Professional Documents
Culture Documents
Gnipst 4 30 5694 2.4
Gnipst 4 30 5694 2.4
Uploaded by
Antar GayenCopyright:
Available Formats
You might also like
- Annex 7: WHO Guidelines On Transfer of Technology in Pharmaceutical ManufacturingDocument25 pagesAnnex 7: WHO Guidelines On Transfer of Technology in Pharmaceutical ManufacturingEkin Duran100% (1)
- Kit Kat Case StudyDocument6 pagesKit Kat Case Studyms.Ahmed0% (1)
- Lab 2Document30 pagesLab 2jianneNo ratings yet
- SOP For Continued Process VerificationDocument9 pagesSOP For Continued Process VerificationMubarak Patel100% (1)
- Technology Transfer in Pharmaceutical IndustryDocument24 pagesTechnology Transfer in Pharmaceutical IndustryPranjal100% (3)
- Parametric ReleaseDocument7 pagesParametric ReleaseshdphNo ratings yet
- Risk Identification For Aseptic ProcessingDocument42 pagesRisk Identification For Aseptic Processingmmmmm100% (2)
- Validation and Validation ProtocolDocument18 pagesValidation and Validation ProtocolLalit Lata JhaNo ratings yet
- Unit - Granularity Technology Transfer - SVKDocument167 pagesUnit - Granularity Technology Transfer - SVKHarshada AgarkarNo ratings yet
- Peer-Reviewed: Medical Devices: Warning LettersDocument1 pagePeer-Reviewed: Medical Devices: Warning LettersSandra RodriguezNo ratings yet
- Guidance For Industry: Process ValidationDocument18 pagesGuidance For Industry: Process ValidationBruno DebonnetNo ratings yet
- Technology Transfer V.1.0Document39 pagesTechnology Transfer V.1.0Syahid AchyarNo ratings yet
- DocumentationDocument46 pagesDocumentationajak16406No ratings yet
- TRS961 - Annex7 WHO Tech TransferDocument25 pagesTRS961 - Annex7 WHO Tech TransferkrasataNo ratings yet
- ISO 11607 PackagingDocument8 pagesISO 11607 PackagingAngel CvetanovNo ratings yet
- Gnipst 4 30 5694 2.1Document5 pagesGnipst 4 30 5694 2.1Antar GayenNo ratings yet
- Comments On Ongoing Process VerifDocument2 pagesComments On Ongoing Process VerifsofianesedkaouiNo ratings yet
- Gnipst 4 30 5694 2.2Document2 pagesGnipst 4 30 5694 2.2Antar GayenNo ratings yet
- ValidationDocument49 pagesValidationAshokPokiriNo ratings yet
- USP-Lifecycle Management of Analytical ProceduresDocument15 pagesUSP-Lifecycle Management of Analytical ProceduresNur AcarNo ratings yet
- Articulo Tecnologia de TransferenciaDocument10 pagesArticulo Tecnologia de Transferencia20172504No ratings yet
- TRS961 Annex7Document25 pagesTRS961 Annex7Tahir KhanNo ratings yet
- ValidationDocument5 pagesValidationjyothisahadevanNo ratings yet
- VAL MANUAL 018 Potential Critical Packaging Process Parameters and Validation Practices SampleDocument3 pagesVAL MANUAL 018 Potential Critical Packaging Process Parameters and Validation Practices SampleRahul VermaNo ratings yet
- 2016Document40 pages2016schumonNo ratings yet
- Chinna Nagamalleswara Reddy Alla: SummaryDocument5 pagesChinna Nagamalleswara Reddy Alla: SummaryVijay LS SolutionsNo ratings yet
- RequestDocument3 pagesRequestiloveit52252No ratings yet
- Pharmaceutical Development Q8 (R2) : International Conference On Harmonisation (ICH)Document11 pagesPharmaceutical Development Q8 (R2) : International Conference On Harmonisation (ICH)Md. Akil Mahmud100% (1)
- Gnipst 4 30 5694 2.5Document4 pagesGnipst 4 30 5694 2.5Antar GayenNo ratings yet
- Types of Process ValidationDocument5 pagesTypes of Process ValidationLuanne Dela CruzNo ratings yet
- Technology Transfer - 20230918 - 201200 - 0000Document9 pagesTechnology Transfer - 20230918 - 201200 - 0000KALP PATELNo ratings yet
- Process Analytical TechnologyDocument57 pagesProcess Analytical Technologycarleen_almiraNo ratings yet
- Process-Validation - General-Principles-and-Practices-10Document1 pageProcess-Validation - General-Principles-and-Practices-10Kendry JoseNo ratings yet
