Professional Documents
Culture Documents
SALTS
SALTS
Uploaded by
aquamogolwaneCopyright:
Available Formats
You might also like
- Project PPT ShubhamDocument17 pagesProject PPT ShubhamRounak Atram100% (1)
- Chapter 8 - Acids, Bases and SaltsDocument16 pagesChapter 8 - Acids, Bases and Saltsjannat amgadNo ratings yet
- Acids RecallDocument4 pagesAcids RecallAssumpta McguckinNo ratings yet
- Pure Chemistry Qualitative Analysis NotesDocument1 pagePure Chemistry Qualitative Analysis NotesVivienne SeowNo ratings yet
- Inorganic Salt AnalysisDocument9 pagesInorganic Salt Analysiswama ojha100% (1)
- IGCSE ChemistryDocument25 pagesIGCSE ChemistryLiliana DamocNo ratings yet
- ATP Notes For Chemistry o LevelDocument25 pagesATP Notes For Chemistry o LevelSaad Arsalan100% (4)
- 3 Experiment ChemistryDocument30 pages3 Experiment ChemistryThangavel SarujanNo ratings yet
- Qualitative Analysis of A Salt - F - 231128 - 000159Document9 pagesQualitative Analysis of A Salt - F - 231128 - 000159Dhairya VeerNo ratings yet
- Qualitative Analysis of Some IonsDocument42 pagesQualitative Analysis of Some IonsShaina Mae ContilloNo ratings yet
- List of Common Anions (Acidic Radicals) For Salt Analysis: Group Cations (Basic Radicals)Document6 pagesList of Common Anions (Acidic Radicals) For Salt Analysis: Group Cations (Basic Radicals)Dhruv PanditaNo ratings yet
- 2024 - 6092 - Notes of Qualitative AnalysisDocument1 page2024 - 6092 - Notes of Qualitative Analysisaleesya1302No ratings yet
- Ion Test PDFDocument11 pagesIon Test PDFAnderson XiaoNo ratings yet
- Notes Updates SaltsDocument32 pagesNotes Updates SaltsLim Jing YeeNo ratings yet
- 2324 Level M (Gr11 UAE-Gulf) Chemistry Chapter 2 NotesDocument19 pages2324 Level M (Gr11 UAE-Gulf) Chemistry Chapter 2 Notesaminata13536No ratings yet
- Identification of Ions and GasesDocument4 pagesIdentification of Ions and GasesMuqaddas FatimaNo ratings yet
- Inorganic Qualitative AnalysisDocument15 pagesInorganic Qualitative AnalysisKev WattsNo ratings yet
- Notes On SaltsDocument4 pagesNotes On SaltsFelix S100% (1)
- Identifying A Simple Salt: Ion ColorDocument15 pagesIdentifying A Simple Salt: Ion ColorNabindra RuwaliNo ratings yet
- Testing For Ions PDFDocument1 pageTesting For Ions PDFClevxyNo ratings yet
- Testing For Cations: Lesson 11.5Document5 pagesTesting For Cations: Lesson 11.5Helpful HandNo ratings yet
- Test For CationsDocument3 pagesTest For CationsBaggyNo ratings yet
- ESSENTIAL CHEMESTRIY Final 45Document14 pagesESSENTIAL CHEMESTRIY Final 45Syrus ZambiaNo ratings yet
- Form 4 Chemistry - SaltDocument6 pagesForm 4 Chemistry - SaltSze NingNo ratings yet
- Salt Analysis (Theory) - EngDocument28 pagesSalt Analysis (Theory) - Engjoxis70026100% (1)
- Imp. Practical Chem. KnowledgeDocument4 pagesImp. Practical Chem. KnowledgedebanivkashyapNo ratings yet
- C12 Chemical Analysis and InvestigationDocument10 pagesC12 Chemical Analysis and InvestigationSarah PendNo ratings yet
- 0 - Organic and Inorganic Tests For AS PDFDocument8 pages0 - Organic and Inorganic Tests For AS PDFAbed AymanNo ratings yet
- Salts FormationDocument19 pagesSalts FormationUrwa Abdul MannanNo ratings yet
