Professional Documents
Culture Documents
Ch5 Workbook On Science 9
Ch5 Workbook On Science 9
Uploaded by
mllupoOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ch5 Workbook On Science 9
Ch5 Workbook On Science 9
Uploaded by
mllupoCopyright:
Available Formats
COMPLETE AND RETURN IT TO MR.
LLUPO ON THE DAY OF THE CHAPTER TEST
Chapter 5 Workbook NAME: ___________________________________________
Section 5.1 Review - Evolution of the Atomic Model
Multiple Choice
1. Which of the following statements is not part of 4. Which scientists suggested models of the atom that did
Dalton’s atomic theory? not include a nucleus?
a. Compounds are created when atoms of different a. Dalton and Rutherford
elements link together. b. Thomson and Dalton
b. Atoms cannot be created or destroyed. c. Rutherford and Bohr
c. Atoms are composed of negatively charged d. Thomson and Bohr
particles that are in energy levels. e. Dalton and Bohr
d. All matter is made up of atoms.
e. The atoms of one element differ in mass and 5. Which list represents the order of atomic theory
size from atoms of other elements. contributors, from earliest to most recent?
a. Bohr, Rutherford, Thomson, Dalton
2. Which of the following is a correct description of b. Rutherford, Bohr, Dalton, Thomson
the model of the atom shown below? c. Dalton, Rutherford, Thomson, Bohr
d. Dalton, Thomson, Rutherford, Bohr
e. Thomson, Dalton, Rutherford, Bohr
6. Which of the following represents how electrons
occupy energy levels?
a. two people on a roller coaster
b. a person on a swing
c. two people on a teeter-totter
d. a person on a ramp
a. a central nucleus with orbiting electrons e. a person on stairs
b. a mass of positive charge with negative
electrons stuck throughout it 7. What led scientists to think that atoms contained
c. a positive nucleus with negative electrons in neutral particles?
energy levels a. The ray in a gas discharge tube travels from the
d. a small, dense indivisible particle cathode to the anode.
e. material with positive and negative charges on b. Neutral particles attract electrons to the nucleus.
its surface c. The nucleus has more mass than can be accounted
for by the number of protons.
d. Hydrogen and oxygen have different properties.
3. What was the surprising observation made during
Rutherford’s gold foil experiment? e. Alpha particles pass through gold foil.
a. Alpha particles bounced back to the source.
b. Most alpha particles passed through the foil. 8. What particles make up the atomic nucleus?
c. The same ray was created in a discharge tube. a. electrons
d. Hydrogen had distinct properties. b. protons
e. Some alpha particles were deflected. c. neutrons
d. protons and neutrons
e. protons and electrons
62 MHR • Unit 2 Atoms, Elements, and Compounds 978-0-07-031851-9
Section 5.1 Review - Evolution of the Atomic Model
Written Answer
9. What did John Dalton experiment with that led him to develop his atomic theory?
10. What discovery resulted in changes to Dalton’s model of the atom, and who made the discovery?
11. One model of the atom is shown below. This model, which was developed based on experimental evidence, is
different from earlier models.
a. What is the main difference between the structure of the atom in this model compared to the atoms shown in
earlier models?
b. Describe the experimental procedure that provided the evidence for this model.
12. Marie Curie played an important role in the evolution of the atomic model. What was her contribution to atomic
theory?
13. Why did many scientists oppose Rutherford’s model of the atom?
14. What was Bohr’s contribution to the current model of the atom?
15. Describe the properties and composition of the nucleus of an atom.
16. What are subatomic particles? List three examples of them.
MHR • Unit 2 Atoms, Elements, and Compounds 63
Section 5.2 Review - The Structure of the Atom
Multiple Choice
For each question below, select the best answer.
1. Which of the following statements about protons, 5. Which of the following statements about forces within an
neutrons, and electrons is true? atom is true?
a. Protons and electrons have about the same a. Protons and electrons repel one another due to electric
mass. charge.
b. Neutrons and electrons have equal but opposite b. Protons and neutrons attract one another due to the
charges. strong force.
c. The mass of a proton is nearly 2000 times c. Electrons and neutrons repel one another due to
greater than the mass of an electron. electric charge.
d. The mass of a proton is nearly 2000 times d. Protons and neutrons attract one another due to
greater than the mass of a neutron. electric charge.
e. Neutrons and electrons are found in the nucleus e. Protons and electrons attract one another due to the
of an atom. strong force.
