Professional Documents
Culture Documents
Enrgy: Average
Enrgy: Average
Uploaded by
zyzy6527Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Enrgy: Average
Enrgy: Average
Uploaded by
zyzy6527Copyright:
Available Formats
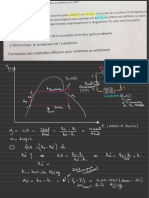
Enrgy of Parudes
( a) NH 3
(d ) Average Kinetio
Hcl
号 dire otly proporeion al
to tlfuperature
什e iuei' o emeryy
'
The arerage
aandis assumedtoBethes searme
atthe suore
for
allgases
(b) He ( sin
HClli temperathure .
NH 3 ( ii )
¢) NH 3 ( ii )
HllLi )
Heliii )
䰞
PV = RT
mass
—
moles =
mass
molar
= 500
44 01 .
g
/ mol
=
B 业
. 98
look
≈ H 36 mol
9Cooky
=
Tkla
g
125 + 2315 5
=
≈ 398 15 , k ,
( 7 066 10 6 atm ) C
5 67 L )
-
No 2
.
.
=
10 082 | L
,
-
atonlono | -
k ) ( 303 . HK }
4 !
~
≈ 1 081 . × 10 mo
E xH 0
Umg
µ =
z
'
5 405 × 10 - rmol
5
≈ .
Mong =Ang x Molar
MassMg
5
≈ (5 405 × l)
glouol )
-
, (0 u0 × ( 24
,
3)
≈ 0. 00131
g ,
35
1H cl :
1 + 35 453 ,
1 + 37 45号
3>
/ H Cl :
.
H 35cl :
2 3 4535 ,
2 H 37 C 1 : 2 +3 } . 45 号
/ H 350 L CIHTCL CIHHEL C 2 HEl
十
而
门
You might also like
- (Historical Atlas of World Mythology) Joseph Campbell - The Way of The Animal Powers-Harper & Row (1983)Document299 pages(Historical Atlas of World Mythology) Joseph Campbell - The Way of The Animal Powers-Harper & Row (1983)Pedro Daniel Egusquiza LeonNo ratings yet
- 1.engaged Scholarship in A Professional SchoolDocument35 pages1.engaged Scholarship in A Professional SchoolGerald Hartman100% (1)
- Stoichiometry 1 Worksheet and KeyDocument4 pagesStoichiometry 1 Worksheet and KeySea Clest100% (1)
- Kimia Us AbyanDocument1 pageKimia Us AbyanYoga PratamaNo ratings yet
- Engine - Repetition Engine - Repetition Air Fuel Mixture (A/F)Document5 pagesEngine - Repetition Engine - Repetition Air Fuel Mixture (A/F)scaniaNo ratings yet
- Correl 2 Hge NotesDocument21 pagesCorrel 2 Hge Notesromelio salumbidesNo ratings yet
- Broschuere Acrylic Und Methacrylic Monomers GlobalDocument5 pagesBroschuere Acrylic Und Methacrylic Monomers GlobalMohamed HalemNo ratings yet
- Particle Swarm Optimization With Area Extension (AEPSO) : A Macroscopic Model of PSO in Robotic SwarmDocument50 pagesParticle Swarm Optimization With Area Extension (AEPSO) : A Macroscopic Model of PSO in Robotic SwarmkkkprotNo ratings yet
- Umhlanga BrochureDocument19 pagesUmhlanga BrochuresafinditNo ratings yet
- Table UW-12 Maximum Allowable Joint Efficiencies For Arc and Gas Welded JointsDocument1 pageTable UW-12 Maximum Allowable Joint Efficiencies For Arc and Gas Welded Jointsluis armandoNo ratings yet
- Exo Thermos 2emsessDocument12 pagesExo Thermos 2emsessإبراهيم الزايدي الكيحلNo ratings yet
- AmirKabir HDEX3 (GM5010T2N)Document1 pageAmirKabir HDEX3 (GM5010T2N)alimotazedi57No ratings yet
- Gaz Parfaits 2Document8 pagesGaz Parfaits 2fifiNo ratings yet
