Professional Documents
Culture Documents
q2 General Chemistry Rev (Complete)
q2 General Chemistry Rev (Complete)
Uploaded by
rosedancel52Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
q2 General Chemistry Rev (Complete)
q2 General Chemistry Rev (Complete)
Uploaded by
rosedancel52Copyright:
Available Formats
GENERAL CHEMISTRY I
Second Quarter
GENERAL CHEMISTRY
QUANTUM NUMBERS
It is the branch of science that deals with the These are the set of numbers used to describe the
properties, composition, structure of elements position, trajectory, movement, and energy of the
and compounds, and the transformations they electron in an atom.
undergo.
It is a group of numerical values that are acceptable
REVIEW by the Schrodinger wave equation for hydrogen atoms.
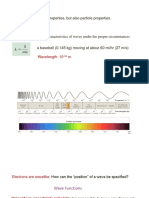
To understand the electronic structure of atoms, one
must understand the nature of electromagnetic radiation. All electrons have four quantum numbers which
describe their atomic orbital.
1. Principal quantum number, denoted by n.
2. Angular momentum quantum number (or
azimuthal quantum number), denoted by l.
3. Magnetic quantum number, denoted by ml.
4. Electron spin quantum number, denoted by
ms.
PRINCIPAL QUANTUM NUMBERS
The principal quantum number (n) designates the
principal electron shell or the size of the orbital (the
distance between the nucleus and the electrons) and the
energy level in an electron is placed in (Fig. 2).
Figure 1. Electromagnetic Radiation Figure 2. Principal Quantum Number
Wave: A wave is a disturbance that travels from one
location to another location.
The highest peak of the wave is called the crest and the
lowest point is named as the trough.
Wavelength (): The distance between corresponding
points on adjacent waves is the wavelength.
Frequency: The number of waves passing a given point
per unit of time is the frequency.
The longer the wavelength, the smaller the frequency,
and vice versa
A collection of orbitals with the same value of n and the
Amplitude: The “height” of wave, maximum outside part of an atom around the atomic nucleus is
displacement of periodic function. frequently called a shell.
All electromagnetic radiation travels the speed of light
ANGULAR MOMENTUM QUANTUM NUMBER
(c), 3.00 108 m/s (in a vacuum). Therefore: c =
The angular momentum quantum number (denoted
by the symbol ‘l’) describes the shape of the given
Particle: A particle is an object which has distinct
orbital, and its value is equal to the total number of
chemical or physical properties such as volume or mass.
angular nodes in the orbital.
A collection of orbitals with the same value of n and l is
QUANTUM MECHANICS
referred to as a subshell.
Erwin Schrödinger derived a complex mathematical
formula to incorporate the wave and particle
A value of (l) can indicate either an s, p, d, or f subshell
characteristics of electrons. Instead of referring to orbits
which vary in shape (Fig. 3)
as in the Bohr model, quantum numbers and wave
functions describe atomic orbitals.
Wave behavior is described with the wave function ψ.
The probability of finding an electron in a certain area of
space is proportional to ψ2 and is called electron density.
The energy states and wave functions are characterized
by a set of quantum numbers.
Figure 3. Angular Momentum Quantum Number
Created by Jorel Donald A. Varon
GENERAL CHEMISTRY I
Second Quarter
Orbitals have shapes that are best described as Example:
spherical (l = 0 or s), polar (l = 1 or p), or cloverleaf (l = 2 3p refers to the third principal quantum number (n=3)
or d). This value depends on (and is capped by) the and the p subshell (l=1).
value of the principal quantum number (n-1).
For example: LESSON SUMMARY
if n =3, the azimuthal quantum number can take on the To summarize quantum numbers:
following values: principal (n) – size
= n-1 angular (l) – shape
= 3-1
magnetic (ml) – orientation
= 0,1, and 2.
electron spin (ms) – direction of spin
When l=0, the resulting subshell is an ‘s’ subshell.
Similarly, when l=1 and l=2, the resulting subshells are
‘p’ and ‘d’ subshells (respectively). Therefore, when n=3,
the three possible subshells are 3s, 3p, and 3d.
MAGNETIC QUANTUM NUMBER
The magnetic quantum number (denoted by ml)
determines the number of orbitals and their orientation
within a subshell. Figure 4. Quantum Numbers
Its value depends on the orbital angular momentum
quantum number l. For a given value of l, the value of ml ELECTRON CONFIGURATION
ranges between the interval -l to +l. The electron configuration describes how the electrons
are distributed in the various atomic orbitals, showing
For example: which ones are filled and which ones remain vacant.
if n = 4 and l = 3 in an atom, the possible values of the
magnetic quantum number are -3, -2, -1, 0, +1, +2, and Electron configurations
+3. of atoms follow a
standard notation in
The total number of orbitals in a given subshell is given which all electron-
by the formula (2 l + 1). containing atomic
subshells (with the
For example: number of electrons
the ‘3d’ subshell (n=3, l =2) contains 5 orbitals (2*2 + 1). they hold written in
Each orbital can accommodate 2 electrons. Therefore, superscript) are placed in a sequence.
the 3d subshell can hold a total of 10 electrons.
For example:
The electron configuration of sodium is 1s22s22p63s1.
ELECTRON SPIN QUANTUM NUMBER
The value of this number gives insight into the direction
in which the electron is spinning, and is denoted by the RULES OF ELECTRON CONFIGURATION
symbol ms. The possible values of the electron spin
quantum number are +1⁄2 and -1⁄2. 1. AUFBAU PRINCIPLE
The positive value of ms implies an upward spin on the The Aufbau principle
electron and is denoted by the symbol ↑, and vice versa. states that electrons are
added to the lowest
energy orbitals first
before moving to higher
NAMING ORBITALS (SHELL AND SUBSHELL) energy orbitals.