- Qualitative AnalysisDocument5 pagesQualitative AnalysisAlex noslenNo ratings yet
- Salt AnalysisDocument7 pagesSalt AnalysisJaya RNo ratings yet
- Form 4 Chem Chapter 3Document12 pagesForm 4 Chem Chapter 3George LeongNo ratings yet
- Notes Salts (Chemistry)Document32 pagesNotes Salts (Chemistry)Darishana100% (1)
- Chapter 8Document2 pagesChapter 8Luna LatisyaNo ratings yet
- SPM Chemistry Formula List Form4Document14 pagesSPM Chemistry Formula List Form4Heng HoweNo ratings yet
- Part IV Acids and Alkalis: LQ 01 (Answer) Properties of Acids and AlkalisDocument12 pagesPart IV Acids and Alkalis: LQ 01 (Answer) Properties of Acids and AlkalisCharmine HolmesNo ratings yet
- Practicles - Identification of AnionsDocument11 pagesPracticles - Identification of AnionsDebasis SatapathyNo ratings yet
- Salt AnalysisDocument7 pagesSalt Analysisdharun200777No ratings yet
- Chemistry Notes Acids Bases and SaltsDocument7 pagesChemistry Notes Acids Bases and SaltsGouri RajNo ratings yet
- Qualitative Analysis TestsDocument2 pagesQualitative Analysis TestsReshmanraaj SingamNo ratings yet
- 9 Notes For Use in Qualitative Analysis Test For Anions: © UCLES 2017 0620/05/SP/20Document2 pages9 Notes For Use in Qualitative Analysis Test For Anions: © UCLES 2017 0620/05/SP/20Mayur VanjaniNo ratings yet
- Cation Anion TestDocument1 pageCation Anion TestPromit SenguptaNo ratings yet
- Csec Identification of Cations and AnionsDocument6 pagesCsec Identification of Cations and AnionsDarrion BruceNo ratings yet
- Making Salts: Neutralisation ReactionsDocument4 pagesMaking Salts: Neutralisation ReactionsPedro Moreno de SouzaNo ratings yet
- Identification of Cations, Anions and GasesDocument2 pagesIdentification of Cations, Anions and GasesMustufa FerozNo ratings yet
- Salt and SolutionDocument33 pagesSalt and SolutionFarhan Altaf100% (1)
- (A) Physical Properties of Salt: Colourless SolutionDocument4 pages(A) Physical Properties of Salt: Colourless SolutionJacelynNo ratings yet
- Qualitative Analysis NotesDocument1 pageQualitative Analysis NotesNaseema MalikNo ratings yet
- Qualitative Analysis NotesDocument1 pageQualitative Analysis NotesNaseema MalikNo ratings yet
- Chapter - 4 - Analytical Chemistry Exercise - 4Document9 pagesChapter - 4 - Analytical Chemistry Exercise - 4parijatbhattacharjee949No ratings yet
- 100L Lecture 4 SaltsDocument6 pages100L Lecture 4 SaltsMichael EhondorNo ratings yet
- Salt PreparationDocument41 pagesSalt Preparationsidsolegend123No ratings yet
- SALTDocument22 pagesSALTparitoshNo ratings yet
- 3E5NA Sci Chem Qualitative Analysis Notes Student'sDocument19 pages3E5NA Sci Chem Qualitative Analysis Notes Student'sAditi Ravi kaushikNo ratings yet
- Tests For Anions and CationsDocument3 pagesTests For Anions and Cationscameron.yeung.08No ratings yet
- Selina Solutions Concise Chemistry For Class 10 Chapter 4Document6 pagesSelina Solutions Concise Chemistry For Class 10 Chapter 4Akash SinghNo ratings yet
- Acid Base and Salts - Part 6-Qualitative AnalysisDocument30 pagesAcid Base and Salts - Part 6-Qualitative AnalysisKronix GamingNo ratings yet
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresFrom EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresRating: 5 out of 5 stars5/5 (1)
