Professional Documents
Culture Documents
Overview of Research
Overview of Research
Uploaded by
lorien86Copyright:
Available Formats
You might also like
- Critical Appraisal Tools and Reporting Guidelines For Evidence-Based PracticeDocument10 pagesCritical Appraisal Tools and Reporting Guidelines For Evidence-Based PracticeSITI KURNIAWATINo ratings yet
- Current Challenges in Clinical Trial Patient Recruitment and EnrollmentDocument9 pagesCurrent Challenges in Clinical Trial Patient Recruitment and EnrollmentAgnes KanimozhiNo ratings yet
- h7d) KCD, 5bc$9zm-5Document29 pagesh7d) KCD, 5bc$9zm-5Kristel AnneNo ratings yet
- A Simple Guide To Reading An: - HcspfactsheetDocument2 pagesA Simple Guide To Reading An: - HcspfactsheetRinawati UnissulaNo ratings yet
- A Descriptive Study To Assess The Knowledge Regarding Venipuncture Among Staff Nurses in A Selected Hospital, Lucknow With A View To Develop An Information BookletDocument7 pagesA Descriptive Study To Assess The Knowledge Regarding Venipuncture Among Staff Nurses in A Selected Hospital, Lucknow With A View To Develop An Information BookletEditor IJTSRDNo ratings yet
- Canadian Fundamentals of Nursing - Chapter 6, 11Document9 pagesCanadian Fundamentals of Nursing - Chapter 6, 11elen.myNo ratings yet
- 22 Cilinical Research 5 Quantitative Data Collection and AnalysisDocument7 pages22 Cilinical Research 5 Quantitative Data Collection and Analysishello thereNo ratings yet
- Clinical Data RepositoriesDocument5 pagesClinical Data RepositoriesvivianschuylerNo ratings yet
- The Use of Clinical Trials in Comparative Effectiv PDFDocument9 pagesThe Use of Clinical Trials in Comparative Effectiv PDFSilverio CasillasNo ratings yet
- 2a3 Rki 18 Sela AmaliaDocument14 pages2a3 Rki 18 Sela Amalia28. Sela AmaliaNo ratings yet
- Week 3 Evidence Based NursingDocument4 pagesWeek 3 Evidence Based NursingFrances BañezNo ratings yet
- Factors That Influence Validity (1) : Study Design, Doses and PowerDocument2 pagesFactors That Influence Validity (1) : Study Design, Doses and PowerHabib DawarNo ratings yet
- Observational Research Choosing The Right Research Approach For The Right Question by Louise Parmenter, PHD, QuintilesDocument4 pagesObservational Research Choosing The Right Research Approach For The Right Question by Louise Parmenter, PHD, QuintilesMatthewNo ratings yet
- MEDECIELO MELO - Chapter 4 SummaryDocument12 pagesMEDECIELO MELO - Chapter 4 SummaryMelo MedecieloNo ratings yet
- He Clinical Integrative Puzzle For Teaching and Assessing Clinical Reasoning Preliminary Feasibility, Reliability, and Validity EvidenceDocument7 pagesHe Clinical Integrative Puzzle For Teaching and Assessing Clinical Reasoning Preliminary Feasibility, Reliability, and Validity EvidenceFrederico PóvoaNo ratings yet
- Nuclear Medicine AIDocument8 pagesNuclear Medicine AIdistruckNo ratings yet
- Chapter 59 - Evidence Based Practi - 2016 - Hand and Upper Extremity RehabilitatDocument4 pagesChapter 59 - Evidence Based Practi - 2016 - Hand and Upper Extremity Rehabilitatjessica hongNo ratings yet