Chemists describe the shell and subshell in which an
orbital belongs with a two-character code. The first The order of occupation
character indicates the shell (n). The second character is as follows:
identifies the subshell (l) (Table 1).
1s<2s<2p<3s<3p<4s<3d
Name of Subshell Value of l <4p<5s<4d<5p<6s<4f<5d
S subshell l=0 <6p<7s<5f<6d<7p
P subshell l=1
D subshell l=2 Figure 5. Electron Orbital Order
F subshell l=3
Created by Jorel Donald A. Varon
GENERAL CHEMISTRY I
Second Quarter
↑↓ ↑↓ ↑↓ ↑↓ ↑
2. HUND’S RULE 1s 2s 2p
To place electrons in orbital diagrams, electrons:
1. Are represented by arrows and the direction of Step 4: Count the number of electrons in each subshell
the arrow is used to represent electron spin. and write them as subscripts of the subshell (e.g., for 2p
2. Fill orbitals in order of increasing energy we have 5 electrons, so it must be written as 2p5).
beginning with 1s, then 2s and 2p…
3. Orbitals can hold a maximum of two electrons. Answer: 1s22s22p5
4. Within sublevels that contain multiple orbitals, _____________________________________________
electrons must be placed in each orbital with We can also abbreviate the electron configuration using
positive ½ spins first before completely pairing the noble gases as a shortcut. Using the same example:
them.
Step 1: Locate the noble gases prior to the element of
According to Hund’s rule, the most stable arrangement interest. In this case, the noble gas prior to Fluorine is
of electrons is the one in which the number of electrons Helium.
with the same spin is maximized. In other words,
electrons must be filled singly first before pairing them. Step 2: Helium has an electron configuration of 1s2
For example: He = 1s2
We can substitute it to shorten the electron
configuration. Thus, from 1s22s22p5 we can write
[He]2s22p5
Answer: [He]2s22p5
Carbon has 6 electrons. According to Hund's Rule, they
must first occupy each of the two degenerate p orbitals,
namely the 2px orbital and 2py orbital with parallel spins
3. PAULI’S EXCLUSION PRINCIPLE
Wolfgang Pauli postulated that each electron can be
described with a unique set of four quantum numbers.
No two electrons in an atom can have the same four
quantum numbers. They have different spin magnetic
quantum numbers.
MAGNETIC BEHAVIOUR
Magnetic materials derive their magnetic behavior from
the magnetic properties of their electrons. The
magnetism of most materials can be categorized as
being diamagnetic, paramagnetic, or ferromagnetic.
EXAMPLES a. Diamagnetic
Determine the electron configuration of Fluorine (F). All electrons are spin paired and the material does not
have a net magnetic field. These materials are slightly
Solution: Fluorine (F) has 9 electrons. repelled by the magnetic field of a strong magnet.
Step 1: According to Aufbau’s principle, electrons must b. Paramagnetic
be added from the lowest energy orbitals first before contain atoms, molecules, or ions with unpaired
moving to higher energy orbitals. electrons. Paramagnetic materials exhibit a weak
attraction towards magnets. They still pull themselves
↑↓ ↑↓ __ __ __ towards magnets, but paramagnetic materials are much
1s 2s 2p weaker than ferromagnetic materials.
Step 2: We still have 5 electrons left, using Hund’s rule, c. Ferromagnetic
we must first fill all orbitals in the P subshell with the paramagnetic materials, contain particles with unpaired
upper spin. electrons. In these materials, however, the individual
magnetic fields align naturally and produce a strong,
↑↓ ↑↓ ↑ ↑ ↑ permanent magnetic field. The common magnets you
1s 2s 2p are familiar with are ferromagnets.
Step 3: The remaining two electrons will be paired up by
Pauli exclusion principle.
Created by Jorel Donald A. Varon
GENERAL CHEMISTRY I
Second Quarter
Therefore, the largest number found in the electron
configuration is the outermost shell. To identify the
number of valence electrons, add up the superscripts
in these shells.
For example,
Chlorine’s electron configuration: 1s22s22p63s23p5.
As we can see, there are seven electrons in the 3s and
3p orbitals combined, which together make up the
PERIODIC TABLE CHEAT SHEET
outermost third orbital.
Using the periodic table alone, we can predict the
electron configuration of an element.
3s2 = 2 electrons
3p5 = 5 electrons.
5 + 2 = 7 electrons
Answer: Chlorine has 7 valence electrons
FINDING THE VALENCE ELECTRONS USING THE
PERIODIC TABLE
To make things easier, we can also find the number of
valence electrons an atom has by looking at the periodic
table. (Fig. 7)
VALENCE ELECTRONS
Valence electrons are
electrons in the
outermost shell of an Figure 7. Valence electrons in Periodic Table
atom. These electrons,
being the furthest
from the nucleus and LEWIS DOT DIAGRAM
thus the least tightly Lewis dot diagram is a very simplified representation of
held by the atom, are the valence shell electrons in a molecule. It is used to
the electrons that show how the electrons are arranged around individual
participate in bonds and reactions. atoms in a molecule. Electrons are shown as dots
This also means that the number of valence electrons Step 1: Write out the chemical symbol
that an element has determines its reactivity, Step 2: Find the number of valence electrons
electronegativity, and the number of bonds it can form. Step 3: Draw dots around the chemical symbol
corresponding to the number of valence electrons.
FINDING THE VALENCE ELECTRONS USING THE
ELECTRON CONFIGURATION
In an electron configuration notation, the shells are
written first in numerical form. (Fig. 6)
Figure 6. Electron Configuration Figure 8. Lewis dot structures
Created by Jorel Donald A. Varon
GENERAL CHEMISTRY I
Second Quarter
LEWIS DOT STRUCTIRES
Lewis structures, also known as Lewis-dot diagrams, For Example,
show the bonding relationship between atoms of a
molecule and the lone pairs of electrons in the molecule.