2. Which of the following can be used to identify an 6. How are the mass number, the atomic number, and the
atom as a certain element? number of neutrons related?
a. the number of neutrons a. atomic number = mass number + number of neutrons
b. the mass number b. mass number = atomic number + number of neutrons
c. the number of protons c. number of neutrons = atomic number + mass number
d. the number of electrons
d. mass number = number of neutrons – atomic number
e. the ion charge
atomic number = number of neutrons – mass number
e.
3. Which statement about the relative number of
subatomic particles in an atom is always true?
a. The mass number is equal to the total number of
7. What is the mass number of an atom with 6 protons and 7
protons and electrons.
neutrons?
b. The number of electrons equals the number of
a. 7
neutrons in a neutral atom.
b. 6
c. The atomic number is equal to the number of
c. 13
neutrons.
d. 1.17
d. The number of neutrons is equal to the mass
e. 0.86
number of an atom.
e. The atomic number is the number of electrons
in a neutral atom. 8. How many electrons does an atom have if the mass
number is 15 and the number of neutrons is 7?
a. 8
4. In which part of an atom is most of the mass
b. 7
located?
c. 15
a. the nucleus
d. 22
b. the electrons
e. 105
c. the protons
d. the neutrons
e. the energy levels surrounding the nucleus
64 MHR • Unit 2 Atoms, Elements, and Compounds 978-0-07-031851-9
Section 5.2 Review - The Structure of the Atom
Multiple Choice
For each question below, select the best answer.
9. Which of the following symbols represents the 13. How are electrons organized (starting from the first
element with an atomic number of 14? energy level) in an atom of potassium (atomic number
a. C 19)?
b. N a. 2, 8, 8, 1
c. Ni b. 19, 0, 0, 0
d. Si c. 2, 10, 7, 0
e. S d. 1, 4, 6, 8
e. 2, 8, 9, 0
10. What is the mass number of an atom of chlorine
that has 18 neutrons? 14. Which of the following statements describes what
a. 1– could be an isotope of the atom represented below?
b. 17
c. 18
d. 35
e. 35.5
11. What is the standard atomic notation for an atom
that has an atomic number of 4 and has 5 neutrons? a. The atom has 0 electrons.
4 b. The atom has 2 neutrons and 1 proton.
a. 5 Be
c. The atom has 2 protons and 1 neutron.
4 d. The atom has 2 electrons.
b. 9 F
9
e. The atom has 0 protons and 1 neutron.
c. 4 Be
d. 9
B 15. A neutral atom has 8 electrons and 8 neutrons. Which
5
of the following correctly represents the atom?
a. carbon-16
4
e. 1 H
b. oxygen-16
12. What element is represented by the Bohr- c. oxygen-24
Rutherford diagram below? d. oxygen-8
e. sulfur-8
16. How many protons, neutrons, and electrons are in an
atom of calcium-42 (atomic number 20)?
a. 22 protons, 20 neutrons, and 20 electrons
b. 21 protons, 20 neutrons, and 21 electrons
c. 20 protons, 20 neutrons, and 22 electrons
d. 42 protons, 0 neutrons, and 42 electrons
a. potassium e. 20 protons, 22 neutrons, and 20 electrons
b. calcium
c. hydrogen
d. argon
e. yttrium
MHR • Unit 2 Atoms, Elements, and Compounds 65
Section 5.2 Review - The Structure of the Atom
Written Answer
17. Fill in the missing information about subatomic particles.
Properties of Subatomic Particles
Subatomic Particle Electric Charge Location in the Atom Relative Mass
electron 1
proton
neutron
18. What is the role of the strong force in an atom?
19. If you know the number of protons in the nucleus of a neutral atom, what else do you know about that atom?
20. An atom has 7 protons, 7 electrons, and 7 neutrons. What is its atomic number?
21. An atom with a mass number of 7 and an atomic number of 3. How many neutrons does it have?
22. An atom has a mass number of 55 and an atomic number of 25.
a. What is its element? How do you know?
b. How many protons, neutrons, and electrons are in the atom?
23. An isotope of iodine(I) that is used for the medical diagnosis of thyroid problems has 78 neutrons and 53 protons.
Write the standard atomic notation for this isotope.
