Professional Documents
Culture Documents
Organic Flow Chart MrSyed PDF New
Organic Flow Chart MrSyed PDF New
Uploaded by
muhammadim2007Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Organic Flow Chart MrSyed PDF New
Organic Flow Chart MrSyed PDF New
Uploaded by
muhammadim2007Copyright:
Available Formats
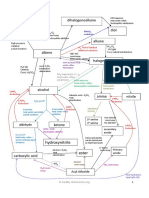
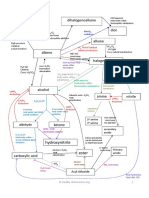
• Tertiary alcohol do not undergo oxidation * The order of reactivity of halogenoalkanes is R-I > R-Br>R-Cl>R-F * The order of reactivity
ctivity of halogenoalkanes is Tertiary > secondary >primary
No reaction silver mirror red brown ppt, Cu2O
CO2/Alde
hyde/Ket
polym
ones [Ag(NH3)2]+Tollen’s Alkali, Cu2+Fehling’s [Ag(NH3)2]+Tollen’s (oxi) Alkali, Cu2+Fehling’s(oxidation)
Diol ers
Hydroxy alkane
Yellow ppt HCN nitrile
Cold&dil hot & conc 2,4-DNPH nucleophilic addition
ketones Aldehydes
H+/KMnO4/K2Cr2O7 H+/KMnO4/K2Cr2O7 Redu/H2/LiALH4 H+/KMnO4/K2Cr2O7 H+/KMnO4/K2Cr2O7 Redu/H2/LiALH4
Cracking /Al2O3/SiO2 H+/H2O/electrophilic addition secondary primary
Alkanes Alkene Alcohol Carboxylic acid
H2/Ni/Pt /150C0 concH2SO4/reflux/heat
Primary:SN2/sec:SN1/SN2Ter:SN1 H+/heat H2O/H+
X2/HX/Electrophilic addition KOH(aq)/ Reflux /heat esters
Heat/ Reflux/Nacl+H2SO4/
PCl3/PCl5/SOCl2/p+I2 NH3 Heat/ Reflux/Nacl+H2SO4/
X2/UV/free radicle substitution KOH(alc) nucleophilic substitution PCl3/PCl5/SOCl2/p+I2
H2O
H2O/H+/heat reflux
Acyle NH3/Nucleophilic sub Amide+
Amines Halogeno HCl
Alkane chloride
NH3 /nucleophilic subs alkane HCN/Nucleophilic substitution
nitrile
(Compounds containing CH3-CO- can undergo iodoform reactiob in which , they form yellow ppt of CHI3(Iodoform) when reacted with Alkali(ex:NaOH) and Iodine) By:MR.Syed
You might also like
- Module 2 Lesson 3 MISSIONARY RESPONSEDocument1 pageModule 2 Lesson 3 MISSIONARY RESPONSEHarriz Diether DomingoNo ratings yet
- Material Safety Data Sheet: 1. Identification of The Material and SupplierDocument5 pagesMaterial Safety Data Sheet: 1. Identification of The Material and Suppliersylvester rasheedNo ratings yet
- The Model Town Part I-1 PDFDocument41 pagesThe Model Town Part I-1 PDFNadeem Malik100% (2)
- Excavation Working in Trenches Swms 10281-6Document17 pagesExcavation Working in Trenches Swms 10281-6JamesNo ratings yet
- Synthetic Routes (A Level) - Reaction Pathways Aliphatic CompoundsDocument6 pagesSynthetic Routes (A Level) - Reaction Pathways Aliphatic CompoundsJunior GonzalesNo ratings yet
- Organic Chemistry Synthesis IedxcelDocument10 pagesOrganic Chemistry Synthesis IedxcelAliya Rahman100% (2)
- Crux and Reagents of Organic ChemDocument4 pagesCrux and Reagents of Organic ChemBILL RUSSO100% (5)
- Cost Accounting 7 8 - Solution Manual Cost Accounting 7 8 - Solution ManualDocument27 pagesCost Accounting 7 8 - Solution Manual Cost Accounting 7 8 - Solution ManualMARIA100% (1)
- Alkene N BenzeneDocument2 pagesAlkene N Benzenemikumo81No ratings yet
- 6carboxylic AcidsDocument1 page6carboxylic AcidssharmimiameerasanadyNo ratings yet