- Diagramespsicrometrics High TempDocument1 pageDiagramespsicrometrics High TempJohnny SanchezNo ratings yet
- Atmosphere: Water ResourcesDocument3 pagesAtmosphere: Water ResourcesMathew WebsterNo ratings yet
- Biotechnological App.Document3 pagesBiotechnological App.KaanNo ratings yet
- Flexible Polyurethane Foams: Product OverviewDocument2 pagesFlexible Polyurethane Foams: Product OverviewSergio Marcos KettmayerNo ratings yet
- Free Diseno de Maquinas T5 Gulag FreeDocument12 pagesFree Diseno de Maquinas T5 Gulag FreePablo GarciaNo ratings yet
- Iconesmycologic 01 BoudDocument412 pagesIconesmycologic 01 BoudBranko NikolicNo ratings yet
- Esercitazioni PropulsioneDocument1 pageEsercitazioni PropulsioneMatteo LorenziniNo ratings yet
- BrochureDocument4 pagesBrochurea0439422572No ratings yet
- Rotex: Torsionally Flexible CouplingsDocument1 pageRotex: Torsionally Flexible CouplingsZeeshan SajidNo ratings yet
- Week 02 Lecture 04-1 - 1-1Document1 pageWeek 02 Lecture 04-1 - 1-1Gyfyy FtufuNo ratings yet
- CitrixSummitSolutionsExpo v1Document1 pageCitrixSummitSolutionsExpo v1Citrix SystemsNo ratings yet
- Tema 6 - Derivación DiscretaDocument5 pagesTema 6 - Derivación DiscretaLuyi WangNo ratings yet
- Nemesis Instructiones (ES)Document24 pagesNemesis Instructiones (ES)Rebeca CH DobantonNo ratings yet
- E Li O LDTB Ahitt Exploring Oracle Database ArchitectureDocument54 pagesE Li O LDTB Ahitt Exploring Oracle Database Architecturejay karandikarNo ratings yet
- Calorimetry, Heat Transfer, Thermal Expansion, ElasticityDocument1 pageCalorimetry, Heat Transfer, Thermal Expansion, ElasticityTanay1 MitraNo ratings yet
- htiteetdkt (: FSKF Wa TFDocument1 pagehtiteetdkt (: FSKF Wa TFNguyen DuyNo ratings yet
- Atherosclerosis IllustrationDocument1 pageAtherosclerosis IllustrationDean WoodNo ratings yet
- Ryobi 18 Volt Battery Charger ManualDocument24 pagesRyobi 18 Volt Battery Charger ManualBryan JonesNo ratings yet
- Cone Girls at HalloweenDocument2 pagesCone Girls at HalloweenDaschNo ratings yet
- Mrprintables Cone Girls HalloweenDocument2 pagesMrprintables Cone Girls HalloweenAleksandra Jasik-ŁatkowskaNo ratings yet
- Mrprintables Cone Girls HalloweenDocument2 pagesMrprintables Cone Girls HalloweenMarilia Souza de AlmeidaNo ratings yet
- END TERM EXAM PAPERS (4th Semester)Document14 pagesEND TERM EXAM PAPERS (4th Semester)Chaitanya ShahareNo ratings yet
- Catalogue SP 8II PDFDocument1 pageCatalogue SP 8II PDFPhan Nguyễn AudioNo ratings yet
- D3e802f8707!1!9l Pumpe Duse Tdi Bew and BRMDocument24 pagesD3e802f8707!1!9l Pumpe Duse Tdi Bew and BRMJan HavelNo ratings yet
- Gigantic Hyper S ALE: One Day OnlyDocument2 pagesGigantic Hyper S ALE: One Day OnlyThabang MoeraneNo ratings yet
- Rate Analysis For Academic Block-VDocument16 pagesRate Analysis For Academic Block-VEngr TahseenNo ratings yet