Basic Concepts of Lewis Structures
• Electrons – drawn as dots.
• Single bond – made up of 2 shared electrons
and is represented by a line.
• Double bonds – two lines
• Triple bonds – three lines
• Lone pairs – two dots. 2. Incomplete Octet - Less than 8 electrons in the
central atom after bond forming between the central
atom and the surrounding atom.
Example:
H2CO has a Lewis For example,
structure like this.
BCl3 has an incomplete octet
since Boron only contains 6
electrons, thus not satisfying
the octet rule.
OCTET RULE
The octet rule refers to the tendency of atoms to prefer
to have eight electrons in the valence shell. This is 3. Duet Rule – The duet rule states that hydrogen and
only applicable to main group elements. helium may have no more than two electrons in their
valence shells.
Example:
In this image, both
carbon and oxygen
have eight electrons
around them,
satisfying the octet
rule.
EXCEPTIONS TO THE OCTET RULE STEPS IN DRAWING THE LEWIS STRUCTURES
There are three exceptions to the octet rule. Step 1: Calculate the
number of valence
1. Expanded Octet – expanded octet occurs when an electrons of each
atom is able to have more than 8 valence electrons. element.
Step 2: Choose a central
Expansion of octet is possible only from Period 3
atom.
elements onwards, due to the presence of low-lying
Step 3: Place a bond
empty d orbitals that can accommodate the extra
between each element
electrons.
and make sure the number of electrons add up.
Step 4: Make sure each atom satisfies the octet rule
especially the central atom OR if it is an exception to the
octet rule, ensure that the number of electrons are
correct.
VSEPR THEORY OR MOLECULAR GEOMETRY
The valence shell electron pair repulsion (VSEPR)
theory is a model used to predict 3D molecular geometry
based on the number of valence shell electron bond
pairs among the atoms in a molecule or ion.
This model assumes that electron pairs will arrange
Figure 9. Elements with an Expanded Octet
themselves to minimize repulsion effects from one
another. In other words, the electron pairs are as far
apart as possible.
Parent Shape – These are the 3D models that have no
lone pairs.
Created by Jorel Donald A. Varon
GENERAL CHEMISTRY I
Second Quarter
3. Bent
Number of Bonds or Parent VSEPR Shape • This occurs when there
Electron Groups (No Lone Pairs) are 2 bonds and 2 lone
2 Linear pairs.
3 Trigonal Planar • It has a bond angle less
4 Tetrahedral than 107° (≈105°).
5 Trigonal Bipyramidal
6 Octahedral
Note that this is the same general geometry as the bent
molecule in the 3 electron group, but consists of a
TWO (2) ELECTRON GROUPS
different number of electron pairs.
This group consists only of the linear geometry.
1. Linear
FIVE (5) ELECTRON GROUPS
• Linear geometries occur when there are only 2 The 5 electron group consists of the trigonal bipyramidal,
bonds and 0 lone pairs. see-saw, T-shaped, and linear geometries.
• They are completely straight (Bond angle from
one bond to another is 180°) 1. Trigonal Bipyramidal
• Occurs when there are 5
bonds and 0 lone pairs.
• There are two regions of
bonds in TBPs: the axial
and the equatorial.
• The 2 axial bonds lay
THREE (3) ELECTRON GROUPS
along the axis, whereas
This group consists of trigonal planar molecules and
the 3 equatorials lay on
bent molecules.
the "equator" of the
molecule.
1. Trigonal Planar
• The angle between the 3 equatorial bonds
• In a trigonal
is 120° and the angle between each equatorial
planar molecule, there
and axial bond is 90°.
are 3 bonds and 0 lone
pairs, with bond angles
2. See-saw
of 120°
2. Bent
• Bent molecules have 2
bonds and 1 lone
pair. The bond angle is
less than 120° (≈119°) • Occurs when there are 4 bonds and 1 lone pair.
• This is because lone pairs • Just like the TBP, the see-saw has equatorial
take up more room. and axial regions.
• The linear (straight) portion is the axial whereas
the portion with the bent bonds is the equatorial.
FOUR (4) ELECTRON GROUPS • The equatorial bond angles are slightly less
This group consist of tetrahedral, trigonal pyramidal, and than 90° and the axial bond angles is slightly
bent geometries. less than 120°
1. Tetrahedral 3. T-Shaped
• The tetrahedral geometry • The T-Shaped geometry
exists when there are 4 occurs when there are 3
bonds and 0 lone pairs. bonds and 2 lone pairs.
• The bond angle for • The bond angle between
tetrahedral molecules is all of the bonds is slightly
approximately 109.5°. less than 90°
• The lone pairs sit where the other 2 equatorial
2. Trigonal Pyramidal bonds usually would.
• The trigonal
pyramidal geometry 4. Linear
exists when there are 3
bonds and 1 lone pair.
• The bond angle for
trigonal pyramidal
• This geometry occurs when there are 2 bonds
geometries is less than 109.5° (≈107°).
and 3 lone pairs and looks indistinguishable
from the other linear geometries.
• The bond angle between the two bonds is 180°
Created by Jorel Donald A. Varon
GENERAL CHEMISTRY I
Second Quarter
SIX (6) ELECTRON GROUPS • There is unequal distribution of charge making
The 6 electron group geometries consist of the the molecule unsymmetrical. A partially positive
octahedral, square pyramidal, and square planar end and a partially positive negative end exist.
geometries.
Example:
1. Octahedral The electronegativity of oxygen is 3.5, whereas the
• The octahedral geometry electronegativity of hydrogen is 2.1, making the bod
(right) occurs when there unequal. Thus, OH is an unequal molecule.
are 6 bonds and 0 lone
pairs.
• The bond angles is 90°
between all bonds.
2. Square Pyramidal
• The square
pyramidal has 5
bonds and 1 lone pair.