24. Use standard atomic notation to represent the atoms with the number of protons, neutrons, and electrons listed
below.
a. 12 protons, 12 neutrons, and 12 electrons
b. 10 protons, 10 neutrons, and 10 electrons
c. 15 protons, 16 neutrons, and 15 electrons
d. 7 protons, 7 neutrons, and 7 electrons
e. 13 protons, 14 neutrons, and 13 electrons
f. 18 protons, 22 neutrons, and 18 electrons
66 MHR • Unit 2 Atoms, Elements, and Compounds 978-0-07-031851-9
Section 5.2 Review - The Structure of the Atom
Written Answer
25. What is another name for energy levels?
26. If an atom has 15 electrons, how many energy levels are occupied?
27. Draw a Bohr-Rutherford diagram of an atom of beryllium with 5 neutrons.
28. List two ways in which isotopes of an element differ from one another.
29. “All atoms of sodium isotopes have the same number of neutrons.” Is this statement true?Explain why or why not.
30. How many neutrons are in the isotope of carbon represented by the following symbol?
31. Explain what the names “hydrogen-1” and “hydrogen-2” mean.
32. Research into the structure and composition of atoms continues at laboratories like CERN.
a. Which subatomic particles in an atom are made up of even smaller subatomic particles?
b. What are these smaller particles called?
MHR • Unit 2 Atoms, Elements, and Compounds 67
Section 5.3 Review - The Periodic Table
Multiple Choice
For each question below, select the best answer.
5. Which element in Group 13 is not a metal?
1. What did Mendeleev use as a basis for arranging
a. B
the elements into a periodic table?
b. Al
a. number of neutrons
c. C
b. mass number
d. Ga
c. atomic number
e. S
d. atomic mass
e. number of protons
6. Which of the following properties is characteristic
of metals?
2. How are elements arranged across rows in the most
a. brittle
common form of the modern periodic table?
b. malleable
a. by increasing mass number
c. dull
b. by increasing atomic number
d. poor electrical conductivity
c. by increasing atomic mass
e. gas at room temperature
d. by decreasing mass number
e. by increasing ion charge
7. Which statement about elements and their
relationship in the periodic table is true?
3. Which of the following elements is a non-metal?
a. Chlorine and iodine are alkali metals.
a. Na
b. Potassium and phosphorus are in the same
b. S
group.
c. Al
c. Calcium and magnesium are alkaline-earth
d. Ca
e. Sb metals.
d. Boron and silicon are in the same period.
e. Lead and tin are both non-metals.
4. Which of the following elements is a metalloid?
a. Sn
8. What group of elements is shown below?
b. Sr
c. S a. alkali metals
d. Se b. halogens
c. metalloids
e. Si
d. noble gases
e. alkaline-earth metals
68 MHR • Unit 2 Atoms, Elements, and Compounds 978-0-07-031851-9
Section 5.3 Review - The Periodic Table
Written Answer
9. What was one of the most important features of Mendeleev’s table?
10. Examine the portions of the simplified periodic table shown below. What does the number in each cell represent?
11. Using the periodic table, identify the full name, atomic number, and atomic mass of the following elements.
Identify each element as a metal, a metalloid, or a non-metal.
a. Ge
b. Fe
c. Se
12. Metallic gold can be found in nature and picked up by hand. Yet gold mining has the potential to contaminate
soil and ground water with cyanide solution. Explain why this is so.
13. What metal has contaminated many waterways on Aboriginal lands?
14. Describe the difference between a period and a group in the periodic table.
15. Why is a group in the periodic table also called a family?
16. Name a group in the periodic table that is made up of reactive non-metals.
MHR • Unit 2 Atoms, Elements, and Compounds 69
Section 5.4 Review - Trends in the Periodic Table
Multiple Choice
For each question below, select the best answer.
1. Which of the following elements has atoms with 5. Which of the following non-metals is the most
the least number of valence electrons? reactive?
a. K a. He
b. Ca b. N
c. O c. S
d. Mg d. F
e. C e. O
2. Which of the following statements about the 6. Which element has properties that are similar to the
reactivity of metals is true? properties of sulfur but has atoms that are smaller
a. Metals that have fewer occupied energy levels than atoms of sulfur?
are more reactive than metals with more a. chlorine
occupied energy levels. b. oxygen
b. Metals become more reactive as their number of c. phosphorus
valence electrons increases. d. selenium
c. Metals become more reactive as their atoms get e. argon
smaller.
d. Metals become more reactive as you move from 7. Which of the following atoms is the largest in size?
left to right across a period. a. Sr
e. Metals become more reactive as you move from b. Mg
top to bottom in a group. c. I
d. Br
3. Which group in the periodic table does the atom e. Xe
represented below belong to?