- Later InstagramIndustry BenchmarkReport 2022Document22 pagesLater InstagramIndustry BenchmarkReport 2022Linh NguyenNo ratings yet
- It Ai: ? L Ii. NSDocument8 pagesIt Ai: ? L Ii. NSKunal JainNo ratings yet
- EMSR568 AOI01 DEL PRODUCT r1 RTP01 v1Document1 pageEMSR568 AOI01 DEL PRODUCT r1 RTP01 v1Nélio FernandoNo ratings yet
- Tutorial 01 CRE A186207Document33 pagesTutorial 01 CRE A186207NURUL SYAHIRAH BINTI ABDUL HALIMNo ratings yet
- Theft From Motor Vehicle Maps For Sept. 16 To 22, 2019Document2 pagesTheft From Motor Vehicle Maps For Sept. 16 To 22, 2019Teresa VerencaNo ratings yet
- (9024SOD2) C15) : All MyDocument1 page(9024SOD2) C15) : All Myqxpvgd66mmNo ratings yet
- Routes 203 - 204 WeekdaysDocument1 pageRoutes 203 - 204 WeekdaysMathew WebsterNo ratings yet
- Buffalo Mt. Drive Detour MapDocument1 pageBuffalo Mt. Drive Detour MapsummitdailyNo ratings yet
- Polynomial: IsomorphismDocument2 pagesPolynomial: Isomorphism陳研希No ratings yet
- LectureNotes9 PDFDocument8 pagesLectureNotes9 PDFGuoXuanChanNo ratings yet
- 2013 Ucc ResultsDocument2 pages2013 Ucc ResultsJane PollyNo ratings yet
- CERF Annual Results Report 2021Document45 pagesCERF Annual Results Report 2021MustafaNo ratings yet
- Tips In) : - Ik Uke On Drawing and Designing House PlansDocument114 pagesTips In) : - Ik Uke On Drawing and Designing House Planscomputergator2014No ratings yet
- Moving Charges and Magnetism-1Document4 pagesMoving Charges and Magnetism-1nandanasatheesh01No ratings yet
- Sheet 4 - Armour, Crit, Damage, Death 1.23Document1 pageSheet 4 - Armour, Crit, Damage, Death 1.23Sam PowellNo ratings yet
- Savan BunglowsDocument1 pageSavan BunglowsKanjariya YashvantNo ratings yet
- BK DR C Fi Ti Backup and Recovery: ConfigurationDocument18 pagesBK DR C Fi Ti Backup and Recovery: Configurationjay karandikarNo ratings yet
- PDF Updated Class 11 Physics Formula Sheet CompressDocument22 pagesPDF Updated Class 11 Physics Formula Sheet CompressdrjbjpNo ratings yet
- Ewd 1Document18 pagesEwd 1Antonio GomezNo ratings yet
- Ejercicios No EstacionarioDocument18 pagesEjercicios No Estacionarioangi.acero0No ratings yet
- x04 Cost Volume Profit RelationshipsDocument49 pagesx04 Cost Volume Profit Relationshipsrachel banana hammockNo ratings yet
- It's So Easy Going Green: An Interactive, Scientific Look at Protecting Our EnvironmentFrom EverandIt's So Easy Going Green: An Interactive, Scientific Look at Protecting Our EnvironmentNo ratings yet
- Pertemuan - 4ATK2 - VAPORIZATION AND COOLING OF MIXTUREDocument9 pagesPertemuan - 4ATK2 - VAPORIZATION AND COOLING OF MIXTUREdonaNo ratings yet
- Lampiran BDocument38 pagesLampiran BsoniaNo ratings yet
- Laporan Sementara Praktikum Farmasi Fisika Objek 3 Pengaruh Pelarut Campur Terhadap Kelarutan ZatDocument4 pagesLaporan Sementara Praktikum Farmasi Fisika Objek 3 Pengaruh Pelarut Campur Terhadap Kelarutan ZatPutri RiduanNo ratings yet