The 1 lone pair sits on
the "bottom" of the NON-POLAR BONDS
molecule. • The polarities of the two bonds are exactly equal
• The result is that the in magnitude and opposite in direction. They
bond angles are all cancel each other out, making the molecule
slightly lower than 90° nonpolar.
• Any molecule in which the relationship of the
bonded atoms is completely symmetrical- linear,
3. Square Planar trigonal planar, or tetrahedral molecules.
• The last geometry is
the square Example:
planar which is just 4 Since Iodine’s electronegativity cancel each other out,
bonds and 2 lone pairs. they are nonpolar.
• The two lone pairs sit
on "top" and "bottom"
of the molecule,
effectively balancing each other out.
• This results in the bond angles being exactly 90°
POLARITY OF MOLECULES
Electronegativity is the tendency for an atom of a given
chemical element to attract shared electrons when IONIC BONDS
forming a chemical bond. The more electronegative • A molecule is said to be ionic when the
atoms get more of the share. Thus, the molecule electronegative difference between the two is
becomes unsymmetrical or a dipole or polar. greater than 2.0
Dipole - A molecule becomes a dipole or polar when
there is unequal distribution or sharing of charge. SUMMARY
In summary, we must consider two factors when
deciding whether a molecule is polar: the bond polarity
and the molecular shape.
a. If the bonds are polar and the molecular shape
is not symmetrical, then molecule is polar.
b. If all the bonds are nonpolar or if the molecular
shape is symmetrical, the molecule is nonpolar.
Figure 9. Electronegativity Trend in the Periodic Table
POLAR AND NON-POLAR BONDS
POLAR BONDS
• are bonded atoms that share electrons
unequally, resulting in partial charges on the Table 1. Electronegativity values and polarity
atoms.
Created by Jorel Donald A. Varon
GENERAL CHEMISTRY I
Second Quarter
ALKANES
Alkanes are hydrocarbons in which the carbon atoms
are held together by single bonds.
Alkanes are called PARAFFINS because they have little
affinity towards general reagents under normal
conditions.
PHYSICOCHEMICAL PROPERTIES
Physical Properties
• Alkanes have no color.
• Alkanes are lighter than water and have a lower
density.
• Alkanes dissolve more readily in non-polar than
polar solvents because they are nonpolar
molecules.
• Alkanes do not dissolve in water.
• The melting and boiling temperatures of shorter
chain alkanes are low, but as the number of
carbon atoms in the carbon chain rises, the
melting and boiling values of alkanes rise.
Chemical Properties
• Alkanes have low reactivity.
• Strong acids, bases, oxidizing agents and Figure 10. IUPAC Naming
reducing agents do not react with alkanes.
• Alkanes are valuable as fuels because they burn
and release energy.
BOND-LINE FORMULA
Each carbon atom, as well
as, the corresponding
FORMULA AND STRUCTURE
hydrogen atoms are
General Formula: CnH2n+2
represented by each corner
General Structure: 2 4 6
of the bond line structure.
Each carbon atom is singly
For example, for hexane,
bonded together with 1 3 5
we have 6 carbon atoms, so
hydrogen. Carbon can only
the bond line structure would
have 4 bondings.
look like:
Hexane
IUPAC NAMING CYCLOALKANES
In naming alkanes, the class of hydrocarbons having a ring-like structure.
you just add “-ane” to This ring is formed due to their saturated nature, and
the prefix based on they have three compounds of alkane present in the
the number of structure which helps them in forming a ring.
carbons.
Example: PHYSICOCHEMICAL PROPERTIES
C4H10 Temperature
• The first four classes of cycloalkanes are said to
This alkane has 4 be in gaseous state in the room temperature.
carbon atoms, so we Boiling Points
use the prefix “but” • These saturated hydrocarbons are said to have
and add “ane” their boiling points ranging between 10 – 20 K.
Structure
Thus, it is named as • These are also called as saturated
Butane. hydrocarbons since saturated compounds form
ring structure.
Melting Points
• These compounds are also reported exhibiting
higher melting points and densities.
Created by Jorel Donald A. Varon
GENERAL CHEMISTRY I
Second Quarter
molecular mass or chain length, this indicates
FORMULA AND STRUCTURE that the intermolecular attractions become
General Formula: CnH2n stronger with the increase in the size of the
molecule.
Step 1: Determine the
number of carbons.
Step 2: Connect each carbon FORMULA AND STRUCTURE
atom to form a ring. General Formula: CnH2n
Step 3: Add two (2) hydrogen
atoms per carbon atom to Alkenes have at least
satisfy carbon’s valency. one carbon-carbon
Cyclopentane double bond.
For example,
IUPAC NAMING AND BOND LINE If we have 2 carbons
Use the prefix for then we have:
the corresponding
number of carbon C2H4
atoms and insert ETHENE
“cyclo-“ and “-
ane”
For example:
C5H10
Since we have 5
carbon atoms, we
will use “pent”
and add “cyclo” (Ethene)
and “ane”
Cyclopentane
For 3 carbons:
Tip: Whenever
you have
multiple carbon
bonds, Imagine
the molecule as
an alkane first
and remove two
hydrogen atoms
and make a
double bond for
the carbons.
ALKENES
Alkenes are a class of hydrocarbons (e.g, containing IUPAC NAMING
only carbon and hydrogen) unsaturated compounds with Step 1: Based on the
at least one carbon-to-carbon double bond. number of carbon
atoms, fixate an
Another term used to describe alkenes is OLEFINS. appropriate prefix.
Alkenes are more reactive than alkanes due to the Step 2: Add “ene” as
presence of the double bond. a suffix.
For example,
PHYSICOCHEMICAL PROPERTIES If we have 3 carbon
1. Physical state –The members containing two or atoms:
four carbon atoms are gases, five to seventeen,
liquids, eighteen onwards, solids at room Use “prop” and add
temperature and they burn in air with a luminous the suffix “ene:
smoky flame.