- A User's Guide To Research in Palliative Care: Melissa D.A. Carlson, Ph.D. and R. Sean Morrison, M.DDocument6 pagesA User's Guide To Research in Palliative Care: Melissa D.A. Carlson, Ph.D. and R. Sean Morrison, M.DPutri Atthariq IlmiNo ratings yet
- NCM 114 Core Elements of Evidenced Based Gerontological Practice and Violence and Elder Mistreatment Mrs. OrprezaDocument5 pagesNCM 114 Core Elements of Evidenced Based Gerontological Practice and Violence and Elder Mistreatment Mrs. OrprezaRoyce Vincent TizonNo ratings yet
- Patient-Centered Framework For Rehabilitation Research in Outpatient SettingsDocument9 pagesPatient-Centered Framework For Rehabilitation Research in Outpatient SettingscarolciveNo ratings yet
- A Study To Evaluate The Effectiveness of Video Assisted Teaching (VAT) On Knowledge Regarding Stroke Rehabilitation Among The Caregivers of Stroke Patients in Selected Hospitals, Hassan, KarnatakaDocument15 pagesA Study To Evaluate The Effectiveness of Video Assisted Teaching (VAT) On Knowledge Regarding Stroke Rehabilitation Among The Caregivers of Stroke Patients in Selected Hospitals, Hassan, KarnatakaInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Journal Article Critique Nursing 665Document5 pagesJournal Article Critique Nursing 665api-214213767No ratings yet
- Long Finals JurisDocument22 pagesLong Finals JurisJeannezelle Anne Mariz GazaNo ratings yet
- Sample - Critical Review of ArticleDocument11 pagesSample - Critical Review of Articleacademicproffwritter100% (1)
- Medicina Basada en La EvidenciaDocument3 pagesMedicina Basada en La EvidenciaAdriana M Rueda CastellanosNo ratings yet
- Patient Satisfaction While Enrolled in Clinical TrialsDocument13 pagesPatient Satisfaction While Enrolled in Clinical TrialsAlessandra BendoNo ratings yet
- Evidence-Based Medicine: David L. SackettDocument3 pagesEvidence-Based Medicine: David L. SackettNicolas MarinNo ratings yet
- Methodology For Research I.6Document6 pagesMethodology For Research I.6Estilita BazánNo ratings yet
- Drug RDocument8 pagesDrug Rwww.santhoshvjd123No ratings yet
- Drug Recommendation System Based On Sentiment Analysis of Drug Reviews Using Machine LearningDocument8 pagesDrug Recommendation System Based On Sentiment Analysis of Drug Reviews Using Machine LearningtafNo ratings yet
- Locating Credible Databases and ResearchDocument7 pagesLocating Credible Databases and ResearchTrex WritersNo ratings yet
- Page 2012 RESEARCH DESIGNS IN SPORTS PHYSICAL THERAPY PDFDocument11 pagesPage 2012 RESEARCH DESIGNS IN SPORTS PHYSICAL THERAPY PDFValeRichardNo ratings yet
- 2010 The Informationist - Building Evidence For An EmergingDocument10 pages2010 The Informationist - Building Evidence For An EmergingxaviergeorgeNo ratings yet
- Principles of Drug Literature Evaluation For Observational Study DesignsDocument13 pagesPrinciples of Drug Literature Evaluation For Observational Study Designs李姵瑩No ratings yet
- Journal of Clinical Nursing - 2022 - Burke - Factors That Influence Hospital Nurses Escalation of Patient Care in ResponseDocument50 pagesJournal of Clinical Nursing - 2022 - Burke - Factors That Influence Hospital Nurses Escalation of Patient Care in ResponsePaulaNo ratings yet