- CLAUSE 8.5 Production and Service ProvisionDocument10 pagesCLAUSE 8.5 Production and Service ProvisionNavnath TamhaneNo ratings yet
- 4.1.5 The Quality Assurance ManagerDocument1 page4.1.5 The Quality Assurance ManagerthisiscookingNo ratings yet
- Documentation: Good Manufacturing Practices Modul-3Document29 pagesDocumentation: Good Manufacturing Practices Modul-3ChristinaNo ratings yet
- Taticek-Product Monitoring & Post-Approval Lifecycle Management of Biotech ProductsDocument36 pagesTaticek-Product Monitoring & Post-Approval Lifecycle Management of Biotech Products刘朝阳No ratings yet
- M4 - Lesson 4 - Continued Process VerificationDocument1 pageM4 - Lesson 4 - Continued Process VerificationWilliam DC RiveraNo ratings yet
- Prospective, Concurrent and Retrospective Validation-GOOD ARTICLEDocument8 pagesProspective, Concurrent and Retrospective Validation-GOOD ARTICLEraju1559405No ratings yet
- Adopting The Product Lifecycle ApproachDocument4 pagesAdopting The Product Lifecycle Approach刘朝阳No ratings yet
- DR KyQUALITY by DESIGN:From Theory To PracticeDocument25 pagesDR KyQUALITY by DESIGN:From Theory To PracticemanipallavanNo ratings yet
- Technology Transfer - 20230927 - 114610 - 0000Document10 pagesTechnology Transfer - 20230927 - 114610 - 0000KALP PATELNo ratings yet
- Qualification Existing Equipment FinalDocument16 pagesQualification Existing Equipment FinalAjay Kumar100% (1)
- 3-2. Assessing Production Documents:: Executed and Master RecordsDocument48 pages3-2. Assessing Production Documents:: Executed and Master RecordsAmer RahmahNo ratings yet
- ValidationDocument49 pagesValidationSwathi Battula100% (1)
- Validation of Pharmaceutical PackagingDocument28 pagesValidation of Pharmaceutical PackagingNaheed Malik100% (3)
- Role and Responsibility of Pharmaceutical Industry Plant PersonnelFrom EverandRole and Responsibility of Pharmaceutical Industry Plant PersonnelNo ratings yet
- Informe 40Document104 pagesInforme 40HOSMANECHEVERRIANo ratings yet
- Annex 4 Supplementary Guidelines On GoodDocument72 pagesAnnex 4 Supplementary Guidelines On GoodHiếu Ngô QuangNo ratings yet
- (920 923) Jul Aug14Document4 pages(920 923) Jul Aug14surafelNo ratings yet
- ASEAN PV (Version 3 1) Includes All AnnexesDocument39 pagesASEAN PV (Version 3 1) Includes All AnnexesAndy RojasNo ratings yet
- Basic Principles of GMP Basic Principles of GMPDocument33 pagesBasic Principles of GMP Basic Principles of GMPTueNo ratings yet
- CGMP GuidelinesDocument23 pagesCGMP GuidelinesRyan 1112No ratings yet
- M4 - Lesson 1 - Introduction To Process ValidationDocument4 pagesM4 - Lesson 1 - Introduction To Process ValidationWilliam DC RiveraNo ratings yet
- Drugs and Health Products: Validation Guidelines For Pharmaceutical Dosage Forms (GUIDE-0029)Document12 pagesDrugs and Health Products: Validation Guidelines For Pharmaceutical Dosage Forms (GUIDE-0029)upadhyayparag01No ratings yet
- 3-2. Assessing Production DocumentsDocument48 pages3-2. Assessing Production DocumentsSandeep sharmaNo ratings yet
- Basic Principles of GMP Basic Principles of GMPDocument33 pagesBasic Principles of GMP Basic Principles of GMPTahir KhanNo ratings yet
- Validation: Presented To: Prof. H.S. Keerthy Department of Pharmaceutics Mallige College of PharmacyDocument26 pagesValidation: Presented To: Prof. H.S. Keerthy Department of Pharmaceutics Mallige College of PharmacyAfdal Naim100% (1)
- Contribute To The Development of A Strategic Plan ILM Assessment Guidance (ML45)Document4 pagesContribute To The Development of A Strategic Plan ILM Assessment Guidance (ML45)Mk Seifu EthioNo ratings yet
- Nursery Car Seat Supplement 2023Document40 pagesNursery Car Seat Supplement 2023doniNo ratings yet