- Acumedia Cross ReferenceDocument6 pagesAcumedia Cross ReferenceGRACIELANo ratings yet
- Pre-Lab Expt 3-Salivary Digestion and Factors Affecting Enzyme ActivityDocument2 pagesPre-Lab Expt 3-Salivary Digestion and Factors Affecting Enzyme ActivityMaria Isabella Francesca C. BargayoNo ratings yet
- LRYEQ16AY1Document4 pagesLRYEQ16AY1Hamid BichriNo ratings yet
- 7 0-AlkenesDocument84 pages7 0-AlkenesAj MirandaNo ratings yet
- Technical Data SheetDocument2 pagesTechnical Data Sheetshyamdas38892853No ratings yet
- Construction and Building MaterialsDocument15 pagesConstruction and Building MaterialsZakaria MohdNo ratings yet
- Course-Planner XDocument1 pageCourse-Planner XVikasNo ratings yet
- Baymer Spray 270 E: (Trial Product)Document5 pagesBaymer Spray 270 E: (Trial Product)Teodorerescu DanielNo ratings yet
- Small Volume Parentrals: Dr.Y.Anand KumarDocument25 pagesSmall Volume Parentrals: Dr.Y.Anand Kumarsaloni patelNo ratings yet
- 2018 CNGold Nanoparticlesandtheir Applicationsin Cancer TreatmentDocument19 pages2018 CNGold Nanoparticlesandtheir Applicationsin Cancer TreatmentShinyNo ratings yet
- Sample Calculations Bio-OilDocument8 pagesSample Calculations Bio-OilJames Matthew LimpinNo ratings yet
- A Process Flow Diagram For An Acetone - Acid Acetic Distillation ColumnDocument1 pageA Process Flow Diagram For An Acetone - Acid Acetic Distillation ColumnVinh Lê KhảiNo ratings yet
- Poster 230 The Space Group List Project 230 Dina0 CDocument2 pagesPoster 230 The Space Group List Project 230 Dina0 CrataburguerNo ratings yet
- Vitocrossal 200-cm2 SM gw6b TDMDocument16 pagesVitocrossal 200-cm2 SM gw6b TDMCiprian BalcanNo ratings yet
- HND Polymer Note Part OneDocument46 pagesHND Polymer Note Part OnemuhammadmaihadisiNo ratings yet
- Chem Principles 7e ISM Focus 01 Even FINALDocument26 pagesChem Principles 7e ISM Focus 01 Even FINALSelma MeloNo ratings yet
- 2018 Sec 4 NA Science Chemistry SA2 Unity SecondaryDocument25 pages2018 Sec 4 NA Science Chemistry SA2 Unity SecondaryWinnie TanNo ratings yet
- Epimeya: Ribulose S-Phosprade Isomer YaseDocument1 pageEpimeya: Ribulose S-Phosprade Isomer YaseOmpriya SNo ratings yet
- QP 000803 04Document6 pagesQP 000803 04Ajay RayNo ratings yet
- VSC Operators Manual Method 3 9.17.20Document21 pagesVSC Operators Manual Method 3 9.17.20INGENIERIA Y CONSULTORIA GLOBALNo ratings yet
- Ultra Sanicro35 Datasheet FINAL Digital-1Document6 pagesUltra Sanicro35 Datasheet FINAL Digital-1Vaibhav KaleNo ratings yet
- Yellowing Mechanisms of Epoxy and Vinyl Ester Resins Under Thermal, UV and Natural Aging Conditions and Protection MethodsDocument14 pagesYellowing Mechanisms of Epoxy and Vinyl Ester Resins Under Thermal, UV and Natural Aging Conditions and Protection MethodszrpyhjtzztNo ratings yet
- Strong Acid-Strong Base TitrationsDocument4 pagesStrong Acid-Strong Base TitrationsNigatu MAmoNo ratings yet
- Chimiaadmin,+2021 0027Document6 pagesChimiaadmin,+2021 0027Edgar Blanco AcuñaNo ratings yet
- HTS CatalystDocument16 pagesHTS CatalystMuhammad Junaid100% (1)