2. Density – Alkenes are lighter than water. Propene
3. Solubility – Alkenes are insoluble in water and
soluble in organic solvents such as benzene etc.
4. Boiling point – The boiling points of alkenes
show a gradual increase with an increase in the
Created by Jorel Donald A. Varon
GENERAL CHEMISTRY I
Second Quarter
IUPAC NAMING
Step 1: Determine
the number of
carbons and fixate
the appropriate
prefix.
Step 2: Then, add
“yne” to the end.
Step 3: For any
alkynes with
substituents,
determine the
location of the
substituent and
name it.
In this case, methyl is the
BOND LINE STRUCTURE substituent, and 4 is
To signify a double bond, add another line on top of an number of the carbon
“alkane” bond line structure to make it into alkene. atom in which it is
attached
For example:
For Pentene, add another
line on top of the structure BOND LINE STRUCTURE
to where the double bond For alkynes, triple bonds
has been placed. are represented by three
lines in the bond line
Double bond structure.
For example, for
Hexyne
ALKYNES
An alkyne is an unsaturated hydrocarbon containing at
least one carbon-carbon triple bond. AROMATIC HYDROCARBONS
Originally named because of their fragrant properties,
are unsaturated hydrocarbon ring structures that exhibit
PHYSICOCHEMICAL PROPERTIES special properties, including unusual stability, due to
• Alkynes are nonpolar, unsaturated hydrocarbons their aromaticity.
with physical properties similar to alkanes and
alkenes.
• Alkynes dissolve in organic solvents, have slight PHYSICOCHEMICAL PROPERTIES
solubility in polar solvents, and are insoluble in • have high melting and boiling points
water. compared to other organic compounds. This is
• Compared to alkanes and alkenes, alkynes have due to the strong intermolecular forces between
slightly higher boiling points. the molecules.
• An alkyne shows acidic nature only if it contains • The term "aromatic" in organic chemistry now
terminal hydrogen. means that the molecule contains benzene, or
• The existence of two π-bonds in alkynes allows its structural relatives.
them to undergo addition reactions twice, • Benzene itself is a clear, colorless, highly
making them more reactive compared to flammable liquid, which melts at 5.5°C.
alkenes. • stable unsaturated compounds.
• The triple bond in alkynes is relatively stronger. • nonpolar and immiscible with water
FORMULA AND STRUCTURE
FORMULA AND STRUCTURE 1. Draw the Benzene
General Formula: CnH2n-2 structure.
2. Decide where to
Alkynes like propyne are connect the other
represented by ≡ to show compound.
the triple bond between 3. Connect the structure
carbon atoms, omitting without the letters.
hydrogen atoms for
simplicity. Benzene
Created by Jorel Donald A. Varon
GENERAL CHEMISTRY I
Second Quarter
ALCOHOL
Alcohols are organic molecules containing the
“hydroxyl” functional group. (OH directly bonded to
carbon)
BOND LINE STRUCTURE
Alcohols are characterized by the presences of “OH” or
the hydroxyl group. Thus to make the bond line
structure, just attach OH to another carbon molecule.
The carbon directly attached to OH is technically called
the “carbinol” carbon, although this nomenclature is
often not introduced in introductory classes.
CARBOXYLIC ACID
IUPAC NAMING Carboxylic acids are a class of organic compounds that
contain a carboxyl functional group, which consists of a
carbonyl group (C=O) and a hydroxyl group (-OH)
attached to the same carbon atom.
PHYSICOCHEMICAL PROPERTIES
• Acidic Nature:
Carboxylic acids are weak acids. They can donate a
proton (H+) from the hydroxyl group, making them acidic
in nature.
• Reaction with Bases:
Carboxylic acids react with bases to form salts
(carboxylate salts) and water in a neutralization reaction.
• Esterification:
Example: Carboxylic acids react with alcohols in the presence of
an acid catalyst to form esters and water.
• Decarboxylation:
Some carboxylic acids undergo decarboxylation,
especially under high-temperature conditions, leading to
the loss of a carbon dioxide molecule.
• Physical State
Carboxylic acids are generally found in the liquid state at
room temperature. The smaller carboxylic acids (up to
about C5) are soluble in water due to hydrogen bonding,
Created by Jorel Donald A. Varon
GENERAL CHEMISTRY I
Second Quarter
but solubility decreases with increasing molecular size.
IUPAC NAMING
• Hydrogen Bonding:
Carboxylic acids can form hydrogen bonds with
themselves and with other molecules, contributing to
their higher boiling points and solubility in water.
• Polarity:
Carboxylic acids are polar molecules due to the
electronegativity difference between oxygen and carbon.
The presence of the carbonyl group enhances this
polarity.
FORMULA AND STRUCTURE
General Formula: CnH2n+1COOH
Step 1: Determine the number of carbon atoms and ESTER
draw it in a chain. An ester is a chemical compound derived from an acid
Step 2: Add the Carboxyl group at any point of the (organic or inorganic) in which at least one –OH hydroxyl
chain. group is replaced by an –O– alkyl (alkoxy) group.
Step 3: Add the corresponding Hydrogen atoms that can
satisfy the valency of the carbon atom. Esters are also usually derived from carboxylic acids.
When a carboxylic acid reacts with an alcohol it removes
For example: H2O forming an ester (see image below).
C2H5CO2H (Propanoic Acid)
FORMULA AND GENERAL STRUCTURE
• where R can be a
hydrogen atom, an
alkyl group, or an
aryl group
• and R′ can be an
alkyl group or an
aryl group but not a
hydrogen atom.
IUPAC NAMING
The names of the esters are based on the names of both
the alcohol and the carboxylic acid.
• The first part of the ester’s name comes from the
alcohol, with the suffix -anol being changed to -
yl.