- Calculating Equilibrium ConstantDocument3 pagesCalculating Equilibrium ConstantRei RomeroNo ratings yet
- Amjid Ali Bs-Physics Sse (Science) Ghs 147 JB Chiniot 0344-7763733Document2 pagesAmjid Ali Bs-Physics Sse (Science) Ghs 147 JB Chiniot 0344-7763733MuhammadAliNo ratings yet
- Cao Mgo Fe O Al O Sio So: Clinker AnalysisDocument2 pagesCao Mgo Fe O Al O Sio So: Clinker AnalysisFlorenceNo ratings yet
- 3 Review Stoichiometry Chemistry Practice Quiz and AnswersDocument3 pages3 Review Stoichiometry Chemistry Practice Quiz and AnswersMichael CaiNo ratings yet
- Stoichiometry Worksheet and Key: 2 Kclo 2 KCL + 3 ODocument4 pagesStoichiometry Worksheet and Key: 2 Kclo 2 KCL + 3 ORobin WongNo ratings yet
- Latihan Soal TitrasiDocument15 pagesLatihan Soal TitrasifirstcaNo ratings yet
- Calculadora DQODocument4 pagesCalculadora DQOOmar KallufNo ratings yet
- Hidrolisis Dan PenyanggaDocument10 pagesHidrolisis Dan PenyanggaDylfa AprinaNo ratings yet
- Isotermas DefinitivoDocument11 pagesIsotermas DefinitivoTelomeros de la CienciaNo ratings yet
- Neraca MassaDocument38 pagesNeraca MassaGunNo ratings yet
- This Study Resource Was: Data Sheet Experiment 3 Synthesis of Potassium Tris (Oxalato) Ferrate (III) TrihydrateDocument3 pagesThis Study Resource Was: Data Sheet Experiment 3 Synthesis of Potassium Tris (Oxalato) Ferrate (III) TrihydrateSolutions MasterNo ratings yet
- 1Document2 pages1axznpsychoNo ratings yet
- Polarity in Covalent BondsDocument15 pagesPolarity in Covalent BondsMarcoNo ratings yet
- Formulario de TermodinamicaDocument2 pagesFormulario de TermodinamicaPablo Lobatón HildaNo ratings yet
- Modul 4 Stoi, Bab 4Document11 pagesModul 4 Stoi, Bab 4Agus Ari BowoNo ratings yet
- CHEM 181 DL1: Final ReportDocument3 pagesCHEM 181 DL1: Final ReportNeally WeallyNo ratings yet
- Soal BufferDocument6 pagesSoal BufferFitria Rizky100% (1)
- C C1+C2T+C3T C 4 T C 4 T C: Iv - Specific Heat (J/KGK)Document2 pagesC C1+C2T+C3T C 4 T C 4 T C: Iv - Specific Heat (J/KGK)Andrew OracionNo ratings yet
- Zogadi Qimiis KursiDocument153 pagesZogadi Qimiis KursiTata Gazashvili100% (1)
- Stoichiometry Questions & AnswersDocument2 pagesStoichiometry Questions & Answersnosirat aladeNo ratings yet
- Sita RamDocument1 pageSita RamSampa MukherjeeNo ratings yet
- Scie6043 Tcda TP1 W2 S3 R1Document6 pagesScie6043 Tcda TP1 W2 S3 R1Muhammad Lutfi HidayatNo ratings yet
- 059317001Document14 pages059317001Nirmani HansiniNo ratings yet
- Perhitungan Asidi AlkalimetriDocument4 pagesPerhitungan Asidi AlkalimetriChristoperQ HutaurukNo ratings yet
- 00 Perhitungan Alat Besar 1-DeSKTOP-NJGKKH8Document359 pages00 Perhitungan Alat Besar 1-DeSKTOP-NJGKKH8Muhammad FakhrizalNo ratings yet
- Spesifikasi Tangki PenampungDocument65 pagesSpesifikasi Tangki PenampungBunga Rajhana Ragil GayatriNo ratings yet