8. Which of the following lists of atoms is in order
from largest to smallest?
a. Sr, Ca, Rb, Ga, B, O
b. Ga, B, O, Rb, Sr, Ca
c. Rb, Sr, Ca, Ga, B, O
d. Rb, Sr, Ga, Ca, O, B
a. noble gases e. O, B, Ga, Ca, Sr, Rb
b. metalloids
c. alkaline-earth metals
d. alkali metals
e. halogens
4. Which of the following metals is the most reactive?
a. Al
b. Sr
c. Cs
d. K
e. Mg
70 MHR • Unit 2 Atoms, Elements, and Compounds 978-0-07-031851-9
Section 5.4 Review - Trends in the Periodic Table
Written Answer
9. How many valence electrons does an atom of each of the following elements have?
a. B
b. Ne
c. S
d. Mg
10. Why are elements in Group 1 and in Group 17 very reactive?
11. The number of valence electrons follows a trend in the periodic table.
a. Describe the trend in valence electrons as you move from left to right across a period.
b. Describe the trend in valence electrons as you move from top to bottom within a group.
12. Which group of elements has atoms that have a full set of valence electrons?
13. Why do elements in a group tend to react in similar ways?
14. How does the size of an atom relate to how easily the atom loses an electron?
15. Why is lithium less reactive than sodium?
16. Based on the Bohr-Rutherford diagrams of the atoms shown below, explain the trend in atomic sizes as you move
from top to bottom within a group of the periodic table.
MHR • Unit 2 Atoms, Elements, and Compounds 71
Chapter 5 Review - Understanding the Properties of Elements
Multiple Choice
For each question below, select the best answer.
1. Which scientists suggested models of the atom that 5. How many electrons does an atom have if the mass
included electrons? number is 31 and the number of neutrons is 16?
a. Dalton and Thomson a. 15
b. Bohr and Dalton b. 16
c. Rutherford, Thomson, and Dalton c. 31
d. Bohr, Rutherford, and Thomson, d. 47
e. Bohr, Rutherford, Thomson, and Dalton e. The number of electrons cannot be determined
from the information given.
2. Which scientist first suggested the presence of
particles within an atom? 6. How many valence electrons does an atom of oxygen
a. Curie have?
b. Thomson a. 6
c. Bohr b. 16
d. Dalton c. 32
e. Rutherford d. 8
e. 18
3. Which statement about forces within an atom
is false? 7. The table below gives the atomic radius of the atoms
a. Protons and electrons attract one another due to of several elements. What trend do the data in the
electric charge. table illustrate?
b. Protons and neutrons attract one another due to
Atomic Radii of Selected Elements
the strong force.
c. Electrons and neutrons neither attract nor repel Element Radius Element Radius
one another due to electric charge. (nm) (nm)
d. Protons and protons repel one another due to Mg 0.160 P 0.110
electric charge. Al 0.143 Cl 0.099
e. Protons and electrons repel one another due to Si 0.117 Ar 0.094
the strong force. a. Atoms get more reactive from top to bottom
within a group
4. What is the atomic number of aluminum? b. Non-metal atoms have more valence electrons than
a. 13 metal atoms.
b. 27.0 c. Metals are more reactive than non-metals.
c. 3+ d. Atoms get smaller from top to bottom within a
d. 14 group.
e. 3 e. Atoms get smaller from left to right within
a period.
8. Which of the following atoms is the smallest?
a. Po
b. Se
c. O
d. Ba
e. Cs
72 MHR • Unit 2 Atoms, Elements, and Compounds 978-0-07-031851-9
Chapter 5 Review - Understanding the Properties of Elements
Written Answer
9. Which subatomic particle was discovered through the use of a gas discharge tube?
10. What characteristic of the nucleus allowed most of the alpha particles to pass through the gold foil in Rutherford’s
experiment?
11. Why are the atomic number and mass number whole numbers, but the atomic mass is not?
12. How is the organizing principle behind the order of elements in the modern periodic table different from
Mendeleev’s original periodic table?
13. List three properties of metals.
14. How many valence electrons does an atom of each of the following elements have?
a. Ca
b. Ne
15. What are three ways in which an atom’s outer energy level can become filled?
16. For each group marked A, B, C, and D in the diagram below, write the name of the group, describe its reactivity,
and write the number of valence electrons in the atoms
of the elements.
MHR • Unit 2 Atoms, Elements, and Compounds 73
Chapter 5 Review - Understanding the Properties of Elements
Written Answer
17. What evidence supports the statement that the direct communication and sharing of information between
scientists led to the current model of the atom?
18. You overhear someone in class say that learning about the atom is not useful. Write a short statement explaining
why this is not the case and provide an example of a current application that was developed from scientists’ better
understanding of the atom.