Created by Jorel Donald A. Varon
GENERAL CHEMISTRY I
Second Quarter
• The second part of the ester’s name comes from For example,
the carboxylic acid, with the suffix -oic acid
replaced by -oate. in formaldehyde (HCHO), the alkyl
group (R) is hydrogen.
For example:
To name the ester in the diagram below we must firstly
count the number of carbon atoms in each of the
carboxylic acid and alcohol chains:
In acetaldehyde (CH3CHO), the
alkyl group is methyl (CH3). The
specific identity of the alkyl or aryl
group determines the particular
aldehyde compound.
There are three carbon atoms on the left of the ester
meaning that the carboxylic acid contained three carbon IUPAC NAMING
atoms. The carboxylic acid must therefore be propanoic The IUPAC system of nomenclature assigns a
acid. characteristic suffix -al to aldehydes.
There is one carbon atom on the right of the ester For example, H2C=O is methanal, more commonly
meaning that the alcohol called formaldehyde.
only contained one carbon
atom. The alcohol used Note:
must therefore have been The stems for the common names of the first four
methanol. aldehydes are as follows:
• 1 carbon atom: form-
The name of this ester is • 2 carbon atoms: acet-
therefore methyl • 3 carbon atoms: propion-
propanoate.
• 4 carbon atoms: butyr-
ALDEHYDE
Aldehydes refer to any of a class of organic compounds
in which a carbon atom shares:
• a double bond with an
oxygen atom
• a single bond with a
hydrogen atom, and;
• a single bond with
another atom or group
of atoms (designated KETONE
R in general chemical An organic compound with the structure R−C(=O)−R',
formulas and structure where R and R' can be a variety of carbon-containing
diagrams); the R group. substituents. Ketones contain a carbonyl group −C(=O)−
(which contains a carbon-oxygen double bond C=O)
Electrophiles – electron-loving molecules attract the
electrons from molecules called nucleophiles.
FORMULA AND STRCUTURE
The general molecular formula for an aldehyde is
RCHO, where "R" represents an alkyl or aryl group. In
more specific terms:
• R: Represents an alkyl group (a group IUPAC NAMING
composed only of carbon and hydrogen atoms) 1. Name the parent compound by finding the
or an aryl group (a group containing a benzene longest continuous chain that contains the
ring). carbonyl group. Change the -e at the end of the
• CHO: The functional group of the aldehyde, name of the alkane to -one.
consisting of a carbon atom doubly bonded to an 2. Number the carbon atoms in the chain in a way
oxygen atom and singly bonded to a hydrogen so that the carbonyl group has the lowest
atom. possible number.
Created by Jorel Donald A. Varon
GENERAL CHEMISTRY I
Second Quarter
3. Add the numerical prefix into the name before are located using “N” as the position number. More
the name of the ketone. complex primary amines are named with —NH2 as the
4. Use a hyphen between the number and the amino substituent.
name of the ketone.
Aromatic amines: named as derivatives of the parent
Example: compound aniline. Substituents attached to the nitrogen
are indicated by using “N-” as the location number.
AMINE
Amines are organic derivatives of ammonia, NH3, in
which one or more of the three H’s is replaced by a
carbon group.
Amines are classified according to the number of carbon
atoms bonded directly to the nitrogen atom.
A primary (1°) amine has one alkyl (or aryl) group on the
nitrogen atom, a secondary (2°) amine has two, and a
tertiary (3°) amine has three.
IUPAC NAMING
Simple 1°, 2°, and 3° amines: common (trivial) names
are obtained by alphabetically arranging the names of
the alkyl substituents on the nitrogen and adding the
suffix -amine (e.g., ethylmethylamine).
Amines in the IUPAC system: the “e” ending of the
alkane name for the longest chain is replaced with –
amine. The amine group is located by the position
number. Groups that are attached to the nitrogen atom
Created by Jorel Donald A. Varon
You might also like
- Detailed Lesson Plan On Transverse and Longitudinal WavesDocument7 pagesDetailed Lesson Plan On Transverse and Longitudinal WavesMae Amor Enorio100% (4)
- StoneLight30, Brochure, 2016Document4 pagesStoneLight30, Brochure, 2016Servicio TécnicoNo ratings yet
- DME I Year CHEDocument91 pagesDME I Year CHERadha KrishnaNo ratings yet
- Chapter 3 - Electronic Structure of Elements - RozainaDocument75 pagesChapter 3 - Electronic Structure of Elements - RozainaEzzarenNo ratings yet
- CHM131 - Chapter 2 - Structure of Atom - PeriodicityDocument97 pagesCHM131 - Chapter 2 - Structure of Atom - PeriodicityLeo PietroNo ratings yet
- Atomic Orbitals: Quantum NumbersDocument16 pagesAtomic Orbitals: Quantum NumberslostgirlNo ratings yet
- Lesson Atomic Structure 2 - MDocument11 pagesLesson Atomic Structure 2 - MZia OulNo ratings yet
