Professional Documents
Culture Documents
Endometrialcarcinoma r0416 7941
Endometrialcarcinoma r0416 7941
Uploaded by
crisibarra911Copyright:
Available Formats
You might also like
- Pharmacy Calculation Workbook: 250 Questions to Prepare for the NAPLEX and PTCB ExamFrom EverandPharmacy Calculation Workbook: 250 Questions to Prepare for the NAPLEX and PTCB ExamRating: 5 out of 5 stars5/5 (2)
- Acute Renal Failure - PPT 1Document36 pagesAcute Renal Failure - PPT 1Jay Paul50% (2)
- Lung Cancer Treatment Regimens (Part 1 of 4)Document4 pagesLung Cancer Treatment Regimens (Part 1 of 4)Rubana Reaz TanaNo ratings yet
- Breast Cancer RegimensDocument6 pagesBreast Cancer RegimensMohammad Arwan HamdaniNo ratings yet
- Head Neck 0515 7935Document5 pagesHead Neck 0515 7935Lucia FNo ratings yet
- Brain Cancer Treatment Regimens (Part 1 of 5)Document5 pagesBrain Cancer Treatment Regimens (Part 1 of 5)Rubana Reaz TanaNo ratings yet
- Perioperative Chemotherapy (Neoadjuvant or Adjuvant)Document4 pagesPerioperative Chemotherapy (Neoadjuvant or Adjuvant)c.ramNo ratings yet
- Non-Hodgkin Lymphoma (NHL)Document11 pagesNon-Hodgkin Lymphoma (NHL)archanaNo ratings yet
- Squamous Cell Cancers: Primary Systemic Therapy + Concurrent RadiotherapyDocument5 pagesSquamous Cell Cancers: Primary Systemic Therapy + Concurrent RadiotherapyArjun Singh ChoudharyNo ratings yet
- B-Cell Lymphomas Treatment Regimens - Follicular Lymphoma (Grade 1-2) - Cancer Therapy Advisor PDFDocument12 pagesB-Cell Lymphomas Treatment Regimens - Follicular Lymphoma (Grade 1-2) - Cancer Therapy Advisor PDFOleques Rodriguês MuahequeNo ratings yet
- Primary Therapy-Chronic Smoldering: ContinuedDocument2 pagesPrimary Therapy-Chronic Smoldering: ContinuedYuliana Eka RusdiyantiNo ratings yet
- EC-DH Epirubicin Cyclophosphamide Followed by Docetaxel With Trastuzumab Protocol V1.1Document11 pagesEC-DH Epirubicin Cyclophosphamide Followed by Docetaxel With Trastuzumab Protocol V1.1smokkerNo ratings yet
- Ovarian Cancer Treatment Regimens - Print Article - Cancer Therapy AdvisorDocument5 pagesOvarian Cancer Treatment Regimens - Print Article - Cancer Therapy AdvisorAnonymous g1hIPZNo ratings yet
- Leukemia Treatment Regimens 7937Document3 pagesLeukemia Treatment Regimens 7937Irfan FathurrahmanNo ratings yet
- Acute Lymphoblastic Leukemia Treatment RegimensDocument4 pagesAcute Lymphoblastic Leukemia Treatment Regimensnur-aisyahkNo ratings yet
- Castration-Recurrent Prostate Cancer First-Line Therapy: No Visceral MetastasesDocument2 pagesCastration-Recurrent Prostate Cancer First-Line Therapy: No Visceral Metastasesalberto cabelloNo ratings yet
- Regimen Kanker RektumDocument8 pagesRegimen Kanker RektumNurul Kamilah SadliNo ratings yet
- Hodgkin Lymphoma RegimensDocument5 pagesHodgkin Lymphoma RegimensLuana MNo ratings yet
- Chemotherapy ProtocolsDocument42 pagesChemotherapy ProtocolswilsoncarbonhealthNo ratings yet
- EC-D With HP Epirubicin Cyclophosphamide Followed by Docetaxel Trastuzumab Pertuzumab Neo-Adjuvant and Adjuvant Protocol V2.1Document20 pagesEC-D With HP Epirubicin Cyclophosphamide Followed by Docetaxel Trastuzumab Pertuzumab Neo-Adjuvant and Adjuvant Protocol V2.1smokkerNo ratings yet
- Vejiga. CtaDocument11 pagesVejiga. CtaIsabel Gago CastilloNo ratings yet
- LYRICE ProtocolDocument6 pagesLYRICE ProtocolInas UthmanNo ratings yet
- Bone Cancer Treatment RegimensDocument2 pagesBone Cancer Treatment RegimensAnne Lorraine BringasNo ratings yet
- MultipleMyeloma r0521Document17 pagesMultipleMyeloma r0521Raja Alfian IrawanNo ratings yet
- Teicoplanin Dosing and Monitoring in AdultsDocument3 pagesTeicoplanin Dosing and Monitoring in Adultsdps_1976No ratings yet
- Trasturel & BevacirelDocument28 pagesTrasturel & Bevacireldr.htetaung2017No ratings yet
- Guideline: Antibiotic Drug Monitoring: Aminoglycosides and GlycopeptidesDocument8 pagesGuideline: Antibiotic Drug Monitoring: Aminoglycosides and GlycopeptidesKenRodulfReyesVillaruelNo ratings yet
- Docetaxel Carboplatin Trastuzumab T Carbo H Breast Cancer Adjuvant ProtocolDocument14 pagesDocetaxel Carboplatin Trastuzumab T Carbo H Breast Cancer Adjuvant ProtocolsmokkerNo ratings yet
- Nephrotic Syndrome Relapse Clinical PathwayDocument8 pagesNephrotic Syndrome Relapse Clinical Pathwaydr.salimuzzamanNo ratings yet