- KP53V85 Tech ManualDocument106 pagesKP53V85 Tech ManualrichiegranNo ratings yet
- UK Wooden Pallet 1200 X 1000mmDocument1 pageUK Wooden Pallet 1200 X 1000mmRizwan rizviNo ratings yet
- A. B. C. D. E.: IIA Report Logistics IndustryDocument14 pagesA. B. C. D. E.: IIA Report Logistics IndustryABHISHEK BHARDWAJNo ratings yet
- Business Plan Hindi Pa FinalDocument10 pagesBusiness Plan Hindi Pa FinalMaria Theresa Cortez MendozaNo ratings yet
- Financial Status and Academic AchievementDocument20 pagesFinancial Status and Academic AchievementkalbokalapatoNo ratings yet
- Logic Controller: Multitechnology Know-HowDocument12 pagesLogic Controller: Multitechnology Know-HowPrIsmatIIcONo ratings yet
- Pipe Flange FormulaDocument57 pagesPipe Flange FormulaRiandi HartartoNo ratings yet
- ABLE Contract Approval.Document5 pagesABLE Contract Approval.Ferris FerrisNo ratings yet
- Tobacco IndustryDocument9 pagesTobacco IndustryjoséNo ratings yet
- TERPS PANS-OPS Approach Brief With Circling Considerations - NBAADocument39 pagesTERPS PANS-OPS Approach Brief With Circling Considerations - NBAAMos DetNo ratings yet
- Accounting Information SystemsDocument11 pagesAccounting Information SystemsMohamed ZakyNo ratings yet
- Social Responsibility Theory FixDocument8 pagesSocial Responsibility Theory Fixsyifa fauziahNo ratings yet
- Absolute Containers Brochure 2019 2 27 PDFDocument19 pagesAbsolute Containers Brochure 2019 2 27 PDFEduardo SolanoNo ratings yet
- Struktur Kelas 10 Ipa 3Document17 pagesStruktur Kelas 10 Ipa 3Okvie AjaNo ratings yet
- Communication Design Insights From The Creative Industries - 2Document33 pagesCommunication Design Insights From The Creative Industries - 2Grace PsycheNo ratings yet
- Hannah Gonzales ResumeDocument2 pagesHannah Gonzales Resumeapi-500481504No ratings yet
- Pig FarmingDocument25 pagesPig Farmingসপ্তক মন্ডল100% (1)
- MZ Instruction and Maintenance ManualDocument25 pagesMZ Instruction and Maintenance ManualLakiLakicNo ratings yet
- Revit-Structural Bim SpecialistDocument5 pagesRevit-Structural Bim SpecialistYoukhanna ZayiaNo ratings yet
- Matrix Korelasi ISO IntergrasiDocument3 pagesMatrix Korelasi ISO IntergrasiAgungMahendraNo ratings yet
- CV Structural EngineerDocument5 pagesCV Structural EngineerMuhammad Ridha100% (1)
- How To Identify Maketing Research ProblemDocument2 pagesHow To Identify Maketing Research ProblempavanNo ratings yet
- Business Schools of AsiaDocument12 pagesBusiness Schools of AsiaRahul JainNo ratings yet
- SGX-Listed Hatten Land Diversifies Beyond Melaka Through Proposed Acquisition of Unicity in Seremban, MalaysiaDocument3 pagesSGX-Listed Hatten Land Diversifies Beyond Melaka Through Proposed Acquisition of Unicity in Seremban, MalaysiaWeR1 Consultants Pte LtdNo ratings yet
- Transmission Hydraulic SystemDocument4 pagesTransmission Hydraulic SystemA Ramos Gaby100% (5)
- AMM - 01-Aug-2019 - 79-21-10-000-004-A - Removal of The Lubrication UnitDocument10 pagesAMM - 01-Aug-2019 - 79-21-10-000-004-A - Removal of The Lubrication UnitIrfan05100% (1)
Gnipst 4 30 5694 2.4
Gnipst 4 30 5694 2.4
Uploaded by
Antar GayenOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Gnipst 4 30 5694 2.4
Gnipst 4 30 5694 2.4
Uploaded by
Antar GayenCopyright:
Available Formats
GURU NANAK INSTITUTE OF PHARMACEUTICAL SCIENCE & TECHNOLOGY
INDUSTRIAL PHARMACY II
PT716A
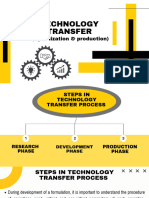
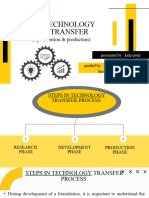
Transfer from R & D to production (Process, packaging and cleaning)
• It should be established at the outset whether the intention is to perform single-batch
manufacture, continuous production or campaigns, and whether the RU can
accommodate the intended production capacity.