19. Create a diagram to describe the atomic structure of an element to a student who does not speak English.
20. An atom has 11 protons, 12 neutrons, and 11 electrons.
a. What is the element? How do you know?
b. Draw a Bohr-Rutherford diagram on the right to represent the atom.
21. An atom has a mass number of 11 and an atomic number of 5.
a. What is the element? How do you know?
b. Draw a Bohr-Rutherford diagram on the right to represent the atom.
c. Draw a Bohr-Rutherford diagram of an isotope of this atom.
22. How do you think modern scientists have used the periodic table to help synthesize new elements?
23. You read a posting on the Internet in which someone claims to have made a rare element that belongs between
gold and platinum in the periodic table. Why should you be skeptical about such a claim?
24. How does the physical location of metalloids in the periodic table reflect the properties of metalloids?
74 MHR • Unit 2 Atoms, Elements, and Compounds 978-0-07-031851-9
Chapter 5 Review - Understanding the Properties of Elements
Written Answer
25. You and a friend have developed a new line of titanium jewellery. Develop an online advertisement for it,
emphasizing why it is an excellent alternative to gold jewellery.
26. Why might eating fish caught in certain lakes and rivers be hazardous?
27. Answer the following questions based on the diagram shown below.
a. Describe the physical properties of the element represented. Explain how you know.
b. Provide an example of an element that is more reactive than the element represented. Explain
your reasoning.
28. Why are there only two elements in the first period of the periodic table?
29. A helium atom has 2 valence electrons, but helium is at the top of Group 18 in the periodic table.
a. Based only on the number of its valence electrons, what group should helium belong to?Explain your
reasoning.
b. Why is Group 18 the proper place for helium?
30. A portion of the periodic table with Bohr-Rutherford models of atoms of somenon-metals is shown above. Use
this diagram to answer the following questions.
a. Describe the trend in atomic size shown in these diagrams. Explain how hese models illustrate this pattern
successfully.
b. What trend in atomic size is not shown in these diagrams? Explain whythese models do not illustrate this
pattern.
31. Which element is more reactive: lithium or sodium? Explain.
32. Some compounds exist in which atoms of noble gases share electrons with atoms of other elements. Why would
noble gases near the bottom of the group in the periodic table tend to form these compounds more easily than
noble gases at the top of the group?
MHR • Unit 2 Atoms, Elements, and Compounds 75
You might also like
- Atomic Structure (Grade 8) - Free Printable Tests and Worksheets - HelpTeaching PDFDocument1 pageAtomic Structure (Grade 8) - Free Printable Tests and Worksheets - HelpTeaching PDFnick2107067% (3)
- Selina Concise Chemistry Solutions Class 8 Chapter 4 Atomic StructureDocument17 pagesSelina Concise Chemistry Solutions Class 8 Chapter 4 Atomic StructurelolNo ratings yet
- Peter Atkins Julio de Paula Ron Friedman Physical Chemistry Quanta (0868-0918)Document51 pagesPeter Atkins Julio de Paula Ron Friedman Physical Chemistry Quanta (0868-0918)Administracion OTIC IVICNo ratings yet
- Atomic Theory Quiz and Teacher KeyDocument3 pagesAtomic Theory Quiz and Teacher KeyJomar CarabotNo ratings yet
- Atomic StructureDocument2 pagesAtomic StructureTeang LamNo ratings yet
- Atomic Structure and The Periodic TableDocument3 pagesAtomic Structure and The Periodic TableKhoer Ummah100% (1)
- Chemistry Review Question For Grade 9 Unit 3Document5 pagesChemistry Review Question For Grade 9 Unit 3mtadesse158No ratings yet