- Bioethanol Production From Renewable Raw Materials and Its Separation and Purification - A ReviewDocument38 pagesBioethanol Production From Renewable Raw Materials and Its Separation and Purification - A Reviewnabeelkhaliq323No ratings yet
- PP 1100zc DatasheetDocument2 pagesPP 1100zc Datasheetphanplastic299No ratings yet
- AE Impex DIY Prodects Catalog 1Document16 pagesAE Impex DIY Prodects Catalog 1t bhavanaNo ratings yet
- MSDS PropaneDocument3 pagesMSDS Propaneyogeshmalkar1232002No ratings yet
SALTS
SALTS
Uploaded by
aquamogolwaneOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
SALTS
SALTS
Uploaded by
aquamogolwaneCopyright:
Available Formats
SALTS
A salt is a compound that contains a positive and a negative ion, the negative ion of which comes
from an acid.
N.B; salts are named from the acid in which they are obtained from, e.g. hydrochloric acid forms
chloride, sulphuric acid forms sulphates.
A good number of salts are soluble in water while other salts are insoluble, knowledge of
solubility of salts is important when preparing salts
“ refer to the solubility rules”
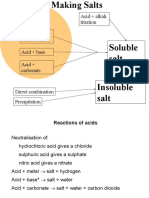
Methods of preparing soluble salts
1. Reaction of an acid with a metal
e.g. Mg(s) + HCl(aq) MgCl2(aq) + H2(g)
2. Reaction of acid with alkali
e.g. HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l)
3. Reaction of an acid with a carbonate
e.g. 2HCl(aq) + CaCO3(s) CaCl(aq) + CO2(g) + H2O(l)
4. Reaction of acid with metal oxide
e.g. 2HCl(aq) + CaO(s) CaCl2(aq) + H2O(l)
General steps of salt preparation
1. Reaction – add metal/carbonate/metal oxide in excess to make sure all the acid is used up
2. Filtration – to remove the excess
3. Heating and Evaporation – to saturate or concentrate the solution
4. Cooling and crystalisation – allow saturated solution to cool and start forming crystals
5. Filter and dry the crystals – filter out any remaining water and dry the crystals.
Example using the reaction of acid + metal oxide;
Preparation of Insoluble salts
Insoluble salts are prepared by a method known as Precipitation.
This involves mixing two solutions of soluble salts. When these are mixed, a reaction takes place
in which the salt is formed as a precipitate ( a solid which is formed in a solution).
Soluble salt + Soluble salt Insoluble salt(precipitate) + soluble salt
Steps
1.Solutions of the two soluble salts are prepared separately
2. the two solutions are then mixed together
3. A precipitate is formed, this is the insoluble salt
4. The precipitate is filtered off is collected as the residue
5. it is washed by pouring plenty of distilled water on ot
6. it is the dried by pressing between filter papers.
E.g
To prepare Barium Sulphate (BaSO4), We need Barium salt which is soluble and sulphate salt
which is soluble
Na2SO4(aq) + BaNO3(aq) BaSO4(s) + NaNO3(aq)
Water of crystallization
It is the water contained in some crystals.
Hydrated crystals- these are crystals which contain water of crystallization e.g. CuSO4.5H2O
Anhydrous crystals- these are crystals that do not contain water of crystallisatio. E.g NaCl
Qualitative Analysis (Test for ions)
It is the analysis of ions from samples using colour of their precipitates and types of gases
produced in a chemical reaction.