- Evidence-Based Nursing (Qualitative Research)Document6 pagesEvidence-Based Nursing (Qualitative Research)focus16hoursgmailcom100% (1)
- SJNHC 55 105-106 ewJDdEJDocument2 pagesSJNHC 55 105-106 ewJDdEJhassan mahmoodNo ratings yet
- Clinical Research Study Designs: The EssentialsDocument8 pagesClinical Research Study Designs: The EssentialsKanar MahmoodNo ratings yet
- Ictm111 Week 15Document2 pagesIctm111 Week 15KATHREEN CRYSTALLE SALVADORNo ratings yet
- Measuring A Caring Culture in Hospitals: A Systematic Review of InstrumentsDocument10 pagesMeasuring A Caring Culture in Hospitals: A Systematic Review of InstrumentsWinataria YantiNo ratings yet
- Drug Recommendation SystemDocument7 pagesDrug Recommendation SystemRichard WaniNo ratings yet
- DOCUMENTATIONDocument9 pagesDOCUMENTATIONNashleyah AnayatinNo ratings yet
- Sample Size Calculation in Clinical Trials: MedicineDocument5 pagesSample Size Calculation in Clinical Trials: MedicineKarolina PolskaNo ratings yet
- Statistics V: Introduction To Clinical Trials and Systematic ReviewsDocument4 pagesStatistics V: Introduction To Clinical Trials and Systematic Reviewsnabil mansourNo ratings yet
- Evidence Based MedicineDocument4 pagesEvidence Based MedicineCarlos Antonio Párraga GómezNo ratings yet
- Marco Abuaitah DPDMSDocument21 pagesMarco Abuaitah DPDMSAdnan RafiNo ratings yet
- Persepsi Caring Case-JurnalDocument7 pagesPersepsi Caring Case-JurnalYuni PurwatiNo ratings yet
- Clinical Examination of The ShoulderDocument195 pagesClinical Examination of The ShouldernikithaNo ratings yet
- Research Papers Evidence Based PracticeDocument7 pagesResearch Papers Evidence Based Practicegz3ezhjc100% (1)
- Writing A Clinically Appraised Topic Is A Team SpoDocument2 pagesWriting A Clinically Appraised Topic Is A Team Spo大敏No ratings yet
- Virtual Trials and Real-World Evidence Data CollectionDocument4 pagesVirtual Trials and Real-World Evidence Data CollectionRoopali AggarwalNo ratings yet
- Tyra Jackson Workshop Draft 2 Research PapeDocument13 pagesTyra Jackson Workshop Draft 2 Research Papeapi-457810629No ratings yet
- Scholarly Research Paper - Reagan ToddDocument6 pagesScholarly Research Paper - Reagan Toddapi-735430277No ratings yet
- Diya Sana K N - Research MethodologyDocument22 pagesDiya Sana K N - Research MethodologydiywritesanunknownwriterNo ratings yet
- Evidence-Based Nursing PracticeDocument14 pagesEvidence-Based Nursing PracticeRose TeodosioNo ratings yet
- A Practical Approach To Evidence-Based Dentistry: VIDocument12 pagesA Practical Approach To Evidence-Based Dentistry: VIDiego Pinto PatroniNo ratings yet
- Analisis Kuantitatif PDFDocument10 pagesAnalisis Kuantitatif PDFSiti QamariaNo ratings yet
- MBE Evaluacion de TrabajosDocument6 pagesMBE Evaluacion de TrabajosPaolo StrozziNo ratings yet
- Pi Is 0884217519300061Document2 pagesPi Is 0884217519300061PANAO, BABYLYN T.No ratings yet
- Cavite State University Don Severino de Las Alas CampusDocument7 pagesCavite State University Don Severino de Las Alas CampusCorrine IvyNo ratings yet
- Clinical Research Associate - The Comprehensive Guide: Vanguard ProfessionalsFrom EverandClinical Research Associate - The Comprehensive Guide: Vanguard ProfessionalsNo ratings yet