- Chemo Protocol PermetrexedDocument4 pagesChemo Protocol PermetrexedSangireddy NarreddyNo ratings yet
- Picu NotesDocument65 pagesPicu NotesAhmed Mohammed100% (2)
- Trastuzumab (Intravenous) Including Biosimilars of Trastuzumab - Drug Information - UpToDateDocument20 pagesTrastuzumab (Intravenous) Including Biosimilars of Trastuzumab - Drug Information - UpToDateDenys Romero100% (1)
- Enoxaparin Info SheetDocument7 pagesEnoxaparin Info SheetjafarkassimNo ratings yet
- Therapeutic Choices For The Medical Management of Feline LymphomaDocument8 pagesTherapeutic Choices For The Medical Management of Feline LymphomaFran PećnjakNo ratings yet
- Management MalariaDocument2 pagesManagement MalariaMuhammad NuansaNo ratings yet
- Antibiotic ConversionDocument9 pagesAntibiotic ConversionfabianNo ratings yet
- Bone Cancer Treatment RegimensDocument2 pagesBone Cancer Treatment RegimensAndreiMunteanuNo ratings yet
- Pediatric DosesDocument5 pagesPediatric Dosesa2r91No ratings yet
- National Malaria Drug PolicyDocument22 pagesNational Malaria Drug Policydocsaurabh777No ratings yet
- IfosfamideDocument4 pagesIfosfamideNurkholis AminNo ratings yet
- Prevention SepsisDocument25 pagesPrevention SepsisckNo ratings yet
- Drug Dosages in PediatricsDocument4 pagesDrug Dosages in Pediatricsmarry.as2012No ratings yet
- Medication Fact Sheets: 3rd Edition ContributorsDocument50 pagesMedication Fact Sheets: 3rd Edition ContributorsIndumathi ThangathirupathiNo ratings yet
- Answers To Practice Problem Set #10Document3 pagesAnswers To Practice Problem Set #10Raju NiraulaNo ratings yet
- Cyclosporine PPT F (1)Document32 pagesCyclosporine PPT F (1)Omkar KulkarniNo ratings yet
- Medication Math 430Document12 pagesMedication Math 430aliNo ratings yet
- Id 397 TeicoplaninDocument2 pagesId 397 TeicoplaninStacey WoodsNo ratings yet
- Assignment-Intravenous Infusion-StudentDocument7 pagesAssignment-Intravenous Infusion-StudentpinkhasovdNo ratings yet
- TCHP-Docetaxel Carboplatin Trastuzumab Pertuzumab Neoadjuvant Adjuvant Protocol V2.2Document21 pagesTCHP-Docetaxel Carboplatin Trastuzumab Pertuzumab Neoadjuvant Adjuvant Protocol V2.2smokkerNo ratings yet
- Commonly Used Drugs in Pediatric PracticeDocument4 pagesCommonly Used Drugs in Pediatric PracticePraveen’s IphoneNo ratings yet
- Prescribing On The Neonatal Unit Feb2010Document54 pagesPrescribing On The Neonatal Unit Feb2010madimadi11No ratings yet
- Daunorubicin Hydrochloride IV Over 6 Hours On Days 1, 3, and 5, and Etoposide IV Over 4Document3 pagesDaunorubicin Hydrochloride IV Over 6 Hours On Days 1, 3, and 5, and Etoposide IV Over 4Mohammed HaiderNo ratings yet
- Daepoch Protocolo BccancerDocument9 pagesDaepoch Protocolo BccancerLuiz MeloNo ratings yet
- Pediatric Antibiotic DosingDocument7 pagesPediatric Antibiotic DosingAnonymous mm1yTelKNo ratings yet
- جرعات الاطفالDocument50 pagesجرعات الاطفالWael Hamdy100% (1)
- Drug Therapy For PicuDocument32 pagesDrug Therapy For PicuNeethu Mariya MathewNo ratings yet
- Malaria: Public Health Division, Directorate of Health Services Thiruvananthapuram June 2016Document15 pagesMalaria: Public Health Division, Directorate of Health Services Thiruvananthapuram June 2016Ajan MjNo ratings yet
- Darzalex Epar Product Information - enDocument102 pagesDarzalex Epar Product Information - ensmich73No ratings yet
- 1 s2.0 S0163445316300123 MainDocument2 pages1 s2.0 S0163445316300123 MainZara SaeedNo ratings yet
- Antibiotic Treatment Guidelines For Urinary Tract Infections in Children (60 Days Through 17 Years)Document3 pagesAntibiotic Treatment Guidelines For Urinary Tract Infections in Children (60 Days Through 17 Years)Zahid AbbasNo ratings yet
- Antibiotic Guidelines For PediatricsDocument33 pagesAntibiotic Guidelines For PediatricsVarshini Tamil SelvanNo ratings yet
- Protocollo Fazio CovidDocument12 pagesProtocollo Fazio CoviddonNo ratings yet
- 05 N017 2931Document13 pages05 N017 2931anju negalurNo ratings yet
- Diet Readiness QuestionnaireDocument3 pagesDiet Readiness Questionnairesavvy_as_98100% (1)
- A StudyDocument12 pagesA StudyNichchanun LertnuwatNo ratings yet
- Tuberculin PPD RT 23Document10 pagesTuberculin PPD RT 23Irene Olivia SalimNo ratings yet