FOR CATIONS; Aqueous Sodium Hydroxide and Ammonia solutions are used
Cation Effect of Aqueous Sodium Effect of aqueos Ammonia
Hydroxide
Copper ion (Cu2+) Light blue precipitate, insoluble in Light blue precipitate,
excess soluble in excess giving a
dark blue solution
Iron (ii) ion (Fe2+) Dirty green precipitate, insoluble in Dirty green precipitate,
excess. insoluble in excess
Iron (iii) ion (Fe3+) Red-brown precipitate, insoluble in Red-brown precipitate,
excess. insoluble in excess.
Zinc ion (Zn2+) White precipitate, soluble in excess White precipitate, soluble in
giving a colourless solution excess giving a colourless
solution
Aluminium ion (Al3+) White precipitate, soluble in excess White precipitate, insoluble
giving a colourless solution. in excess
Calcium ion (Ca2+) White precipitate, insoluble in No precipitate
excess
Ammonium ion Ammonia produced on warming _
(NH4+)
• Zn2+, Al3+, Ca2+ all show white precipitate hence excess hydroxide and ammonia solutions
may be used to differentiate them.
FOR ANIONS; specif solutions are used to test for specific ions
Anion Test Test result
Carbonate (CO32-) Add dilute acid Effervescence, Carbon
dioxide gas produced ( turns
limewater milky)
Chloride (Cl-) Acidify with dilute nitric acid White precipitate
(in solution) then add aqueous silver
nitrate
Sulphate (SO42-) Acidify with dilute nitric White precipitate
(in solution) acid, then add aqueous
barium nitrate
Iodide (I-) Acidify with dilute nitric Yellow precipitate
(in solution) acid, then add aqueous lead
(ii) nitrate
Nitrate (NO3-) Add aqueous sodium Ammonia produced
(in solution) hydroxide then aluminium
foil, warm carefully.
Test for gases
Gas Test and test result
Ammonia (NH3) Turns damp red litmus paper blue
Carbon dioxide (CO2) Turns limewater milky
Hydrogen (H2) “pops” with a lighted splint
Oxygen (O2) Relights a glowing splint
Chlorine (Cl2) Bleaches damp litmus paper
Sulphur dioxide (SO2) Turns aqueous potassium dichromate(vi)
green
TITRATION
It is a technique that is used to analyse solutions. A solution of known concentration and volume
can be used to find the volume and concentration of other solutions.
Steps that are followed
-A burette is filled with solution (acid)
-Another solution (alkali) of known concentration is placed in a conical flask using a pipette.
-Two or three drops of a suitable indicator ( e.g. methy orange) are added in to a solution in the
conical flask
-The solution from the burette is run in to the conical flask until the indicator just changes colour
-the steps above are repeated until consistent results are obtained, the average of the volume
used is calculated, it is used to calculate the concentration of the acid.
End point in a titration is the point at which the indicator changes its colour indicating that the
reaction is complete.