- Read How To InstallDocument1 pageRead How To Installlorien86No ratings yet
- I9 Acceptable Documents-2Document1 pageI9 Acceptable Documents-2lorien86No ratings yet
- South Carolina Department of Motor Vehicles Response To Insurance NoticeDocument1 pageSouth Carolina Department of Motor Vehicles Response To Insurance Noticelorien86No ratings yet
- Demystifying Buprenorphine Regulations For Pharmacists and CliniciansDocument10 pagesDemystifying Buprenorphine Regulations For Pharmacists and Clinicianslorien86No ratings yet
- 7yefxymwtsdd-RCE0848RSVPanel FinalHandoutforEnduring 7.27.22Document62 pages7yefxymwtsdd-RCE0848RSVPanel FinalHandoutforEnduring 7.27.22lorien86No ratings yet
- Document Medication Waste at The Time of RemovalDocument1 pageDocument Medication Waste at The Time of Removallorien86No ratings yet
- BD Pyxis™ Medstation™ Es: Device SettingsDocument1 pageBD Pyxis™ Medstation™ Es: Device Settingslorien86No ratings yet
- Assign and Load A MedicationDocument2 pagesAssign and Load A Medicationlorien86No ratings yet
- BD Pyxis™ Medstation™ Es: DiscrepanciesDocument1 pageBD Pyxis™ Medstation™ Es: Discrepancieslorien86No ratings yet
- Clinical Data Category (CDC)Document1 pageClinical Data Category (CDC)lorien86No ratings yet
- BD Pyxis™ Medstation™ Es: Block Storage SpaceDocument1 pageBD Pyxis™ Medstation™ Es: Block Storage Spacelorien86No ratings yet
- BD Pyxis™ Medstation™ Es: Destock A MedicationDocument1 pageBD Pyxis™ Medstation™ Es: Destock A Medicationlorien86No ratings yet
- Configure A Storage SpaceDocument4 pagesConfigure A Storage Spacelorien86No ratings yet
- Linzess Package InsertDocument21 pagesLinzess Package Insertlorien86No ratings yet
- Add A Temporary PatientDocument1 pageAdd A Temporary Patientlorien86No ratings yet
- 0PHRM 5202 Course SyllabusDocument7 pages0PHRM 5202 Course Syllabuslorien86No ratings yet
- Susy PPT - Road Map To AccreditationDocument86 pagesSusy PPT - Road Map To AccreditationSUSANTONo ratings yet
- Ehr Poster 1 1Document1 pageEhr Poster 1 1api-542441070No ratings yet
- Telepractica y Fonoaudiologia - Referencias (Retamal-Walter) PDFDocument2 pagesTelepractica y Fonoaudiologia - Referencias (Retamal-Walter) PDFAlejandro QuezadaNo ratings yet
- Kpi Aa Ip 2023Document10 pagesKpi Aa Ip 2023Poppy FitraNo ratings yet
- Cochrane LibraryDocument19 pagesCochrane LibraryBrankaPodgoricaNo ratings yet
- Citation:: Critical Appraisal of A Trial RCT WorksheetDocument4 pagesCitation:: Critical Appraisal of A Trial RCT WorksheetThai CheNo ratings yet
- Hand Hygiene Audit To Study Adherence To Hand Hygiene in A Tertiary Care HospitalDocument4 pagesHand Hygiene Audit To Study Adherence To Hand Hygiene in A Tertiary Care HospitalIJAR JOURNALNo ratings yet
- Stage 1 Meaningful Use - Attestation Worksheet - Core Measures PDFDocument7 pagesStage 1 Meaningful Use - Attestation Worksheet - Core Measures PDFSpit FireNo ratings yet
- June DTRDocument38 pagesJune DTRBrylle FuentesNo ratings yet
- An Interoperable Ehealth Reference Architecture For Primary CareDocument6 pagesAn Interoperable Ehealth Reference Architecture For Primary CareMaleja Camargo Vila100% (1)