- Ferric Carboxymaltose (Hemojet) : Md. Arafat Hossain Brand Executive, SBMD, 01713354512Document5 pagesFerric Carboxymaltose (Hemojet) : Md. Arafat Hossain Brand Executive, SBMD, 01713354512Heranmoy Kumar Bijon100% (1)
- Fuck The System - and How To Live For Free - Published Circa '67Document17 pagesFuck The System - and How To Live For Free - Published Circa '67Tedd St RainNo ratings yet
- Principles of MicrobiologyDocument301 pagesPrinciples of MicrobiologyBenjamin A. Ujlaki76% (17)
- Guidance and Counselling For AdolescentsDocument20 pagesGuidance and Counselling For AdolescentsNalu ChangNo ratings yet
- Development of A Nursing Care Protocol For Care of Neonates With Esophageal Atresia/ Tracheo - Esophageal FistulaDocument14 pagesDevelopment of A Nursing Care Protocol For Care of Neonates With Esophageal Atresia/ Tracheo - Esophageal FistulaSanket TelangNo ratings yet
- Kanaan: Sample Papers?Document8 pagesKanaan: Sample Papers?Kulanthaivelu R SicaNo ratings yet
- Yellow: The Primary ColoursDocument20 pagesYellow: The Primary ColourstripNo ratings yet
- Blanche GrubeDocument21 pagesBlanche GrubesangroniscarlosNo ratings yet
- Primary Health Care Systems (Primasys) : Case Study From CameroonDocument16 pagesPrimary Health Care Systems (Primasys) : Case Study From CameroonEyock PierreNo ratings yet
- Test 2Document49 pagesTest 2Kanish AggarwalNo ratings yet
- Immunohistochemical Characterization of Urethral Polyps in WomenDocument4 pagesImmunohistochemical Characterization of Urethral Polyps in WomenCentral Asian StudiesNo ratings yet
- Nursing Care of Children-QuestionsDocument11 pagesNursing Care of Children-QuestionsRamon Carlo AlmiranezNo ratings yet
- Ledderhose Disease: Pathophysiology Diagnosis and ManagementDocument3 pagesLedderhose Disease: Pathophysiology Diagnosis and ManagementLeandro PolancoNo ratings yet
- Communicable and Non-Communicable DiseaseDocument44 pagesCommunicable and Non-Communicable DiseaseSajida Bibi NoonariNo ratings yet
- The Rokeach Value Survey (RVS) : Milton Rokeach - Was A Polish-American Social Psychologist Who Developed The RVSDocument7 pagesThe Rokeach Value Survey (RVS) : Milton Rokeach - Was A Polish-American Social Psychologist Who Developed The RVSAngel May L. LopezNo ratings yet
- AbbreviationsDocument9 pagesAbbreviationsYuuki Chitose (tai-kun)No ratings yet
- Algorithm 1 Algorithm 2Document51 pagesAlgorithm 1 Algorithm 2Zimm RrrrNo ratings yet
- Plasmapheresis For AtherosclerosisDocument10 pagesPlasmapheresis For AtherosclerosisEliDavidNo ratings yet
- 2 Vol. 8 Issue 5 May 2017 IJPSR RE 2098Document13 pages2 Vol. 8 Issue 5 May 2017 IJPSR RE 2098KhokonNo ratings yet
- Pathology Solved Papers 2015Document35 pagesPathology Solved Papers 2015Lakshmi Venkataraman100% (3)
- Consensus For EGFR Mutation Testing in Non-Small Cell PDFDocument8 pagesConsensus For EGFR Mutation Testing in Non-Small Cell PDFCahrun CarterNo ratings yet
- Faculty of Community Medicine and PublichealthDocument21 pagesFaculty of Community Medicine and PublichealthFarhan Hassan DarNo ratings yet
- The 4000 English Words Essential For An Educated VocabularyDocument139 pagesThe 4000 English Words Essential For An Educated VocabularyLike GhazalNo ratings yet
- Pediatric Medical DevicesDocument25 pagesPediatric Medical DevicesDuck Mann-ConsulNo ratings yet
Endometrialcarcinoma r0416 7941
Endometrialcarcinoma r0416 7941
Uploaded by
crisibarra911Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Endometrialcarcinoma r0416 7941
Endometrialcarcinoma r0416 7941
Uploaded by
crisibarra911Copyright:
Available Formats
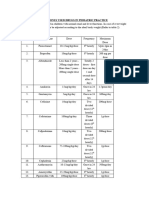
ENDOMETRIAL CARCINOMA TREATMENT REGIMENS (Part 1 of 2)
Clinical Trials: The NCCN recommends cancer patient participation in clinical trials as the gold standard for treatment.
Cancer therapy selection, dosing, administration, and the management of related adverse events can be a complex process that should
be handled by an experienced healthcare team. Clinicians must choose and verify treatment options based on the individual patient;
drug dose modifications and supportive care interventions should be administered accordingly. The cancer treatment regimens below
may include both U.S. Food and Drug Administration-approved and unapproved indications/regimens. These regimens are only provided
to supplement the latest treatment strategies.