- Organic Chemistry ReactionDocument3 pagesOrganic Chemistry ReactionGAMEPORIUMNo ratings yet
- Screenshot 2024-04-16 at 22.57.21Document6 pagesScreenshot 2024-04-16 at 22.57.21fpjbrqfyktNo ratings yet
- Organic RevisionDocument4 pagesOrganic RevisionalicejessicapreesNo ratings yet
- BenzaldehydeDocument1 pageBenzaldehydemikumo81No ratings yet
- 12 Organic SynthesisDocument8 pages12 Organic SynthesisDanyal AhmadNo ratings yet
- 6 14 Organic SynthesisDocument8 pages6 14 Organic SynthesisPedro Moreno de SouzaNo ratings yet
- 20 Organic Chemistry Synthesis Iedxcel PDFDocument10 pages20 Organic Chemistry Synthesis Iedxcel PDFMohammedNo ratings yet
- Reagent ListDocument9 pagesReagent ListArka MukhopadhyayNo ratings yet
- 11 Alcohols Phenols and EthersDocument2 pages11 Alcohols Phenols and EthersVarun Sankpal100% (1)
- Reactions of Alkene: CH CH Markovnikov AdditionDocument8 pagesReactions of Alkene: CH CH Markovnikov AdditionRaye VolvoNo ratings yet
- Reagent Organic Chemistry HandbookDocument3 pagesReagent Organic Chemistry Handbookdodeda4744No ratings yet
- aldehydesketonescarboxylicacids-221214045703-92b48d54Document86 pagesaldehydesketonescarboxylicacids-221214045703-92b48d54monika.rani.fasvNo ratings yet
- Chemical ReactionsDocument31 pagesChemical Reactionsmercedes.caamalNo ratings yet
- 45 Hydrocarbons AlkanesDocument7 pages45 Hydrocarbons Alkanessujalgupta0123456789No ratings yet
- X Uv H /PT HX Mno4 H or Oh NH (Alc) Heat NH (Alc) : PCL PCL Socl ZN/HCLDocument1 pageX Uv H /PT HX Mno4 H or Oh NH (Alc) Heat NH (Alc) : PCL PCL Socl ZN/HCLEmily McCullochNo ratings yet
- HydrocarbonsDocument17 pagesHydrocarbonsManoj MathewNo ratings yet
- Ncea L3 Organic Chemistry NotesDocument14 pagesNcea L3 Organic Chemistry NoteszepphiagonzalesNo ratings yet
- 6 2 5 Revision Guides Organic SynthesisDocument5 pages6 2 5 Revision Guides Organic SynthesisAddan AddanNo ratings yet
- 1714246684533772Document3 pages1714246684533772kaikhasraw09No ratings yet
- Orgaic DigramDocument1 pageOrgaic DigrammimiarmyhpNo ratings yet
- Organic Chemistry Bible of Chemical ReactionsDocument6 pagesOrganic Chemistry Bible of Chemical ReactionsVIVIAN MOYONo ratings yet
- Organic Reactions Summary SheetDocument2 pagesOrganic Reactions Summary Sheetthacheee64% (11)
- Alcohol SummaryDocument2 pagesAlcohol Summarymikumo81No ratings yet
- Aldehydes 1Document1 pageAldehydes 1mikumo81No ratings yet
- Done By: Kaijie, Elias, Chenxi, Ashwini, Sahana, Kelly From 0901 and 0914 Compiled From 2007 and 2008 H2 Chemistry Prelim PapersDocument10 pagesDone By: Kaijie, Elias, Chenxi, Ashwini, Sahana, Kelly From 0901 and 0914 Compiled From 2007 and 2008 H2 Chemistry Prelim Papersdiejunqs sNo ratings yet
- Acfrogcxqpd3dqml GQHMZQ B0c089di81vpcrvgphrwcu4gh Bzezvshldjt4clxztdrke4cieuxds1wlvk6scla 0byn2rmeu4btdaq8ckybm0chweegnztu7u2olcnli2lia5txmf386nyikuDocument6 pagesAcfrogcxqpd3dqml GQHMZQ B0c089di81vpcrvgphrwcu4gh Bzezvshldjt4clxztdrke4cieuxds1wlvk6scla 0byn2rmeu4btdaq8ckybm0chweegnztu7u2olcnli2lia5txmf386nyikuAchal ParekhNo ratings yet