- Chapter 15 - Word DocsDocument15 pagesChapter 15 - Word DocsAbigail Ann BinalayNo ratings yet
- Chapter Two EhealthDocument10 pagesChapter Two Ehealthaynu gebreNo ratings yet
- Ffiffl: Office of The Controller of ExaminationsDocument4 pagesFfiffl: Office of The Controller of Examinationssunny rahmanNo ratings yet
- NURS FPX 6030 Assessment 6 Final Project SubmissionDocument11 pagesNURS FPX 6030 Assessment 6 Final Project Submissionzadem5266No ratings yet
- Information Technology and Community HealthDocument18 pagesInformation Technology and Community HealthDj Gwyn MandigmaNo ratings yet
- Model Pengadaan Obat Dengan Metode ABC VEN Di RS X Semarang: Jurnal Manajemen Kesehatan IndonesiaDocument5 pagesModel Pengadaan Obat Dengan Metode ABC VEN Di RS X Semarang: Jurnal Manajemen Kesehatan IndonesiaMumu AFNo ratings yet
- Ni 2 WordDocument2 pagesNi 2 WordLeigh Angelika Dela CruzNo ratings yet
- Interventions To Improve The Appropriate Use ofDocument213 pagesInterventions To Improve The Appropriate Use ofFarmacia Clínica FES ZaragozaNo ratings yet
- NURS FPX 6412 Assessment 2 Presentation To The OrganizationDocument8 pagesNURS FPX 6412 Assessment 2 Presentation To The Organizationzadem5266No ratings yet
- Report Feb 7 - 11Document15 pagesReport Feb 7 - 11birhane gebreegziabiherNo ratings yet
- NURS FPX 6612 Assessment 2 Quality Improvement ProposalDocument4 pagesNURS FPX 6612 Assessment 2 Quality Improvement Proposaljoohnsmith070No ratings yet
- Nurs FPX 4020 Assessment 1 Enhancing Quality and SafetyDocument6 pagesNurs FPX 4020 Assessment 1 Enhancing Quality and Safetyjoohnsmith070No ratings yet
- NHS FPX 6008 Assessment 2 Needs Analysis For ChangeDocument8 pagesNHS FPX 6008 Assessment 2 Needs Analysis For Changejoohnsmith070No ratings yet
- Electronic Health Record (EHR) Work Group (WG) - Attendance: HL7 WG Meeting - May 2019 - Montréal, QCDocument44 pagesElectronic Health Record (EHR) Work Group (WG) - Attendance: HL7 WG Meeting - May 2019 - Montréal, QCSridhar AppasamyNo ratings yet
- NURS FPX 6416 Assessment 2 Technology Needs Assessment Summary and Implementation PlanDocument4 pagesNURS FPX 6416 Assessment 2 Technology Needs Assessment Summary and Implementation PlanEmma WatsonNo ratings yet
- JBI Levels of Evidence 2014 0Document5 pagesJBI Levels of Evidence 2014 0Rita MarquesNo ratings yet
- Nursing Informatics: "In Data We Trust"Document18 pagesNursing Informatics: "In Data We Trust"Melissa Marie Custodio100% (1)
- NURS FPX 6612 Assessment 1 Triple Aim Outcome MeasuresDocument8 pagesNURS FPX 6612 Assessment 1 Triple Aim Outcome MeasuresEmma WatsonNo ratings yet
- Pharmacy College ListDocument6 pagesPharmacy College ListHNo ratings yet
Overview of Research
Overview of Research
Uploaded by
lorien86Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Overview of Research
Overview of Research
Uploaded by
lorien86Copyright:
Available Formats
OVERVIEW OF RESEARCH
Epic integrates clinical care and research to improve the patient experience, optimize patient care, and
enhance operational research efficiency. Clinicians’ documentation in your system creates a rich data set
to support research, discovery, and innovation.