These Guidelines are a work in progress that may be refined as often as new significant data becomes available. The NCCN Guidelines®
are a consensus statement of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to
apply or consult any NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circum-
stances to determine any patient’s care or treatment. The National Comprehensive Cancer Network makes no warranties of any kind
whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way.
Systemic Therapy for Recurrent, Metastatic, or High-Risk Endometrial Carcinoma1
Note: All recommendations are category 2A unless otherwise indicated.
Chemotherapy Regimens
REGIMEN DOSING
Carboplatin + paclitaxel2,3 Day 1: Carboplatin AUC 5–6mg•min/mL IV over 1 hour + paclitaxel
175mg/m2 IV over 3 hours.
Repeat cycle every 3 weeks for 6 to 9 cycles.
Cisplatin + doxorubicin4,5a Day 1: Doxorubicin 60mg/m2 IV followed by cisplatin 50mg/m2 over
1 hour
Day 2–11 (optional): Granulocyte colony-stimulating factor
5mcg/kg/day subcutaneously.
Repeat every 3 weeks for maximum of 7 cycles.
Cisplatin + doxorubicin + paclitaxel4,5b Day 1: Doxorubicin 45mg/m2 IV + cisplatin 50mg/m2 IV
Day 2: Paclitaxel 160mg/m2 IV over 3 hours
Days 3–12: Filgrastim 5mcg/kg SQ (or pegfilgrastim 6mg on
day 3 only).
Repeat every 3 weeks for 6–7 cycles.
Carboplatin + docetaxel6–8c Day 1: Docetaxel 60–75mg/m2 IV over 1 hour; followed by
carboplatin AUC 6mg•min/mL IV over 1 hour.
Repeat every 3 weeks for 6 cycles.
Ifosfamide + paclitaxel (Category 1 for Day 1: Paclitaxel 135mg/m2 IV over 3 hours
carcinosarcoma)9 Days 1–3: Ifosfamide 1.6g/m2/day IV (reduced to 1.2g/m2/day if
patient received prior radiation).
Repeat cycle every 3 weeks for 8 cycles.
Cisplatin + ifosfamide (for Days 1–4: Cisplatin 20mg/m2/day IV over 15 minutes + ifosfamide
carcinosarcoma)10 1.5g/m2/day IV over 1 hour
Day 1: Mesna 120mg/m2 IV bolus over 15 minutes (loading dose)
Days 1–4: Mesna 1.5g/m2/day continuous IV infusion.
Repeat cycle every 3 weeks for 3 cycles.
Cisplatin11 Day 1: Cisplatin 50mg/m2 IV.
Repeat cycle every 3 weeks.
Carboplatin12 Day 1: Carboplatin 400mg/m2 IV.
Repeat cycle every 3 weeks
Doxorubicin13 Day 1: Doxorubicin 60mg/m2 IV.
Repeat cycle every 4 weeks.
Liposomal doxorubicin14 Day 1: Liposomal doxorubicin 50mg/m2 IV over 1 hour.
Repeat cycle every 4 weeks.
Paclitaxel15 Day 1: Paclitaxel 110–200mg/m2 IV.
Repeat cycle every 3 weeks.
Topotecan16 Days 1–5: Topotecan 1.5mg/m2/day IV (reduced to 1.2mg/m2 if
patients received prior pelvic radiation).
Repeat cycle every 3 weeks.
Bevacizumab17 Day 1: Bevacizumab 15mg/kg IV.
Repeat cycle every 3 weeks.
Temsirolimus18 Temsirolimus 25mg IV weekly.
Repeat cycle every 4 weeks.
Docetaxel (Category 2B)19 Days 1, 8, and 15: Docetaexel 36mg/m2 IV over 1 hour.
Repeat cycle every 4 weeks.
Ifosfamide (for carcinosarcoma)9 Days 1–3: Ifosfamide 2g/m2/day IV + mesna 2g IV beginning
15 minutes before ifosfamide infusion.
Repeat cycle every 3 weeks for 8 cycles.
continued
ENDOMETRIAL CARCINOMA TREATMENT REGIMENS (Part 2 of 2)
Systemic Therapy for Recurrent, Metastatic, or High-Risk Endometrial Carcinoma1
Hormonal Therapyd
REGIMEN DOSING
Megestrol + alternating tamoxifen20 Megestrol acetate 80mg orally twice daily for 3 weeks alternating with
tamoxifen 20mg orally twice daily for 3 weeks.
Tamoxifen21 Tamoxifen 20mg orally twice daily until disease progression or
unacceptable toxicity.
Progesterone agents This regimen was included in the NCCN guidelines but no reference
was provided to indicate appropriate agents or dosages, as no
particular dose, drug, or schedule has been found to be superior.
Aromatase inhibitors This regimen was included in the NCCN guidelines but no reference
was provided to indicate appropriate agents or dosages, as no
particular dose, drug, or schedule has been found to be superior.
General treatment notes:
• Participation in clinical trial is strongly recommended.
• Multi-agent chemotherapy regimens preferred over single agents, if tolerated.
• Cisplatin, carboplatin, liposomal doxorubicin, paclitaxel, and docetaxel may cause drug reactions.