- Naming Reaction FinalDocument9 pagesNaming Reaction FinalRajendra ThamerciNo ratings yet
- Chemistry - Overview of Aliphatic Organic ChemistryDocument1 pageChemistry - Overview of Aliphatic Organic Chemistryhelixate100% (5)
- Hydrocarbon LatestDocument23 pagesHydrocarbon LatestHimanshu100% (1)
- 3.14 Revision Guide Organic Synthesis AqaDocument7 pages3.14 Revision Guide Organic Synthesis AqaRutba SafdarNo ratings yet
- GOC Class11thDocument38 pagesGOC Class11thAnju SehrawatNo ratings yet
- Orgo 2 Cheat SheetDocument1 pageOrgo 2 Cheat SheetMartha DejaNo ratings yet
- Important Reagents TreDocument3 pagesImportant Reagents Treraghava123456No ratings yet
- Organic ReactionsDocument1 pageOrganic ReactionsMasrulIsmailNo ratings yet
- Organic ReactionsDocument1 pageOrganic ReactionsFerro FlowNo ratings yet
- Chemical Reactions of Alkanes: Mechanism Reaction Reagent Condition Catalysts Product(s)Document9 pagesChemical Reactions of Alkanes: Mechanism Reaction Reagent Condition Catalysts Product(s)Sam LeeNo ratings yet
- Hasan Sayginel: Edexcel A Level Organic ChemistryDocument41 pagesHasan Sayginel: Edexcel A Level Organic ChemistryDEEBANNo ratings yet
- Organic Laboratory Preparation Class 12: Nepal APF School DP Paneru MS.C ChemistryDocument25 pagesOrganic Laboratory Preparation Class 12: Nepal APF School DP Paneru MS.C ChemistryDamaru Paneru100% (1)
- Organic NotesDocument8 pagesOrganic NotesChrisNo ratings yet
- Flow Chart - HydrocarbonsDocument77 pagesFlow Chart - HydrocarbonsKalyan Reddt100% (2)
- Reagents in Organic ChemistryDocument3 pagesReagents in Organic ChemistryParam SoniNo ratings yet
- Chapter 13 - Hydrocarbons Revision NotesDocument14 pagesChapter 13 - Hydrocarbons Revision NotesSREE GANESHNo ratings yet
- Oxidation ReductionnewDocument16 pagesOxidation ReductionnewanjalilalshaNo ratings yet
- 16 Hydroxyl compound-24-STDDocument38 pages16 Hydroxyl compound-24-STDManh Doan DucNo ratings yet
- Haloalkanes and Haloarenes - Short NotesDocument7 pagesHaloalkanes and Haloarenes - Short Notesbajrang07388No ratings yet
- HaloalkaneDocument2 pagesHaloalkanemikumo81No ratings yet
- Summary of Organic ReactionsDocument6 pagesSummary of Organic ReactionsAbudi Alsagoff100% (6)
- Chemistry Unit 3 Edexcel (AS LEVEL) NotesDocument1 pageChemistry Unit 3 Edexcel (AS LEVEL) Notes--------29% (7)
- FOR STUDENTS - Organic Reactions, Reagents, Conditions, Products SummaryDocument12 pagesFOR STUDENTS - Organic Reactions, Reagents, Conditions, Products Summaryh4rrywastakenNo ratings yet
- 2080 New Course XII HaloalkaneDocument57 pages2080 New Course XII HaloalkaneSangam PaudelNo ratings yet
- Reaction Summary: ALKENES: Reagent Conditions Products Observations Example/DiagramDocument1 pageReaction Summary: ALKENES: Reagent Conditions Products Observations Example/DiagramVictoria KairooNo ratings yet
- 1-4 Road MapDocument4 pages1-4 Road Mapipsita lahiriNo ratings yet