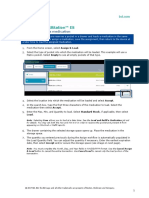
Explore Study Feasibility Collect Patient-Reported Outcomes
SlicerDicer enables researchers to explore Patients can answer questionnaires for a
the feasibility of a study. They can apply specific research study in MyChart.
study-specific criteria to your entire patient
population to explore characteristics of a Review Contraindicated Medications
potential study population. When the study team is working in a patient
encounter, they can see any of the patient's
Empower Patients current medications that are
In MyChart, patients can indicate their contraindicated for a research study.
research-related preferences, such as areas
Inform Clinical Staff
of research they might be interested in. They
Non-research staff can see an indicator on
can also review details about studies they’ve
the patient's chart with information about
participated in or might be eligible for.
research studies the patient is participating
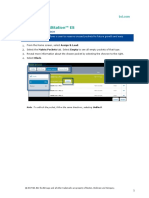
Keep Research Studies Organized in, allowing them to follow up with
Research study administrative records help questions or concerns about the patient's
study teams stay organized. Information participation.
such as the study status, NCT number, Record Adverse Events
ethics board approval number, and key staff Study teams can document and track
members can be found all in one place. adverse events for their study patients.
Recruit Participants Capture Study-Specific Data
Point of care recruitment notifications let Create forms to collect supplemental study-
clinicians know when a patient they’re specific data. Staff can complete forms
seeing is a potential candidate for a study. multiple times, allowing you to track
Patients can also receive study participation changes over time.
opportunities through MyChart. Support External Monitors
Coordinators are automatically notified if EpicCare Link enables external monitors to
the patient is interested. access a read-only chart to review source
data in Epic for specific patients associated
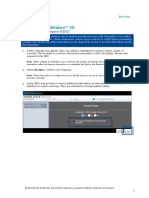
Collect Research Consents with the study they are monitoring.
Coordinators can use e-signatures to
capture a patient’s informed consent for a Stay Research Billing Compliant
study. Consent can be captured either Adjudicate research-related charges and
electronically through MyChart or Welcome, automate NCT number, condition codes,
or by uploading a signed paper form. and appropriate modifiers onto the claim to
reduce the manual per-patient effort
Track Participant Accrual Progress required to ensure compliant billing.
Study teams can monitor their progress
Access Community-Wide Data
towards participant accrual targets and
Expand your research and discovery by
timelines on a dashboard.
using Cosmos to explore EHR data on
EpicUUID: 1EF8D95C-BEC6-47A4-BFFC-B8471C4AEC55
hundreds of millions of patients.
© 2024 Epic Systems Corporation Last Updated: January 26, 2024
You might also like
- Critical Appraisal Tools and Reporting Guidelines For Evidence-Based PracticeDocument10 pagesCritical Appraisal Tools and Reporting Guidelines For Evidence-Based PracticeSITI KURNIAWATINo ratings yet
- Current Challenges in Clinical Trial Patient Recruitment and EnrollmentDocument9 pagesCurrent Challenges in Clinical Trial Patient Recruitment and EnrollmentAgnes KanimozhiNo ratings yet
- h7d) KCD, 5bc$9zm-5Document29 pagesh7d) KCD, 5bc$9zm-5Kristel AnneNo ratings yet
- A Simple Guide To Reading An: - HcspfactsheetDocument2 pagesA Simple Guide To Reading An: - HcspfactsheetRinawati UnissulaNo ratings yet
- A Descriptive Study To Assess The Knowledge Regarding Venipuncture Among Staff Nurses in A Selected Hospital, Lucknow With A View To Develop An Information BookletDocument7 pagesA Descriptive Study To Assess The Knowledge Regarding Venipuncture Among Staff Nurses in A Selected Hospital, Lucknow With A View To Develop An Information BookletEditor IJTSRDNo ratings yet