- How To Use Quantum Numbers To Describe AnDocument11 pagesHow To Use Quantum Numbers To Describe AnAntonio Exal ColladoNo ratings yet
- P.S Week 5Document18 pagesP.S Week 5nicole MenesNo ratings yet
- General Chemistry Lecture 3Document80 pagesGeneral Chemistry Lecture 3Aaron Dela CruzNo ratings yet
- Chapter 2 Electronic StructureDocument62 pagesChapter 2 Electronic StructureLivan TuahNo ratings yet
- Quantum Numbers: Learning Objective Key PointsDocument3 pagesQuantum Numbers: Learning Objective Key PointsCandyAnonymousNo ratings yet
- Quantum Theory and Atomic StructureDocument42 pagesQuantum Theory and Atomic StructureMBalbuena, Daryll A.No ratings yet
- Wave Model - Hydrogen Case - 2023-2024Document36 pagesWave Model - Hydrogen Case - 2023-2024rahmaderradji23No ratings yet
- Quantum Numbers 2. Azimuthal Quantum Number: (L)Document2 pagesQuantum Numbers 2. Azimuthal Quantum Number: (L)Selva ManiNo ratings yet
- Quantum NumbersDocument31 pagesQuantum NumbersDianne de la TorreNo ratings yet
- Quantum NumbersDocument2 pagesQuantum Numbersdaysi canalesNo ratings yet
- Atomic and Molecular Struc.Document25 pagesAtomic and Molecular Struc.lelouchvibritannia0502No ratings yet
- Quantum Numbers and ECDocument3 pagesQuantum Numbers and ECShraddha Deshmukh-KelkarNo ratings yet
- 1A The Shapes and Structures of Molecules Student Handout Part Two 2022.23Document70 pages1A The Shapes and Structures of Molecules Student Handout Part Two 2022.23Music MaestroNo ratings yet
- CHEM 2101 Lecture 2 (Qunatum Number)Document5 pagesCHEM 2101 Lecture 2 (Qunatum Number)Asif UddinNo ratings yet
- Electron Configuration and Quantum NumbersDocument50 pagesElectron Configuration and Quantum NumbersGustavo LarrazabalNo ratings yet
- 1 Quantum NumbersDocument30 pages1 Quantum NumbersKAYE AIRA DE LEONNo ratings yet
- Chapter 2 - Structure of Atom PeridiocityDocument97 pagesChapter 2 - Structure of Atom PeridiocityAina AthirahNo ratings yet
- Atomic StructureDocument19 pagesAtomic StructureJhoanna Marie Manuel-AbelNo ratings yet
- L3 Che101Document28 pagesL3 Che101Musa Ahammed MahinNo ratings yet
- Chapter 1Document41 pagesChapter 1Nguyen NhatNo ratings yet
- Quantum Numbers NotesDocument8 pagesQuantum Numbers NotesMamidanna SashankNo ratings yet
- SLG Chem1 LG 3.2 Quantum NumbersDocument11 pagesSLG Chem1 LG 3.2 Quantum NumbersLaw of Attraction Come trueNo ratings yet
- SummaryDocument2 pagesSummaryamina tahir amina tahirNo ratings yet
- Structure of AtomDocument6 pagesStructure of AtomAbhishekkpNo ratings yet
- Hydrogen Spectrum and Atomic Terms NotesDocument32 pagesHydrogen Spectrum and Atomic Terms Notesoyamo markNo ratings yet
- Screenshot - 2023-01-07-23-13-07-99 - (7 Files Merged)Document7 pagesScreenshot - 2023-01-07-23-13-07-99 - (7 Files Merged)Prashant ChaureNo ratings yet
- Chapter2 StructureofatomDocument47 pagesChapter2 StructureofatomwriterajpawarNo ratings yet
- General Chemistry: Use Quantum Numbers To Describe An Electron in An AtomDocument16 pagesGeneral Chemistry: Use Quantum Numbers To Describe An Electron in An AtomDenver John Caloza LamarcaNo ratings yet
- CHM131 - CH 3 - The Electronic Structure of Atoms and Periodic Table PDFDocument102 pagesCHM131 - CH 3 - The Electronic Structure of Atoms and Periodic Table PDFRabiatul AdawiyyahNo ratings yet
- Energy StateDocument30 pagesEnergy StateTahirullah KhanNo ratings yet
- Chemistry PresentationDocument15 pagesChemistry PresentationSubham KalwarNo ratings yet
- De Broglie Relation: EinsteinDocument19 pagesDe Broglie Relation: EinsteinMaisha MaimunaNo ratings yet
- tilde : (N,, M) Define An Orbital (N,, M, M) Define An Electron in An Determined OrbitalDocument1 pagetilde : (N,, M) Define An Orbital (N,, M, M) Define An Electron in An Determined OrbitalRichardA.TorresNo ratings yet
- Atomic Structure - 3Document27 pagesAtomic Structure - 3suhiermai3No ratings yet
- Quantum Numbers: Introduction To ChemistryDocument24 pagesQuantum Numbers: Introduction To Chemistryhimanshu yadavNo ratings yet
- Dual Behaviour of MatterDocument22 pagesDual Behaviour of Matterasadahmed.saadNo ratings yet
- Quantum Mechanical ModelDocument26 pagesQuantum Mechanical ModelMaye AporadorNo ratings yet
- Exercises No. 2Document6 pagesExercises No. 2John Miguel BarbaNo ratings yet
- Atomic Theory 3Document2 pagesAtomic Theory 3Husen HasenNo ratings yet
- Module 1-3 NotesDocument18 pagesModule 1-3 Notesjared.greenwood93No ratings yet
- CEM1008F - 3. The Periodic Table and Atomic Structure 2024 - Lecture - NotesDocument41 pagesCEM1008F - 3. The Periodic Table and Atomic Structure 2024 - Lecture - Notesparlypalesa90No ratings yet
- Basic Chemistry Lecture Notes 2Document34 pagesBasic Chemistry Lecture Notes 2youngNo ratings yet
- Structure of AtomDocument29 pagesStructure of AtomAbhisek DehuriNo ratings yet
- The Quantum Mechanical ModelDocument15 pagesThe Quantum Mechanical ModelNatalia SadekNo ratings yet