Finding Concentration of acid solution
• Step 1; use info on the standard solution
How many moles of alkali are in the flask
• Step 2; use the chemical equation
How many moles of acid are used
• Step 3; use the titration value
What is the concentration of the acid
Example;
You might also like
- Project PPT ShubhamDocument17 pagesProject PPT ShubhamRounak Atram100% (1)
- Chapter 8 - Acids, Bases and SaltsDocument16 pagesChapter 8 - Acids, Bases and Saltsjannat amgadNo ratings yet
- Acids RecallDocument4 pagesAcids RecallAssumpta McguckinNo ratings yet
- Pure Chemistry Qualitative Analysis NotesDocument1 pagePure Chemistry Qualitative Analysis NotesVivienne SeowNo ratings yet
- Inorganic Salt AnalysisDocument9 pagesInorganic Salt Analysiswama ojha100% (1)
- IGCSE ChemistryDocument25 pagesIGCSE ChemistryLiliana DamocNo ratings yet
- ATP Notes For Chemistry o LevelDocument25 pagesATP Notes For Chemistry o LevelSaad Arsalan100% (4)
- 3 Experiment ChemistryDocument30 pages3 Experiment ChemistryThangavel SarujanNo ratings yet
- Qualitative Analysis of A Salt - F - 231128 - 000159Document9 pagesQualitative Analysis of A Salt - F - 231128 - 000159Dhairya VeerNo ratings yet
- Qualitative Analysis of Some IonsDocument42 pagesQualitative Analysis of Some IonsShaina Mae ContilloNo ratings yet
- List of Common Anions (Acidic Radicals) For Salt Analysis: Group Cations (Basic Radicals)Document6 pagesList of Common Anions (Acidic Radicals) For Salt Analysis: Group Cations (Basic Radicals)Dhruv PanditaNo ratings yet
- 2024 - 6092 - Notes of Qualitative AnalysisDocument1 page2024 - 6092 - Notes of Qualitative Analysisaleesya1302No ratings yet
- Ion Test PDFDocument11 pagesIon Test PDFAnderson XiaoNo ratings yet
- Notes Updates SaltsDocument32 pagesNotes Updates SaltsLim Jing YeeNo ratings yet
- 2324 Level M (Gr11 UAE-Gulf) Chemistry Chapter 2 NotesDocument19 pages2324 Level M (Gr11 UAE-Gulf) Chemistry Chapter 2 Notesaminata13536No ratings yet
- Identification of Ions and GasesDocument4 pagesIdentification of Ions and GasesMuqaddas FatimaNo ratings yet
- Inorganic Qualitative AnalysisDocument15 pagesInorganic Qualitative AnalysisKev WattsNo ratings yet
- Notes On SaltsDocument4 pagesNotes On SaltsFelix S100% (1)
- Identifying A Simple Salt: Ion ColorDocument15 pagesIdentifying A Simple Salt: Ion ColorNabindra RuwaliNo ratings yet
- Testing For Ions PDFDocument1 pageTesting For Ions PDFClevxyNo ratings yet
- Testing For Cations: Lesson 11.5Document5 pagesTesting For Cations: Lesson 11.5Helpful HandNo ratings yet
- Test For CationsDocument3 pagesTest For CationsBaggyNo ratings yet
- ESSENTIAL CHEMESTRIY Final 45Document14 pagesESSENTIAL CHEMESTRIY Final 45Syrus ZambiaNo ratings yet
- Form 4 Chemistry - SaltDocument6 pagesForm 4 Chemistry - SaltSze NingNo ratings yet
- Salt Analysis (Theory) - EngDocument28 pagesSalt Analysis (Theory) - Engjoxis70026100% (1)
- Imp. Practical Chem. KnowledgeDocument4 pagesImp. Practical Chem. KnowledgedebanivkashyapNo ratings yet
- C12 Chemical Analysis and InvestigationDocument10 pagesC12 Chemical Analysis and InvestigationSarah PendNo ratings yet
- 0 - Organic and Inorganic Tests For AS PDFDocument8 pages0 - Organic and Inorganic Tests For AS PDFAbed AymanNo ratings yet
- Salts FormationDocument19 pagesSalts FormationUrwa Abdul MannanNo ratings yet
- Qualitative AnalysisDocument5 pagesQualitative AnalysisAlex noslenNo ratings yet