- Alcohol, Esters and Carboxylic AcidsDocument55 pagesAlcohol, Esters and Carboxylic AcidsHuiru ZhaoNo ratings yet
- Quality Management Principles For Excellence: by - Sacchidanand Gogawale, Zen International SystemsDocument5 pagesQuality Management Principles For Excellence: by - Sacchidanand Gogawale, Zen International SystemsSanjeevani GogawaleNo ratings yet
- Free ElecDocument2 pagesFree ElecPopox CalamongayNo ratings yet
- LoboHernandezySaldamandoBenjumea2012 PDFDocument167 pagesLoboHernandezySaldamandoBenjumea2012 PDFLeandro RodríguezNo ratings yet
- Q.1 Write Short Answers of The Following Questions: Ghazali Guess Chemistry 1 10thDocument9 pagesQ.1 Write Short Answers of The Following Questions: Ghazali Guess Chemistry 1 10thAwais AliNo ratings yet
- Atlan A300 A300xl Pi 9107089 en MasterDocument12 pagesAtlan A300 A300xl Pi 9107089 en MasterHanh NguyenNo ratings yet
- Practice Problems 2012Document5 pagesPractice Problems 2012Anonymous Fj3YPHNo ratings yet
- Electrical Engineering Diploma Based 73 Important MCQDocument12 pagesElectrical Engineering Diploma Based 73 Important MCQchetanNo ratings yet
- Modelling The Human Olfactory Stimulus Response FunctionDocument16 pagesModelling The Human Olfactory Stimulus Response Functionđạt lê tiếnNo ratings yet
- Batter Bread - Mary's NestDocument2 pagesBatter Bread - Mary's Nestsnuzzle babiezNo ratings yet
- K.C.S.E Biology Pp1 Ms-ChampionsDocument47 pagesK.C.S.E Biology Pp1 Ms-ChampionsAlfrickNo ratings yet
- Hardware - SoftwareDocument12 pagesHardware - SoftwareMarie Kelsey Acena MacaraigNo ratings yet
- Aquac Uno HDocument218 pagesAquac Uno Hivancalderon867374No ratings yet
- Reflective Journaling2Document3 pagesReflective Journaling2api-313631358No ratings yet
- 013 WFD-logging Tools and Appliction-Abbas Radhi PDFDocument21 pages013 WFD-logging Tools and Appliction-Abbas Radhi PDFroaa bableNo ratings yet
- Free Shipping Fee For This Month: Read The Text and Answer Questions 1 and 2Document9 pagesFree Shipping Fee For This Month: Read The Text and Answer Questions 1 and 2rajab_kedNo ratings yet
- Fallo Flujo de VentiladoresDocument4 pagesFallo Flujo de Ventiladores3GSERONo ratings yet
- Workbook PDFDocument145 pagesWorkbook PDFRecordTrac - City of OaklandNo ratings yet
- Environmental Law Project WorkDocument11 pagesEnvironmental Law Project Workbinny kumariNo ratings yet
- Jaggery Making: How To Make Jaggery?Document5 pagesJaggery Making: How To Make Jaggery?Subham BhattacharyaNo ratings yet
- Vaksinasi8 25Document24 pagesVaksinasi8 25puskesmas pucakwangi1No ratings yet
- JZ990D43501 eDocument6 pagesJZ990D43501 eМаксим ПасичникNo ratings yet
- (Executive Summary) : With The Assistance ofDocument10 pages(Executive Summary) : With The Assistance ofAshraf AtiqueNo ratings yet
- 02 IVD-R Deep-Dive Deck NewDocument215 pages02 IVD-R Deep-Dive Deck Newrajiveacharya100% (1)
- Psychology Semi-Final ExamDocument2 pagesPsychology Semi-Final ExamMonroe OrtizanoNo ratings yet
- Scope of WorkDocument2 pagesScope of WorkRameshbabu PeramNo ratings yet