• Consideration should be given to the level and depth of detail to be transferred to
support production and any further development or process optimization at the RU as
intended under the transfer project plan.
• The SU and the RU should jointly develop a protocol for the transfer of relevant
information related to the manufacturing process under consideration from the SU to the
RU, as well as the development of an equivalent process at the RU.
Process
The SU should provide a detailed characterization of the product, including its qualitative
and quantitative composition, physical description, method of manufacture, in-process
controls and specifications, packaging components and configurations, and any special
safety and handling considerations. The SU should provide any information on the history
of process development which may be required to enable the RU to perform any further
development and/or process optimization intended after successful transfer. Such
information may include the following:
• information on clinical development, e.g. information on the rationale for the
synthesis, route and form selection, technology selection, equipment, clinical tests,
and product composition;
• information on scale-up activities: process optimization, statistical optimization
of critical process parameters, pilot report and/or information on pilot-scale
development activities indicating the number and disposition of batches
manufactured; and
• information or report on full-scale development activities, indicating the number
and disposition of batches manufactured, and deviation and change control
reports which led to the current manufacturing.
The SU should provide to the RU information on any health, safety and
environmental issues associated with the manufacturing processes to be
transferred, and resulting implications, e.g. need for gowning or protective
clothing.
The SU should provide to the RU information on current processing and testing,
including but not limited to:
• a detailed description of facility requirements and equipment ;
• process technology selection;
• information on starting materials, applicable MSDs and storage requirements for
raw materials and finished products;
• description of manufacturing steps (narrative and process maps or flow charts),
including qualification of in-processing hold times and conditions, order and
method of raw material addition and bulk transfers between processing steps;
• description of analytical methods;
• in-process controls, including, e.g. identification of critical performance aspects
for specific dosage forms, identification of process control points, product quality
attributes and qualification of critical processing parameter ranges, statistical
process control (SPC) charts;
• validation information, e.g. validation plans and reports, and annual product
reviews;
• stability information; and an authorized set of SOPs and work instructions for
manufacturing.
Packaging
It should follow the same procedural patterns as those of the production transfer.
• Information on packaging to be transferred from the SU to the RU include specifications
for a suitable container/closure system, as well as any relevant additional information on
design, packing, processing or labeling requirements needed for qualification of
packaging components at the RU.
• For quality control testing of packaging components, specifications should be provided
for drawings, artwork, and material (glass, card, fibre board, etc.).
Based on the information provided, the RU should perform a suitability study for initial
qualification of the packaging components. Packaging is considered suitable if it provides
adequate protection (preventing degradation of the drug due to environmental
influences), safety (absence of undesirable substances released into the product),
compatibility (absence of interaction possibly affecting drug quality) and performance
(functionality in terms of drug delivery).
Cleaning
During the manufacturing process, pharmaceutical products and APIs can be
contaminated by other pharmaceutical products or APIs if processing different products.
To minimize the risk of contamination and cross-contamination, operator exposure and
environmental effects, adequate cleaning procedures are essential.
The SU should provide information on cleaning procedures in use at the SU to minimize
cross- contamination due to residues from previous manufacturing steps, operator
exposure and environmental impact, including: solubility information of active
ingredients, excipients and vehicles.