- Salt AnalysisDocument7 pagesSalt AnalysisJaya RNo ratings yet
- Form 4 Chem Chapter 3Document12 pagesForm 4 Chem Chapter 3George LeongNo ratings yet
- Notes Salts (Chemistry)Document32 pagesNotes Salts (Chemistry)Darishana100% (1)
- Chapter 8Document2 pagesChapter 8Luna LatisyaNo ratings yet
- SPM Chemistry Formula List Form4Document14 pagesSPM Chemistry Formula List Form4Heng HoweNo ratings yet
- Part IV Acids and Alkalis: LQ 01 (Answer) Properties of Acids and AlkalisDocument12 pagesPart IV Acids and Alkalis: LQ 01 (Answer) Properties of Acids and AlkalisCharmine HolmesNo ratings yet
- Practicles - Identification of AnionsDocument11 pagesPracticles - Identification of AnionsDebasis SatapathyNo ratings yet
- Salt AnalysisDocument7 pagesSalt Analysisdharun200777No ratings yet
- Chemistry Notes Acids Bases and SaltsDocument7 pagesChemistry Notes Acids Bases and SaltsGouri RajNo ratings yet
- Qualitative Analysis TestsDocument2 pagesQualitative Analysis TestsReshmanraaj SingamNo ratings yet
- 9 Notes For Use in Qualitative Analysis Test For Anions: © UCLES 2017 0620/05/SP/20Document2 pages9 Notes For Use in Qualitative Analysis Test For Anions: © UCLES 2017 0620/05/SP/20Mayur VanjaniNo ratings yet
- Cation Anion TestDocument1 pageCation Anion TestPromit SenguptaNo ratings yet
- Csec Identification of Cations and AnionsDocument6 pagesCsec Identification of Cations and AnionsDarrion BruceNo ratings yet
- Making Salts: Neutralisation ReactionsDocument4 pagesMaking Salts: Neutralisation ReactionsPedro Moreno de SouzaNo ratings yet
- Identification of Cations, Anions and GasesDocument2 pagesIdentification of Cations, Anions and GasesMustufa FerozNo ratings yet
- Salt and SolutionDocument33 pagesSalt and SolutionFarhan Altaf100% (1)
- (A) Physical Properties of Salt: Colourless SolutionDocument4 pages(A) Physical Properties of Salt: Colourless SolutionJacelynNo ratings yet
- Qualitative Analysis NotesDocument1 pageQualitative Analysis NotesNaseema MalikNo ratings yet
- Qualitative Analysis NotesDocument1 pageQualitative Analysis NotesNaseema MalikNo ratings yet
- Chapter - 4 - Analytical Chemistry Exercise - 4Document9 pagesChapter - 4 - Analytical Chemistry Exercise - 4parijatbhattacharjee949No ratings yet
- 100L Lecture 4 SaltsDocument6 pages100L Lecture 4 SaltsMichael EhondorNo ratings yet
- Salt PreparationDocument41 pagesSalt Preparationsidsolegend123No ratings yet
- SALTDocument22 pagesSALTparitoshNo ratings yet
- 3E5NA Sci Chem Qualitative Analysis Notes Student'sDocument19 pages3E5NA Sci Chem Qualitative Analysis Notes Student'sAditi Ravi kaushikNo ratings yet
- Tests For Anions and CationsDocument3 pagesTests For Anions and Cationscameron.yeung.08No ratings yet
- Selina Solutions Concise Chemistry For Class 10 Chapter 4Document6 pagesSelina Solutions Concise Chemistry For Class 10 Chapter 4Akash SinghNo ratings yet
- Acid Base and Salts - Part 6-Qualitative AnalysisDocument30 pagesAcid Base and Salts - Part 6-Qualitative AnalysisKronix GamingNo ratings yet
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresFrom EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresRating: 5 out of 5 stars5/5 (1)
- Acumedia Cross ReferenceDocument6 pagesAcumedia Cross ReferenceGRACIELANo ratings yet