• Chemotherapy regimens can be used for all carcinoma histologies. Carcinosarcomas are now considered and
treated as high-grade carcinomas. However, ifosfamide-based regimens were previously used for carcinosarcomas.
a
Patients who have received prior pelvic radiotherapy or who are older than 65 years should receive a reduction in the starting dose
of doxorubicin, to 45mg/m2.
b
The cisplatin/doxorubicin/paclitaxel regimen is not widely used because of concerns about toxicity.
c
Docetaxel may be considered for patients in whom paclitaxel is contraindicated.
d
Hormonal therapy may be used for lower grade endometrioid histologies only (ie, not for G3 endometrioid, serous carcinoma, clear cell car-
cinoma, or carcinosarcoma), preferably in patients with small tumor volume or an indolent growth pace.
References
1. Referenced with permission from the NCCN Clinical Practice 11. Thigpen JT, Blessing JA, Lagasse LD. Phase II trial of cisplatin
Guidelines in Oncology™. Uterine Neoplasms. v 2.2016. as first-line chemotherapy in patients with advanced or recur-
Available at: http://www.nccn.org/professionals/physician_ rent endometrial carcinoma: a Gynecologic Oncology Group
gls/pdf/uterine.pdf. Accessed March 22, 2016. study. Gynecol Oncol. 1989;33(1):68–70.
2. Miller D, Filiaci V, Fleming G, et al. Randomized phase III 12. Van Wijk FH, Lhomme C, Bolis G, et al. Phase II study of
noninferiority trial of first line chemotherapy for metastatic or carboplatin in patients with advanced or recurrent endometrial
recurrent endometrial carcinoma: a Gynecologic Oncology carcinoma: a trial of the EORTC Gynaecological Cancer
Group study [abstract]. Gynecol Oncol. 2012:125:771. Group. Eur J Cancer. 2003;39(1):78–85.
3. Sorbe B, Andersson H, Boman K, et al. Treatment of primary 13. Aapro MS, van Wijk FH, Bolis G, et al. Doxorubicin versus doxoru-
advanced and recurrent endometrial carcinoma with a com- bicin and cisplatin in endometrial carcinoma: definitive results
bination of carboplatin and paclitaxel-long-term follow-up. of a randomized study (55872) by the EORTC Gynaecological
Int J Gynecol Cancer. 2008;18(4):803–808. Cancer Group. Ann Oncol. 2003;14(3):441–448.
4. Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxo- 14. Muggia FM, Blessing JA, Sorosky J, Reid GC. Phase II trial of
rubicin plus cisplatin with or without paclitaxel plus filgrastim the pegylated liposomal doxorubicin in previously treated
in advanced endometrial carcinoma: a Gynecologic Oncology metastatic endometrial cancer: a Gynecologic Oncology
Group Study. J Clin Oncol. 2004;22(11):2159–2166. Group study. J Clin Oncol. 2002;20(9):2360–2364.
5. Homesley HD, Filiaci V, Gibbons SK, et al. A randomized 15. Lincoln S, Blessing JA, Lee RB, Rocereto TF. Activity of paclitaxel
phase III trial in advanced endometrial carcinoma of surgery as second-line chemotherapy in endometrial carcinoma: a
and volume directed radiation followed by cisplatin and Gynecologic Oncology Group study. Gynecol Oncol. 2003;
doxorubicin with or without paclitaxel: A Gynecologic Oncology 88(3):277–281.
Group study. Gynecol Oncol. 2009;112(3):543–552.
16. Wadler S, Levy DE, Lincoln ST, et al. Topotecan is an active
6. Scribner DR Jr, Puls LE, Gold MA. A phase II evaluation of agent in the first-line treatment of metastatic or recurrent
docetaxel and carboplatin followed by tumor volume directed endometrial carcinoma: Eastern Cooperative Oncology Group
pelvic plus or minus paraaortic irradiation for stage III endo- Study E3E93. J Clin Oncol. 2003;21(11):2110–2114.
metrial cancer. Gynecol Oncol. 2012;125(2):388–393.
17. Aghajanian C, Sill MW, Darcy KM, et al. Phase II trial of beva-
7. Geller MA, Ivy JJ, Ghebre R, et al. A phase II trial of carboplatin cizumab in recurrent or persistent endometrial: a Gynecologic
and docetaxel followed by radiotherapy given in a “Sandwich” Oncology Group study. J Clin Oncol. 2011;29(16):2259–2265.
method for stage III, IV, and recurrent endometrial cancer.
Gynecol Oncol. 2011;121(1):112–117. 18. Oza AM, Elit L, Tsao MS, et al. Phase II study of temsirolimus in
women with recurrent or metastatic endometrial cancer: a trial
8. Nomura H, Aoki D, Takahashi F, et al. Randomized phase II of the NCIC Clinical Trials Group. J Clin Oncol. 2011;29(24):
study comparing docetaxel plus cisplatin, docetaxel plus 3278–3285.
carboplatin, and paclitaxel plus carboplatin in patients with
advanced or recurrent endometrial carcinoma: a Japanese 19. Garcia AA, Blessing JA, Nolte S, Mannel RS. A phase II evalua-
Gynecologic Oncology Group study (JGOG2041). Ann Oncol. tion of weekly docetaxel in the treatment of recurrent or
2011;22(3):636–642. persistent endometrial carcinoma: a study by the Gynecologic
Oncology Group. Gynecol Oncol. 2008;111(1):22–26.