You might also like
- Annex 7: WHO Guidelines On Transfer of Technology in Pharmaceutical ManufacturingDocument25 pagesAnnex 7: WHO Guidelines On Transfer of Technology in Pharmaceutical ManufacturingEkin Duran100% (1)
- Kit Kat Case StudyDocument6 pagesKit Kat Case Studyms.Ahmed0% (1)
- Lab 2Document30 pagesLab 2jianneNo ratings yet
- SOP For Continued Process VerificationDocument9 pagesSOP For Continued Process VerificationMubarak Patel100% (1)
- Technology Transfer in Pharmaceutical IndustryDocument24 pagesTechnology Transfer in Pharmaceutical IndustryPranjal100% (3)
- Parametric ReleaseDocument7 pagesParametric ReleaseshdphNo ratings yet
- Risk Identification For Aseptic ProcessingDocument42 pagesRisk Identification For Aseptic Processingmmmmm100% (2)
- Validation and Validation ProtocolDocument18 pagesValidation and Validation ProtocolLalit Lata JhaNo ratings yet
- Unit - Granularity Technology Transfer - SVKDocument167 pagesUnit - Granularity Technology Transfer - SVKHarshada AgarkarNo ratings yet
- Peer-Reviewed: Medical Devices: Warning LettersDocument1 pagePeer-Reviewed: Medical Devices: Warning LettersSandra RodriguezNo ratings yet
- Guidance For Industry: Process ValidationDocument18 pagesGuidance For Industry: Process ValidationBruno DebonnetNo ratings yet
- Technology Transfer V.1.0Document39 pagesTechnology Transfer V.1.0Syahid AchyarNo ratings yet
- DocumentationDocument46 pagesDocumentationajak16406No ratings yet
- TRS961 - Annex7 WHO Tech TransferDocument25 pagesTRS961 - Annex7 WHO Tech TransferkrasataNo ratings yet
- ISO 11607 PackagingDocument8 pagesISO 11607 PackagingAngel CvetanovNo ratings yet
- Gnipst 4 30 5694 2.1Document5 pagesGnipst 4 30 5694 2.1Antar GayenNo ratings yet
- Comments On Ongoing Process VerifDocument2 pagesComments On Ongoing Process VerifsofianesedkaouiNo ratings yet
- Gnipst 4 30 5694 2.2Document2 pagesGnipst 4 30 5694 2.2Antar GayenNo ratings yet
- ValidationDocument49 pagesValidationAshokPokiriNo ratings yet
- USP-Lifecycle Management of Analytical ProceduresDocument15 pagesUSP-Lifecycle Management of Analytical ProceduresNur AcarNo ratings yet
- Articulo Tecnologia de TransferenciaDocument10 pagesArticulo Tecnologia de Transferencia20172504No ratings yet
- TRS961 Annex7Document25 pagesTRS961 Annex7Tahir KhanNo ratings yet
- ValidationDocument5 pagesValidationjyothisahadevanNo ratings yet
- VAL MANUAL 018 Potential Critical Packaging Process Parameters and Validation Practices SampleDocument3 pagesVAL MANUAL 018 Potential Critical Packaging Process Parameters and Validation Practices SampleRahul VermaNo ratings yet
- 2016Document40 pages2016schumonNo ratings yet
- Chinna Nagamalleswara Reddy Alla: SummaryDocument5 pagesChinna Nagamalleswara Reddy Alla: SummaryVijay LS SolutionsNo ratings yet
- RequestDocument3 pagesRequestiloveit52252No ratings yet
- Pharmaceutical Development Q8 (R2) : International Conference On Harmonisation (ICH)Document11 pagesPharmaceutical Development Q8 (R2) : International Conference On Harmonisation (ICH)Md. Akil Mahmud100% (1)
- Gnipst 4 30 5694 2.5Document4 pagesGnipst 4 30 5694 2.5Antar GayenNo ratings yet
- Types of Process ValidationDocument5 pagesTypes of Process ValidationLuanne Dela CruzNo ratings yet
- Technology Transfer - 20230918 - 201200 - 0000Document9 pagesTechnology Transfer - 20230918 - 201200 - 0000KALP PATELNo ratings yet
- Process Analytical TechnologyDocument57 pagesProcess Analytical Technologycarleen_almiraNo ratings yet