- ANSWERS - Review The AtomDocument5 pagesANSWERS - Review The AtomDayana MoreiraNo ratings yet
- Remedial Exam ChemDocument2 pagesRemedial Exam ChemMarvin Tan100% (3)
- ch4 Test BankDocument9 pagesch4 Test BankJerry LouNo ratings yet
- General Organic and Biochemistry 8th Edition Denniston Test Bank PDFDocument17 pagesGeneral Organic and Biochemistry 8th Edition Denniston Test Bank PDFa136596500No ratings yet
- Long Test in Science 8 Atoms and Periodic TableDocument2 pagesLong Test in Science 8 Atoms and Periodic TableThess Curayag FernandezNo ratings yet
- Atom Quiz PracticeDocument4 pagesAtom Quiz PracticeRejNo ratings yet
- Atomic Structure Grade 9Document2 pagesAtomic Structure Grade 9Ratna PuspitasariNo ratings yet
- Module 3.2 - Week 6 - Atoms Inside and OutDocument6 pagesModule 3.2 - Week 6 - Atoms Inside and Outits mr. leorio100% (1)
- General Chemistry 1: First Quarter-Module 2: Atomic StructureDocument26 pagesGeneral Chemistry 1: First Quarter-Module 2: Atomic StructureAlessandra Gabrielle GarezNo ratings yet
- Unit 1 ExamDocument6 pagesUnit 1 Examapi-375738241No ratings yet
- Quiz 5 Atoms, Atomic Theory, Atomic StructureDocument4 pagesQuiz 5 Atoms, Atomic Theory, Atomic StructureJoeylyn BalidioNo ratings yet
- Third Periodic ExamDocument1 pageThird Periodic ExamJunnel MaravillaNo ratings yet
- Structure WorksheetDocument8 pagesStructure Worksheetnitish debbarmaNo ratings yet
- 1 - Q2 ScienceDocument23 pages1 - Q2 Sciencemaximo meridaNo ratings yet
- (Q1) MODULE 2 - Atomic Structure PDFDocument24 pages(Q1) MODULE 2 - Atomic Structure PDFJewel SantiagoNo ratings yet
- 0e729488 02b4 47e5 A21d E7ca032be3d8 - Revision Sheet 2 Answer KeyDocument8 pages0e729488 02b4 47e5 A21d E7ca032be3d8 - Revision Sheet 2 Answer KeySharon BijuNo ratings yet
- Week22 G9 Chapter5.1-5.3Document5 pagesWeek22 G9 Chapter5.1-5.3kwokrenee827No ratings yet
- Atomic Structure: Solved QuestionsDocument4 pagesAtomic Structure: Solved QuestionsItu DeyNo ratings yet
- SCH3U Chemistry Unit 1 MC ReviewDocument16 pagesSCH3U Chemistry Unit 1 MC Review1moeezafNo ratings yet
- Atoms Long Quiz 2nd LQDocument2 pagesAtoms Long Quiz 2nd LQLALINE ZALZOSNo ratings yet
- Test 1: Multiple ChoiceDocument3 pagesTest 1: Multiple ChoiceSam Agustine RosilNo ratings yet
- Atomic Structure: Points To RememberDocument17 pagesAtomic Structure: Points To RememberVidhi AgarwalNo ratings yet
- Questions On Modern Atomic TheoryDocument2 pagesQuestions On Modern Atomic TheoryDr. Sheelu SharmaNo ratings yet
- Formative Test 2.1: GRADE 8: AtomsDocument4 pagesFormative Test 2.1: GRADE 8: AtomsKeisha Gabrielle RabanoNo ratings yet
- Third Grading Examination in Grade 9 Science Name: - Date: - Section: - ScoreDocument4 pagesThird Grading Examination in Grade 9 Science Name: - Date: - Section: - ScoreSharonNo ratings yet
- Full Download General Organic and Biochemistry 8th Edition Denniston Test BankDocument35 pagesFull Download General Organic and Biochemistry 8th Edition Denniston Test Bankwaylayfilsaxaq100% (42)
- 2.1.4 Notes (Chapter 4) 4.2 The Structure of An Atom (P. 108)Document9 pages2.1.4 Notes (Chapter 4) 4.2 The Structure of An Atom (P. 108)api-369706779No ratings yet
- Science 8 - Q3 - Module 3 - Weeks 5-6 - Lesson 3Document17 pagesScience 8 - Q3 - Module 3 - Weeks 5-6 - Lesson 3SanJoseHS67% (3)
- Chemistry Test AtomsDocument3 pagesChemistry Test Atomsangie0812100% (1)
- Activities On Unit 2Document3 pagesActivities On Unit 2Switch JavidownNo ratings yet
- Questions On Modeling AtomDocument2 pagesQuestions On Modeling AtomDr. Sheelu SharmaNo ratings yet