- Schroedinger Equation Atomic Wave Functions Atomic Orbitals Quantum NumbersDocument45 pagesSchroedinger Equation Atomic Wave Functions Atomic Orbitals Quantum NumbersRishit JakhariaNo ratings yet
- Electron Structure of AtomsDocument69 pagesElectron Structure of AtomsnickNo ratings yet
- Max Plank:: Learning Objectives For This ChapterDocument4 pagesMax Plank:: Learning Objectives For This Chapterdomer2011No ratings yet
- 1 Quantum Numbers-1Document34 pages1 Quantum Numbers-1Carlo James SablanNo ratings yet
- Atomic Structure - 1Document38 pagesAtomic Structure - 1DeviNo ratings yet
- Junior ChemistryDocument72 pagesJunior ChemistryjahnavikidambiNo ratings yet
- Quantum NumbersDocument8 pagesQuantum NumbersMoises espinozaNo ratings yet
- Lecture 2 SlidesDocument28 pagesLecture 2 Slidesyusuf tarekNo ratings yet
- 1.3 Electronic Structure of An AtomDocument15 pages1.3 Electronic Structure of An AtomAzrael MoonNo ratings yet
- ACFrOgC xiuXYskoUCylB1F9L1IEJcj Ta KoLk6CTvaGqltDFWA2qAIKTeqUvP 0 7U6kDWb0J0D4WA8tzfPGbl3oDF5E2WbfWJwsRiEHl3syXtQRSt2h85SwNwBgDocument71 pagesACFrOgC xiuXYskoUCylB1F9L1IEJcj Ta KoLk6CTvaGqltDFWA2qAIKTeqUvP 0 7U6kDWb0J0D4WA8tzfPGbl3oDF5E2WbfWJwsRiEHl3syXtQRSt2h85SwNwBgJihanaisyNo ratings yet
- Electron Beam-Specimen Interactions and Simulation Methods in MicroscopyFrom EverandElectron Beam-Specimen Interactions and Simulation Methods in MicroscopyNo ratings yet
- Unsw Mechanical Thesis DatabaseDocument8 pagesUnsw Mechanical Thesis Databaserobynchampagnemanchester100% (2)
- Mathematics in The Modern World: Number Sequence and SeriesDocument12 pagesMathematics in The Modern World: Number Sequence and SeriesCherrylane EdicaNo ratings yet
- Milling Machine FormulasDocument8 pagesMilling Machine Formulastinodaishe guveyaNo ratings yet
- Channel DesignDocument5 pagesChannel DesignAaronNo ratings yet
- Normal DistributionDocument5 pagesNormal DistributionMd. Nazmul Huda 1610805643No ratings yet
- Construction Method For Pile DrivingDocument17 pagesConstruction Method For Pile DrivingsochealaoNo ratings yet
- Lab 8-Measurement of Yield Point of Drilling Mud Sample Using Rheometer.Document12 pagesLab 8-Measurement of Yield Point of Drilling Mud Sample Using Rheometer.Sunny BbaNo ratings yet
- G7 Science Q1 - Week 6-Unsaturated and Saturated SolutionDocument14 pagesG7 Science Q1 - Week 6-Unsaturated and Saturated SolutionJessa-Bhel AlmueteNo ratings yet
- Course Outline MAT 125.9 Summer2023Document6 pagesCourse Outline MAT 125.9 Summer2023Rubayet fahimNo ratings yet
- Study and Comparison of Structure Having Different Infill Materials Using ETABSDocument47 pagesStudy and Comparison of Structure Having Different Infill Materials Using ETABSnarsimha.m107No ratings yet
- NotesDocument420 pagesNotesSkNo ratings yet
- Chapter 1 Physics Assignment 1Document2 pagesChapter 1 Physics Assignment 1Siddhi DubeyNo ratings yet
- Machines For O Level Physics PDFDocument27 pagesMachines For O Level Physics PDFRwabahinda100% (1)
- Probset1 PDFDocument3 pagesProbset1 PDFLee TalierNo ratings yet
- PRACTICE QUESTIONS Unit 4, 5 and 6Document5 pagesPRACTICE QUESTIONS Unit 4, 5 and 6Samrudhi PatilNo ratings yet
- Investigation On The Coal-Based Direct Reduction of Mill Scale Pellets: Statistical Modeling and Characterization StudiesDocument11 pagesInvestigation On The Coal-Based Direct Reduction of Mill Scale Pellets: Statistical Modeling and Characterization StudiesBISWAJEET BEHERANo ratings yet
- Gas Laws / Gases BehaviourDocument35 pagesGas Laws / Gases Behaviour9338-Anmol KatharNo ratings yet
- Water Reticulation Design Calculation LYDocument4 pagesWater Reticulation Design Calculation LYGan Chin PhangNo ratings yet
- Moving Man SimulationDocument12 pagesMoving Man SimulationAlexa AquinoNo ratings yet
- Advance Laboratory Testing Paper PDFDocument30 pagesAdvance Laboratory Testing Paper PDFDariusz GuzikNo ratings yet
- Flo Rite FRDocument4 pagesFlo Rite FRJorge Selim Asaff ArancibiaNo ratings yet
- Introduction To Fluid Mechanics - Herbert OertelDocument172 pagesIntroduction To Fluid Mechanics - Herbert OertelSpiros Chortis33% (3)
- Test Prep For Success Glossary of SAT Math TemsDocument10 pagesTest Prep For Success Glossary of SAT Math Temssendra0285No ratings yet
- Columns (Section 410 NSCP 2015) : Materials (410.2.1)Document8 pagesColumns (Section 410 NSCP 2015) : Materials (410.2.1)Nito AresNo ratings yet
- Direct Hydrocarbon Indicators (DHI)Document11 pagesDirect Hydrocarbon Indicators (DHI)Farooq ArshadNo ratings yet
- Zh. Vychisl. Mat. Mat. FizDocument7 pagesZh. Vychisl. Mat. Mat. FizBroNo ratings yet
- Solar Thermal EnergyDocument56 pagesSolar Thermal EnergyBikash DasNo ratings yet
- Analysis of Planar Vehicle CollisionsDocument16 pagesAnalysis of Planar Vehicle CollisionsIsabela Torres De MeloNo ratings yet