- Process-Validation - General-Principles-and-Practices-10Document1 pageProcess-Validation - General-Principles-and-Practices-10Kendry JoseNo ratings yet
- CLAUSE 8.5 Production and Service ProvisionDocument10 pagesCLAUSE 8.5 Production and Service ProvisionNavnath TamhaneNo ratings yet
- 4.1.5 The Quality Assurance ManagerDocument1 page4.1.5 The Quality Assurance ManagerthisiscookingNo ratings yet
- Documentation: Good Manufacturing Practices Modul-3Document29 pagesDocumentation: Good Manufacturing Practices Modul-3ChristinaNo ratings yet
- Taticek-Product Monitoring & Post-Approval Lifecycle Management of Biotech ProductsDocument36 pagesTaticek-Product Monitoring & Post-Approval Lifecycle Management of Biotech Products刘朝阳No ratings yet
- M4 - Lesson 4 - Continued Process VerificationDocument1 pageM4 - Lesson 4 - Continued Process VerificationWilliam DC RiveraNo ratings yet
- Prospective, Concurrent and Retrospective Validation-GOOD ARTICLEDocument8 pagesProspective, Concurrent and Retrospective Validation-GOOD ARTICLEraju1559405No ratings yet
- Adopting The Product Lifecycle ApproachDocument4 pagesAdopting The Product Lifecycle Approach刘朝阳No ratings yet
- DR KyQUALITY by DESIGN:From Theory To PracticeDocument25 pagesDR KyQUALITY by DESIGN:From Theory To PracticemanipallavanNo ratings yet
- Technology Transfer - 20230927 - 114610 - 0000Document10 pagesTechnology Transfer - 20230927 - 114610 - 0000KALP PATELNo ratings yet
- Qualification Existing Equipment FinalDocument16 pagesQualification Existing Equipment FinalAjay Kumar100% (1)
- 3-2. Assessing Production Documents:: Executed and Master RecordsDocument48 pages3-2. Assessing Production Documents:: Executed and Master RecordsAmer RahmahNo ratings yet
- ValidationDocument49 pagesValidationSwathi Battula100% (1)
- Validation of Pharmaceutical PackagingDocument28 pagesValidation of Pharmaceutical PackagingNaheed Malik100% (3)
- Role and Responsibility of Pharmaceutical Industry Plant PersonnelFrom EverandRole and Responsibility of Pharmaceutical Industry Plant PersonnelNo ratings yet
- Informe 40Document104 pagesInforme 40HOSMANECHEVERRIANo ratings yet
- Annex 4 Supplementary Guidelines On GoodDocument72 pagesAnnex 4 Supplementary Guidelines On GoodHiếu Ngô QuangNo ratings yet
- (920 923) Jul Aug14Document4 pages(920 923) Jul Aug14surafelNo ratings yet
- ASEAN PV (Version 3 1) Includes All AnnexesDocument39 pagesASEAN PV (Version 3 1) Includes All AnnexesAndy RojasNo ratings yet
- Basic Principles of GMP Basic Principles of GMPDocument33 pagesBasic Principles of GMP Basic Principles of GMPTueNo ratings yet
- CGMP GuidelinesDocument23 pagesCGMP GuidelinesRyan 1112No ratings yet
- M4 - Lesson 1 - Introduction To Process ValidationDocument4 pagesM4 - Lesson 1 - Introduction To Process ValidationWilliam DC RiveraNo ratings yet
- Drugs and Health Products: Validation Guidelines For Pharmaceutical Dosage Forms (GUIDE-0029)Document12 pagesDrugs and Health Products: Validation Guidelines For Pharmaceutical Dosage Forms (GUIDE-0029)upadhyayparag01No ratings yet
- 3-2. Assessing Production DocumentsDocument48 pages3-2. Assessing Production DocumentsSandeep sharmaNo ratings yet
- Basic Principles of GMP Basic Principles of GMPDocument33 pagesBasic Principles of GMP Basic Principles of GMPTahir KhanNo ratings yet
- Validation: Presented To: Prof. H.S. Keerthy Department of Pharmaceutics Mallige College of PharmacyDocument26 pagesValidation: Presented To: Prof. H.S. Keerthy Department of Pharmaceutics Mallige College of PharmacyAfdal Naim100% (1)