- Inside Atom MC Review AnswersDocument2 pagesInside Atom MC Review AnswersBelinda LapsitNo ratings yet
- Practice Exam QuestionsDocument5 pagesPractice Exam QuestionsEamon BarkhordarianNo ratings yet
- Science QuizDocument1 pageScience QuizAPRIL EASTER CHUPUICONo ratings yet
- Lecture Notes Nuclear Chemistry-RevisedDocument6 pagesLecture Notes Nuclear Chemistry-RevisedMarj EgiptoNo ratings yet
- Science 9 Summative Test 2Document3 pagesScience 9 Summative Test 2Ma. Socorro Hilario50% (2)
- Chemistry 10Th Edition Whitten Test Bank Full Chapter PDFDocument36 pagesChemistry 10Th Edition Whitten Test Bank Full Chapter PDFpauline.wilson221100% (17)
- Chemistry 10th Edition Whitten Test Bank 1Document48 pagesChemistry 10th Edition Whitten Test Bank 1christopher100% (39)
- Chemistry Lesson 2 MADocument3 pagesChemistry Lesson 2 MAarodaina511No ratings yet
- Unit 4 - Test Questions Humss 1 & Abm 3Document9 pagesUnit 4 - Test Questions Humss 1 & Abm 3Neil GabatoNo ratings yet
- Physical Science 11Document4 pagesPhysical Science 11nelson dante jr.No ratings yet
- Atomic Structure Practice Test 1Document19 pagesAtomic Structure Practice Test 1endalekNo ratings yet
- Unit 3 Test ADocument6 pagesUnit 3 Test AGoldyn FordNo ratings yet
- General Organic and Biochemistry 8th Edition Denniston Test BankDocument25 pagesGeneral Organic and Biochemistry 8th Edition Denniston Test BankCraigVelasquezmcot100% (59)
- Atoms and Period Table Test BDocument3 pagesAtoms and Period Table Test BCamille FrancoNo ratings yet
- All The Elements Have Only One Nucleon (Mass) NumberDocument13 pagesAll The Elements Have Only One Nucleon (Mass) NumberAhmadNo ratings yet
- Second Quarter Examination in Science 9Document2 pagesSecond Quarter Examination in Science 9Teresa Marie CorderoNo ratings yet
- Life The Science of Biology 11th Edition Sadava Hillis Heller Hacker Test BankDocument90 pagesLife The Science of Biology 11th Edition Sadava Hillis Heller Hacker Test Banknick100% (23)
- Filedate - 610download Test Bank For Life The Science of Biology 11Th Edition Sadava Hillis Heller Hacker 1319010164 9781319010164 Full Chapter PDFDocument36 pagesFiledate - 610download Test Bank For Life The Science of Biology 11Th Edition Sadava Hillis Heller Hacker 1319010164 9781319010164 Full Chapter PDFodessa.metzler642100% (11)
- Chemistry Worksheet For Remedial ProgramDocument38 pagesChemistry Worksheet For Remedial ProgramDerso DessieNo ratings yet
- Second Quarter Summative Test in Science 9Document3 pagesSecond Quarter Summative Test in Science 9Rowella Lagalo100% (1)
- 4 Structure-Of-The-AtomDocument9 pages4 Structure-Of-The-Atomsciencee2009No ratings yet
- ch1 Physics 12 Study GuideDocument16 pagesch1 Physics 12 Study GuidemllupoNo ratings yet
- Answers Physics 12 Study GuideDocument3 pagesAnswers Physics 12 Study GuidemllupoNo ratings yet
- Topic 4 Cambridge IB Physics Study GuideDocument18 pagesTopic 4 Cambridge IB Physics Study GuidemllupoNo ratings yet
- Ch9 Workbook ON Science 9Document16 pagesCh9 Workbook ON Science 9mllupoNo ratings yet
- ch1 NEW Physics 12 Study GuideDocument16 pagesch1 NEW Physics 12 Study GuidemllupoNo ratings yet
- Eclass Program Information and Login InstructionsDocument1 pageEclass Program Information and Login InstructionsmllupoNo ratings yet
- ScienceSkills AssignmentDocument7 pagesScienceSkills AssignmentmllupoNo ratings yet
- Ch10 Workbook ON Science 9Document14 pagesCh10 Workbook ON Science 9mllupoNo ratings yet
- Ch8 Workbook ON Science 9Document14 pagesCh8 Workbook ON Science 9mllupoNo ratings yet
- Physics 12C SG Unit4Document38 pagesPhysics 12C SG Unit4mllupoNo ratings yet
- Physics 12C SG Unit3Document90 pagesPhysics 12C SG Unit3mllupoNo ratings yet