9. Homesley HO, Filiaci V, Markman M, et al. Phase III trial of
ifosfamide with or without paclitaxel in advanced uterine 20. Fiorica JV, Brunetto VL, Hanjani P, et al. Phase II trial of alternat-
carcinosarcoma: a Gynecologic Oncology Group Study. ing courses of megestrol acetate and tamoxifen in advanced
J Clin Oncol. 2007:25(5):526–531. endometrial carcinoma: a Gynecologic Oncology Group study.
10. Wolfson AH, Brady MF, Rocereto TF, et al. A gynecologic oncology Gynecol Oncol. 2004;92(1):10–14.
group randomized trial of whole abdominal irradiation (WAI) 21. Thigpen T, Brady MF, Homesley HD, Soper JT, Bell J. Tamoxifen in the
vs cisplatin-ifosfamide-mesna (CIM) in optimally debulked treatment of advanced or recurrent endometrial carcinoma: a Gy-
stage I-IV carcinosarcoma (CS) of the uterus. J Clin Oncol. necologic Oncology Group study. J Clin Oncol.
2006;24(18S):5001. 2001;19(2):364–367.
(Revised 4/2016)
© 2016 by Haymarket Media, Inc.
You might also like
- Pharmacy Calculation Workbook: 250 Questions to Prepare for the NAPLEX and PTCB ExamFrom EverandPharmacy Calculation Workbook: 250 Questions to Prepare for the NAPLEX and PTCB ExamRating: 5 out of 5 stars5/5 (2)
- Acute Renal Failure - PPT 1Document36 pagesAcute Renal Failure - PPT 1Jay Paul50% (2)
- Lung Cancer Treatment Regimens (Part 1 of 4)Document4 pagesLung Cancer Treatment Regimens (Part 1 of 4)Rubana Reaz TanaNo ratings yet
- Breast Cancer RegimensDocument6 pagesBreast Cancer RegimensMohammad Arwan HamdaniNo ratings yet
- Head Neck 0515 7935Document5 pagesHead Neck 0515 7935Lucia FNo ratings yet
- Brain Cancer Treatment Regimens (Part 1 of 5)Document5 pagesBrain Cancer Treatment Regimens (Part 1 of 5)Rubana Reaz TanaNo ratings yet
- Perioperative Chemotherapy (Neoadjuvant or Adjuvant)Document4 pagesPerioperative Chemotherapy (Neoadjuvant or Adjuvant)c.ramNo ratings yet
- Non-Hodgkin Lymphoma (NHL)Document11 pagesNon-Hodgkin Lymphoma (NHL)archanaNo ratings yet
- Squamous Cell Cancers: Primary Systemic Therapy + Concurrent RadiotherapyDocument5 pagesSquamous Cell Cancers: Primary Systemic Therapy + Concurrent RadiotherapyArjun Singh ChoudharyNo ratings yet
- B-Cell Lymphomas Treatment Regimens - Follicular Lymphoma (Grade 1-2) - Cancer Therapy Advisor PDFDocument12 pagesB-Cell Lymphomas Treatment Regimens - Follicular Lymphoma (Grade 1-2) - Cancer Therapy Advisor PDFOleques Rodriguês MuahequeNo ratings yet
- Primary Therapy-Chronic Smoldering: ContinuedDocument2 pagesPrimary Therapy-Chronic Smoldering: ContinuedYuliana Eka RusdiyantiNo ratings yet
- EC-DH Epirubicin Cyclophosphamide Followed by Docetaxel With Trastuzumab Protocol V1.1Document11 pagesEC-DH Epirubicin Cyclophosphamide Followed by Docetaxel With Trastuzumab Protocol V1.1smokkerNo ratings yet
- Ovarian Cancer Treatment Regimens - Print Article - Cancer Therapy AdvisorDocument5 pagesOvarian Cancer Treatment Regimens - Print Article - Cancer Therapy AdvisorAnonymous g1hIPZNo ratings yet
- Leukemia Treatment Regimens 7937Document3 pagesLeukemia Treatment Regimens 7937Irfan FathurrahmanNo ratings yet
- Acute Lymphoblastic Leukemia Treatment RegimensDocument4 pagesAcute Lymphoblastic Leukemia Treatment Regimensnur-aisyahkNo ratings yet
- Castration-Recurrent Prostate Cancer First-Line Therapy: No Visceral MetastasesDocument2 pagesCastration-Recurrent Prostate Cancer First-Line Therapy: No Visceral Metastasesalberto cabelloNo ratings yet
- Regimen Kanker RektumDocument8 pagesRegimen Kanker RektumNurul Kamilah SadliNo ratings yet
- Hodgkin Lymphoma RegimensDocument5 pagesHodgkin Lymphoma RegimensLuana MNo ratings yet
- Chemotherapy ProtocolsDocument42 pagesChemotherapy ProtocolswilsoncarbonhealthNo ratings yet
- EC-D With HP Epirubicin Cyclophosphamide Followed by Docetaxel Trastuzumab Pertuzumab Neo-Adjuvant and Adjuvant Protocol V2.1Document20 pagesEC-D With HP Epirubicin Cyclophosphamide Followed by Docetaxel Trastuzumab Pertuzumab Neo-Adjuvant and Adjuvant Protocol V2.1smokkerNo ratings yet
- Vejiga. CtaDocument11 pagesVejiga. CtaIsabel Gago CastilloNo ratings yet
- LYRICE ProtocolDocument6 pagesLYRICE ProtocolInas UthmanNo ratings yet
- Bone Cancer Treatment RegimensDocument2 pagesBone Cancer Treatment RegimensAnne Lorraine BringasNo ratings yet