- Pre-Lab Expt 3-Salivary Digestion and Factors Affecting Enzyme ActivityDocument2 pagesPre-Lab Expt 3-Salivary Digestion and Factors Affecting Enzyme ActivityMaria Isabella Francesca C. BargayoNo ratings yet
- LRYEQ16AY1Document4 pagesLRYEQ16AY1Hamid BichriNo ratings yet
- 7 0-AlkenesDocument84 pages7 0-AlkenesAj MirandaNo ratings yet
- Technical Data SheetDocument2 pagesTechnical Data Sheetshyamdas38892853No ratings yet
- Construction and Building MaterialsDocument15 pagesConstruction and Building MaterialsZakaria MohdNo ratings yet
- Course-Planner XDocument1 pageCourse-Planner XVikasNo ratings yet
- Baymer Spray 270 E: (Trial Product)Document5 pagesBaymer Spray 270 E: (Trial Product)Teodorerescu DanielNo ratings yet
- Small Volume Parentrals: Dr.Y.Anand KumarDocument25 pagesSmall Volume Parentrals: Dr.Y.Anand Kumarsaloni patelNo ratings yet
- 2018 CNGold Nanoparticlesandtheir Applicationsin Cancer TreatmentDocument19 pages2018 CNGold Nanoparticlesandtheir Applicationsin Cancer TreatmentShinyNo ratings yet
- Sample Calculations Bio-OilDocument8 pagesSample Calculations Bio-OilJames Matthew LimpinNo ratings yet
- A Process Flow Diagram For An Acetone - Acid Acetic Distillation ColumnDocument1 pageA Process Flow Diagram For An Acetone - Acid Acetic Distillation ColumnVinh Lê KhảiNo ratings yet
- Poster 230 The Space Group List Project 230 Dina0 CDocument2 pagesPoster 230 The Space Group List Project 230 Dina0 CrataburguerNo ratings yet
- Vitocrossal 200-cm2 SM gw6b TDMDocument16 pagesVitocrossal 200-cm2 SM gw6b TDMCiprian BalcanNo ratings yet
- HND Polymer Note Part OneDocument46 pagesHND Polymer Note Part OnemuhammadmaihadisiNo ratings yet
- Chem Principles 7e ISM Focus 01 Even FINALDocument26 pagesChem Principles 7e ISM Focus 01 Even FINALSelma MeloNo ratings yet
- 2018 Sec 4 NA Science Chemistry SA2 Unity SecondaryDocument25 pages2018 Sec 4 NA Science Chemistry SA2 Unity SecondaryWinnie TanNo ratings yet
- Epimeya: Ribulose S-Phosprade Isomer YaseDocument1 pageEpimeya: Ribulose S-Phosprade Isomer YaseOmpriya SNo ratings yet
- QP 000803 04Document6 pagesQP 000803 04Ajay RayNo ratings yet
- VSC Operators Manual Method 3 9.17.20Document21 pagesVSC Operators Manual Method 3 9.17.20INGENIERIA Y CONSULTORIA GLOBALNo ratings yet
- Ultra Sanicro35 Datasheet FINAL Digital-1Document6 pagesUltra Sanicro35 Datasheet FINAL Digital-1Vaibhav KaleNo ratings yet
- Yellowing Mechanisms of Epoxy and Vinyl Ester Resins Under Thermal, UV and Natural Aging Conditions and Protection MethodsDocument14 pagesYellowing Mechanisms of Epoxy and Vinyl Ester Resins Under Thermal, UV and Natural Aging Conditions and Protection MethodszrpyhjtzztNo ratings yet
- Strong Acid-Strong Base TitrationsDocument4 pagesStrong Acid-Strong Base TitrationsNigatu MAmoNo ratings yet
- Chimiaadmin,+2021 0027Document6 pagesChimiaadmin,+2021 0027Edgar Blanco AcuñaNo ratings yet
- HTS CatalystDocument16 pagesHTS CatalystMuhammad Junaid100% (1)
- Bioethanol Production From Renewable Raw Materials and Its Separation and Purification - A ReviewDocument38 pagesBioethanol Production From Renewable Raw Materials and Its Separation and Purification - A Reviewnabeelkhaliq323No ratings yet
- PP 1100zc DatasheetDocument2 pagesPP 1100zc Datasheetphanplastic299No ratings yet
- AE Impex DIY Prodects Catalog 1Document16 pagesAE Impex DIY Prodects Catalog 1t bhavanaNo ratings yet
- MSDS PropaneDocument3 pagesMSDS Propaneyogeshmalkar1232002No ratings yet