- Ch2 Workbook On Science 9Document14 pagesCh2 Workbook On Science 9mllupoNo ratings yet
- Physics 12C SG Unit1Document54 pagesPhysics 12C SG Unit1mllupoNo ratings yet
- Physics 12C SG Unit5Document70 pagesPhysics 12C SG Unit5mllupoNo ratings yet
- HF Short CourseDocument95 pagesHF Short CourseSergey Parsegov100% (1)
- Lesson PlanDocument4 pagesLesson PlanRoscela Mae D. Arizo100% (1)
- 7 Step PfmeaDocument132 pages7 Step PfmeaRajdeep SikdarNo ratings yet
- 3b - Serway - MillikanDocument7 pages3b - Serway - Millikanalejandro1rs1orNo ratings yet
- Hydroforming of Typical Hollow Components: S.J. Yuan, G. Liu, X.R. Huang, X.S. Wang, W.C. Xie, Z.R. WangDocument5 pagesHydroforming of Typical Hollow Components: S.J. Yuan, G. Liu, X.R. Huang, X.S. Wang, W.C. Xie, Z.R. WangdavidNo ratings yet
- 8 Vertical Stresses Below Applied LoadsDocument13 pages8 Vertical Stresses Below Applied LoadsSaša MarinNo ratings yet
- (Issued 1 Mar. 1991) CRD-C 307-91 C 307: 1. ScopeDocument2 pages(Issued 1 Mar. 1991) CRD-C 307-91 C 307: 1. Scopemugger400No ratings yet
- Fusion Bonded EpoxyDocument4 pagesFusion Bonded EpoxyShakirNo ratings yet
- 4B4Document7 pages4B4rathish14uNo ratings yet
- EF Choke (Autosaved)Document14 pagesEF Choke (Autosaved)AGT AnalyzersNo ratings yet
- Principle of Metal Magnetic Memory MethodDocument6 pagesPrinciple of Metal Magnetic Memory MethodZIA ULLAHNo ratings yet
- IMO Coating KR PSPCDocument17 pagesIMO Coating KR PSPCJintaek Hong100% (1)
- 2015lubricant Selection GuideEPAW Additives and Lubricity Improvers PDFDocument4 pages2015lubricant Selection GuideEPAW Additives and Lubricity Improvers PDFPhelia KosasihNo ratings yet
- Asme Ii Part D Table 1a CSDocument20 pagesAsme Ii Part D Table 1a CSyar_nlNo ratings yet
- Chemistry MCQDocument3 pagesChemistry MCQZeeshan AslamNo ratings yet
- Ayantika Khanra UG23Document42 pagesAyantika Khanra UG23Anisha GoenkaNo ratings yet
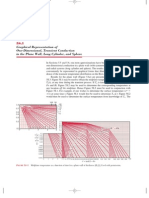
- Graphical Representation of One-Dimensional, Transient Conduction in The Plane Wall, Long Cylinder, and SphereDocument5 pagesGraphical Representation of One-Dimensional, Transient Conduction in The Plane Wall, Long Cylinder, and Spherevincent02hk_57881301No ratings yet
- Introduction To CrystallographyDocument25 pagesIntroduction To Crystallography坏豆腐No ratings yet
- DELTA-DRAIN Technical DataDocument1 pageDELTA-DRAIN Technical DataMauricio Barajas BacaNo ratings yet
- EXAMPLESeparations Pre Lab AX05Document8 pagesEXAMPLESeparations Pre Lab AX05midnightmagicpandaNo ratings yet
- Properties of Extremely Dry Concrete Made With EcoCement and Recycled Coarse AggregateDocument9 pagesProperties of Extremely Dry Concrete Made With EcoCement and Recycled Coarse AggregateCNo ratings yet
- Stress Analysis of Pressure Vessel Nozzle Using Fea IJERTCONV6IS16004Document6 pagesStress Analysis of Pressure Vessel Nozzle Using Fea IJERTCONV6IS16004Kingston RivingtonNo ratings yet
- Lecture 4 Nozzle Theory and DesignDocument26 pagesLecture 4 Nozzle Theory and Design8기이규원No ratings yet
- Alkaline Bottle PDF DetailsDocument15 pagesAlkaline Bottle PDF DetailsNaveenkumar GannaNo ratings yet
- Ib Biology Photosynthesis and RespirationDocument3 pagesIb Biology Photosynthesis and Respirationapi-270967967No ratings yet
- Flue GasDocument2 pagesFlue GasShyne HikaruNo ratings yet
- Paper ChromatographyDocument5 pagesPaper ChromatographyMaria Elena PascualNo ratings yet
- Bonding Wires For Semiconductor TechnologyDocument24 pagesBonding Wires For Semiconductor TechnologyfrdrfdederNo ratings yet
- Aits 2324 Ot I Jeea TD Paper 2 Offline SolDocument14 pagesAits 2324 Ot I Jeea TD Paper 2 Offline SolAshish SharmaNo ratings yet