- MultipleMyeloma r0521Document17 pagesMultipleMyeloma r0521Raja Alfian IrawanNo ratings yet
- Teicoplanin Dosing and Monitoring in AdultsDocument3 pagesTeicoplanin Dosing and Monitoring in Adultsdps_1976No ratings yet
- Trasturel & BevacirelDocument28 pagesTrasturel & Bevacireldr.htetaung2017No ratings yet
- Guideline: Antibiotic Drug Monitoring: Aminoglycosides and GlycopeptidesDocument8 pagesGuideline: Antibiotic Drug Monitoring: Aminoglycosides and GlycopeptidesKenRodulfReyesVillaruelNo ratings yet
- Docetaxel Carboplatin Trastuzumab T Carbo H Breast Cancer Adjuvant ProtocolDocument14 pagesDocetaxel Carboplatin Trastuzumab T Carbo H Breast Cancer Adjuvant ProtocolsmokkerNo ratings yet
- Nephrotic Syndrome Relapse Clinical PathwayDocument8 pagesNephrotic Syndrome Relapse Clinical Pathwaydr.salimuzzamanNo ratings yet
- Chemo Protocol PermetrexedDocument4 pagesChemo Protocol PermetrexedSangireddy NarreddyNo ratings yet
- Picu NotesDocument65 pagesPicu NotesAhmed Mohammed100% (2)
- Trastuzumab (Intravenous) Including Biosimilars of Trastuzumab - Drug Information - UpToDateDocument20 pagesTrastuzumab (Intravenous) Including Biosimilars of Trastuzumab - Drug Information - UpToDateDenys Romero100% (1)
- Enoxaparin Info SheetDocument7 pagesEnoxaparin Info SheetjafarkassimNo ratings yet
- Therapeutic Choices For The Medical Management of Feline LymphomaDocument8 pagesTherapeutic Choices For The Medical Management of Feline LymphomaFran PećnjakNo ratings yet
- Management MalariaDocument2 pagesManagement MalariaMuhammad NuansaNo ratings yet
- Antibiotic ConversionDocument9 pagesAntibiotic ConversionfabianNo ratings yet
- Bone Cancer Treatment RegimensDocument2 pagesBone Cancer Treatment RegimensAndreiMunteanuNo ratings yet
- Pediatric DosesDocument5 pagesPediatric Dosesa2r91No ratings yet
- National Malaria Drug PolicyDocument22 pagesNational Malaria Drug Policydocsaurabh777No ratings yet
- IfosfamideDocument4 pagesIfosfamideNurkholis AminNo ratings yet
- Prevention SepsisDocument25 pagesPrevention SepsisckNo ratings yet
- Drug Dosages in PediatricsDocument4 pagesDrug Dosages in Pediatricsmarry.as2012No ratings yet
- Medication Fact Sheets: 3rd Edition ContributorsDocument50 pagesMedication Fact Sheets: 3rd Edition ContributorsIndumathi ThangathirupathiNo ratings yet
- Answers To Practice Problem Set #10Document3 pagesAnswers To Practice Problem Set #10Raju NiraulaNo ratings yet
- Cyclosporine PPT F (1)Document32 pagesCyclosporine PPT F (1)Omkar KulkarniNo ratings yet
- Medication Math 430Document12 pagesMedication Math 430aliNo ratings yet
- Id 397 TeicoplaninDocument2 pagesId 397 TeicoplaninStacey WoodsNo ratings yet
- Assignment-Intravenous Infusion-StudentDocument7 pagesAssignment-Intravenous Infusion-StudentpinkhasovdNo ratings yet
- TCHP-Docetaxel Carboplatin Trastuzumab Pertuzumab Neoadjuvant Adjuvant Protocol V2.2Document21 pagesTCHP-Docetaxel Carboplatin Trastuzumab Pertuzumab Neoadjuvant Adjuvant Protocol V2.2smokkerNo ratings yet
- Commonly Used Drugs in Pediatric PracticeDocument4 pagesCommonly Used Drugs in Pediatric PracticePraveen’s IphoneNo ratings yet
- Prescribing On The Neonatal Unit Feb2010Document54 pagesPrescribing On The Neonatal Unit Feb2010madimadi11No ratings yet
- Daunorubicin Hydrochloride IV Over 6 Hours On Days 1, 3, and 5, and Etoposide IV Over 4Document3 pagesDaunorubicin Hydrochloride IV Over 6 Hours On Days 1, 3, and 5, and Etoposide IV Over 4Mohammed HaiderNo ratings yet
- Daepoch Protocolo BccancerDocument9 pagesDaepoch Protocolo BccancerLuiz MeloNo ratings yet
- Pediatric Antibiotic DosingDocument7 pagesPediatric Antibiotic DosingAnonymous mm1yTelKNo ratings yet
- جرعات الاطفالDocument50 pagesجرعات الاطفالWael Hamdy100% (1)
- Drug Therapy For PicuDocument32 pagesDrug Therapy For PicuNeethu Mariya MathewNo ratings yet
- Malaria: Public Health Division, Directorate of Health Services Thiruvananthapuram June 2016Document15 pagesMalaria: Public Health Division, Directorate of Health Services Thiruvananthapuram June 2016Ajan MjNo ratings yet
- Darzalex Epar Product Information - enDocument102 pagesDarzalex Epar Product Information - ensmich73No ratings yet