- Contribute To The Development of A Strategic Plan ILM Assessment Guidance (ML45)Document4 pagesContribute To The Development of A Strategic Plan ILM Assessment Guidance (ML45)Mk Seifu EthioNo ratings yet
- Nursery Car Seat Supplement 2023Document40 pagesNursery Car Seat Supplement 2023doniNo ratings yet
- KP53V85 Tech ManualDocument106 pagesKP53V85 Tech ManualrichiegranNo ratings yet
- UK Wooden Pallet 1200 X 1000mmDocument1 pageUK Wooden Pallet 1200 X 1000mmRizwan rizviNo ratings yet
- A. B. C. D. E.: IIA Report Logistics IndustryDocument14 pagesA. B. C. D. E.: IIA Report Logistics IndustryABHISHEK BHARDWAJNo ratings yet
- Business Plan Hindi Pa FinalDocument10 pagesBusiness Plan Hindi Pa FinalMaria Theresa Cortez MendozaNo ratings yet
- Financial Status and Academic AchievementDocument20 pagesFinancial Status and Academic AchievementkalbokalapatoNo ratings yet
- Logic Controller: Multitechnology Know-HowDocument12 pagesLogic Controller: Multitechnology Know-HowPrIsmatIIcONo ratings yet
- Pipe Flange FormulaDocument57 pagesPipe Flange FormulaRiandi HartartoNo ratings yet
- ABLE Contract Approval.Document5 pagesABLE Contract Approval.Ferris FerrisNo ratings yet
- Tobacco IndustryDocument9 pagesTobacco IndustryjoséNo ratings yet
- TERPS PANS-OPS Approach Brief With Circling Considerations - NBAADocument39 pagesTERPS PANS-OPS Approach Brief With Circling Considerations - NBAAMos DetNo ratings yet
- Accounting Information SystemsDocument11 pagesAccounting Information SystemsMohamed ZakyNo ratings yet
- Social Responsibility Theory FixDocument8 pagesSocial Responsibility Theory Fixsyifa fauziahNo ratings yet
- Absolute Containers Brochure 2019 2 27 PDFDocument19 pagesAbsolute Containers Brochure 2019 2 27 PDFEduardo SolanoNo ratings yet
- Struktur Kelas 10 Ipa 3Document17 pagesStruktur Kelas 10 Ipa 3Okvie AjaNo ratings yet
- Communication Design Insights From The Creative Industries - 2Document33 pagesCommunication Design Insights From The Creative Industries - 2Grace PsycheNo ratings yet
- Hannah Gonzales ResumeDocument2 pagesHannah Gonzales Resumeapi-500481504No ratings yet
- Pig FarmingDocument25 pagesPig Farmingসপ্তক মন্ডল100% (1)
- MZ Instruction and Maintenance ManualDocument25 pagesMZ Instruction and Maintenance ManualLakiLakicNo ratings yet
- Revit-Structural Bim SpecialistDocument5 pagesRevit-Structural Bim SpecialistYoukhanna ZayiaNo ratings yet
- Matrix Korelasi ISO IntergrasiDocument3 pagesMatrix Korelasi ISO IntergrasiAgungMahendraNo ratings yet
- CV Structural EngineerDocument5 pagesCV Structural EngineerMuhammad Ridha100% (1)
- How To Identify Maketing Research ProblemDocument2 pagesHow To Identify Maketing Research ProblempavanNo ratings yet
- Business Schools of AsiaDocument12 pagesBusiness Schools of AsiaRahul JainNo ratings yet
- SGX-Listed Hatten Land Diversifies Beyond Melaka Through Proposed Acquisition of Unicity in Seremban, MalaysiaDocument3 pagesSGX-Listed Hatten Land Diversifies Beyond Melaka Through Proposed Acquisition of Unicity in Seremban, MalaysiaWeR1 Consultants Pte LtdNo ratings yet
- Transmission Hydraulic SystemDocument4 pagesTransmission Hydraulic SystemA Ramos Gaby100% (5)
- AMM - 01-Aug-2019 - 79-21-10-000-004-A - Removal of The Lubrication UnitDocument10 pagesAMM - 01-Aug-2019 - 79-21-10-000-004-A - Removal of The Lubrication UnitIrfan05100% (1)