensation On Spray DropletsDocument10 pagesOxygen Stripping in Deaerator Feed Water: Condensation On Spray DropletsVina DwitaNo ratings yet
- Lecture - 9 Fettling and Casting DefectsDocument43 pagesLecture - 9 Fettling and Casting DefectsSara KiNo ratings yet
- Why Salt and CharcoalDocument3 pagesWhy Salt and CharcoalMU Len GANo ratings yet
- Ammonia TechnologyDocument13 pagesAmmonia TechnologyMihaela Popescu-NeagoeNo ratings yet
- Final Project - Fluid Mechanics MCE 3403Document16 pagesFinal Project - Fluid Mechanics MCE 3403ShehbazShoukatNo ratings yet
- RSC Advances: PaperDocument8 pagesRSC Advances: PaperAngélica Andrea SalinasNo ratings yet
- ThermJet PCA (USA)Document2 pagesThermJet PCA (USA)Alejandro Ignacio Araneda WordenNo ratings yet
- Flax FiberDocument33 pagesFlax FiberM Athar RiazNo ratings yet
- Lurgi PSI Evaporator PaperDocument13 pagesLurgi PSI Evaporator PaperMariAle Droz CastroNo ratings yet
- TDC OliveDocument31 pagesTDC OliveFrancesco La Cara100% (1)
- 125 A Mid 2 Chemistry-1Document24 pages125 A Mid 2 Chemistry-1syeda ruqaiyah ashfaqNo ratings yet
- Take Home Pack Gr. 7 Natural Sciences T3Document21 pagesTake Home Pack Gr. 7 Natural Sciences T3debbiemotlatle22No ratings yet
- NPE-2 User's - Manual - EN - 201202Document48 pagesNPE-2 User's - Manual - EN - 201202Tyler RoachNo ratings yet
- The Solubility of Gases in Liquids: Introductory InformationDocument7 pagesThe Solubility of Gases in Liquids: Introductory InformationEwindNo ratings yet
- Materials, Equipments and Fabrication TechniqueDocument17 pagesMaterials, Equipments and Fabrication TechniqueNagarjun CherukuriNo ratings yet
- Covalent Bond: Molecules and Molecular CompoundsDocument24 pagesCovalent Bond: Molecules and Molecular CompoundsIlina DameskaNo ratings yet
- Mass Spectrometric Methods For Trace Analysis of MetalsDocument8 pagesMass Spectrometric Methods For Trace Analysis of MetalsDdd BbbNo ratings yet
- Adva Cast 620Document2 pagesAdva Cast 620naconnetNo ratings yet
- Reactions of Synthetic ImportanceDocument28 pagesReactions of Synthetic ImportanceRx Nadeem ChhipaNo ratings yet
- Genapol PF 10Document2 pagesGenapol PF 10Mohamed HalemNo ratings yet
- Fire ExtinguisherDocument8 pagesFire ExtinguisherAzrif MoskamNo ratings yet
- Specimen QP - Paper 3 Edexcel Chemistry A-LevelDocument36 pagesSpecimen QP - Paper 3 Edexcel Chemistry A-LeveljessissimaessoNo ratings yet
- Prime CoatDocument2 pagesPrime CoatBilal AhmadNo ratings yet
- Kod de BajaDocument1 pageKod de BajaGabrielito PachacamaNo ratings yet
- 5 Methods To Determine Preheat TemperatureDocument4 pages5 Methods To Determine Preheat TemperatureMohamed AtefNo ratings yet
- Norochcholai Power PlantDocument5 pagesNorochcholai Power PlantansudasinghaNo ratings yet
- Chem NotesDocument148 pagesChem Noteskiruba devi .kNo ratings yet