- 1 s2.0 S0163445316300123 MainDocument2 pages1 s2.0 S0163445316300123 MainZara SaeedNo ratings yet
- Antibiotic Treatment Guidelines For Urinary Tract Infections in Children (60 Days Through 17 Years)Document3 pagesAntibiotic Treatment Guidelines For Urinary Tract Infections in Children (60 Days Through 17 Years)Zahid AbbasNo ratings yet
- Antibiotic Guidelines For PediatricsDocument33 pagesAntibiotic Guidelines For PediatricsVarshini Tamil SelvanNo ratings yet
- Protocollo Fazio CovidDocument12 pagesProtocollo Fazio CoviddonNo ratings yet
- 05 N017 2931Document13 pages05 N017 2931anju negalurNo ratings yet
- Diet Readiness QuestionnaireDocument3 pagesDiet Readiness Questionnairesavvy_as_98100% (1)
- A StudyDocument12 pagesA StudyNichchanun LertnuwatNo ratings yet
- Tuberculin PPD RT 23Document10 pagesTuberculin PPD RT 23Irene Olivia SalimNo ratings yet
- Ferric Carboxymaltose (Hemojet) : Md. Arafat Hossain Brand Executive, SBMD, 01713354512Document5 pagesFerric Carboxymaltose (Hemojet) : Md. Arafat Hossain Brand Executive, SBMD, 01713354512Heranmoy Kumar Bijon100% (1)
- Fuck The System - and How To Live For Free - Published Circa '67Document17 pagesFuck The System - and How To Live For Free - Published Circa '67Tedd St RainNo ratings yet
- Principles of MicrobiologyDocument301 pagesPrinciples of MicrobiologyBenjamin A. Ujlaki76% (17)
- Guidance and Counselling For AdolescentsDocument20 pagesGuidance and Counselling For AdolescentsNalu ChangNo ratings yet
- Development of A Nursing Care Protocol For Care of Neonates With Esophageal Atresia/ Tracheo - Esophageal FistulaDocument14 pagesDevelopment of A Nursing Care Protocol For Care of Neonates With Esophageal Atresia/ Tracheo - Esophageal FistulaSanket TelangNo ratings yet
- Kanaan: Sample Papers?Document8 pagesKanaan: Sample Papers?Kulanthaivelu R SicaNo ratings yet
- Yellow: The Primary ColoursDocument20 pagesYellow: The Primary ColourstripNo ratings yet
- Blanche GrubeDocument21 pagesBlanche GrubesangroniscarlosNo ratings yet
- Primary Health Care Systems (Primasys) : Case Study From CameroonDocument16 pagesPrimary Health Care Systems (Primasys) : Case Study From CameroonEyock PierreNo ratings yet
- Test 2Document49 pagesTest 2Kanish AggarwalNo ratings yet
- Immunohistochemical Characterization of Urethral Polyps in WomenDocument4 pagesImmunohistochemical Characterization of Urethral Polyps in WomenCentral Asian StudiesNo ratings yet
- Nursing Care of Children-QuestionsDocument11 pagesNursing Care of Children-QuestionsRamon Carlo AlmiranezNo ratings yet
- Ledderhose Disease: Pathophysiology Diagnosis and ManagementDocument3 pagesLedderhose Disease: Pathophysiology Diagnosis and ManagementLeandro PolancoNo ratings yet
- Communicable and Non-Communicable DiseaseDocument44 pagesCommunicable and Non-Communicable DiseaseSajida Bibi NoonariNo ratings yet
- The Rokeach Value Survey (RVS) : Milton Rokeach - Was A Polish-American Social Psychologist Who Developed The RVSDocument7 pagesThe Rokeach Value Survey (RVS) : Milton Rokeach - Was A Polish-American Social Psychologist Who Developed The RVSAngel May L. LopezNo ratings yet
- AbbreviationsDocument9 pagesAbbreviationsYuuki Chitose (tai-kun)No ratings yet
- Algorithm 1 Algorithm 2Document51 pagesAlgorithm 1 Algorithm 2Zimm RrrrNo ratings yet
- Plasmapheresis For AtherosclerosisDocument10 pagesPlasmapheresis For AtherosclerosisEliDavidNo ratings yet
- 2 Vol. 8 Issue 5 May 2017 IJPSR RE 2098Document13 pages2 Vol. 8 Issue 5 May 2017 IJPSR RE 2098KhokonNo ratings yet
- Pathology Solved Papers 2015Document35 pagesPathology Solved Papers 2015Lakshmi Venkataraman100% (3)
- Consensus For EGFR Mutation Testing in Non-Small Cell PDFDocument8 pagesConsensus For EGFR Mutation Testing in Non-Small Cell PDFCahrun CarterNo ratings yet
- Faculty of Community Medicine and PublichealthDocument21 pagesFaculty of Community Medicine and PublichealthFarhan Hassan DarNo ratings yet
- The 4000 English Words Essential For An Educated VocabularyDocument139 pagesThe 4000 English Words Essential For An Educated VocabularyLike GhazalNo ratings yet
- Pediatric Medical DevicesDocument25 pagesPediatric Medical DevicesDuck Mann-ConsulNo ratings yet