- Canadian Fundamentals of Nursing - Chapter 6, 11Document9 pagesCanadian Fundamentals of Nursing - Chapter 6, 11elen.myNo ratings yet
- 22 Cilinical Research 5 Quantitative Data Collection and AnalysisDocument7 pages22 Cilinical Research 5 Quantitative Data Collection and Analysishello thereNo ratings yet
- Clinical Data RepositoriesDocument5 pagesClinical Data RepositoriesvivianschuylerNo ratings yet
- The Use of Clinical Trials in Comparative Effectiv PDFDocument9 pagesThe Use of Clinical Trials in Comparative Effectiv PDFSilverio CasillasNo ratings yet
- 2a3 Rki 18 Sela AmaliaDocument14 pages2a3 Rki 18 Sela Amalia28. Sela AmaliaNo ratings yet
- Week 3 Evidence Based NursingDocument4 pagesWeek 3 Evidence Based NursingFrances BañezNo ratings yet
- Factors That Influence Validity (1) : Study Design, Doses and PowerDocument2 pagesFactors That Influence Validity (1) : Study Design, Doses and PowerHabib DawarNo ratings yet
- Observational Research Choosing The Right Research Approach For The Right Question by Louise Parmenter, PHD, QuintilesDocument4 pagesObservational Research Choosing The Right Research Approach For The Right Question by Louise Parmenter, PHD, QuintilesMatthewNo ratings yet
- MEDECIELO MELO - Chapter 4 SummaryDocument12 pagesMEDECIELO MELO - Chapter 4 SummaryMelo MedecieloNo ratings yet
- He Clinical Integrative Puzzle For Teaching and Assessing Clinical Reasoning Preliminary Feasibility, Reliability, and Validity EvidenceDocument7 pagesHe Clinical Integrative Puzzle For Teaching and Assessing Clinical Reasoning Preliminary Feasibility, Reliability, and Validity EvidenceFrederico PóvoaNo ratings yet
- Nuclear Medicine AIDocument8 pagesNuclear Medicine AIdistruckNo ratings yet
- Chapter 59 - Evidence Based Practi - 2016 - Hand and Upper Extremity RehabilitatDocument4 pagesChapter 59 - Evidence Based Practi - 2016 - Hand and Upper Extremity Rehabilitatjessica hongNo ratings yet
- A User's Guide To Research in Palliative Care: Melissa D.A. Carlson, Ph.D. and R. Sean Morrison, M.DDocument6 pagesA User's Guide To Research in Palliative Care: Melissa D.A. Carlson, Ph.D. and R. Sean Morrison, M.DPutri Atthariq IlmiNo ratings yet
- NCM 114 Core Elements of Evidenced Based Gerontological Practice and Violence and Elder Mistreatment Mrs. OrprezaDocument5 pagesNCM 114 Core Elements of Evidenced Based Gerontological Practice and Violence and Elder Mistreatment Mrs. OrprezaRoyce Vincent TizonNo ratings yet
- Patient-Centered Framework For Rehabilitation Research in Outpatient SettingsDocument9 pagesPatient-Centered Framework For Rehabilitation Research in Outpatient SettingscarolciveNo ratings yet
- A Study To Evaluate The Effectiveness of Video Assisted Teaching (VAT) On Knowledge Regarding Stroke Rehabilitation Among The Caregivers of Stroke Patients in Selected Hospitals, Hassan, KarnatakaDocument15 pagesA Study To Evaluate The Effectiveness of Video Assisted Teaching (VAT) On Knowledge Regarding Stroke Rehabilitation Among The Caregivers of Stroke Patients in Selected Hospitals, Hassan, KarnatakaInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Journal Article Critique Nursing 665Document5 pagesJournal Article Critique Nursing 665api-214213767No ratings yet
- Long Finals JurisDocument22 pagesLong Finals JurisJeannezelle Anne Mariz GazaNo ratings yet
- Sample - Critical Review of ArticleDocument11 pagesSample - Critical Review of Articleacademicproffwritter100% (1)
- Medicina Basada en La EvidenciaDocument3 pagesMedicina Basada en La EvidenciaAdriana M Rueda CastellanosNo ratings yet
- Patient Satisfaction While Enrolled in Clinical TrialsDocument13 pagesPatient Satisfaction While Enrolled in Clinical TrialsAlessandra BendoNo ratings yet
- Evidence-Based Medicine: David L. SackettDocument3 pagesEvidence-Based Medicine: David L. SackettNicolas MarinNo ratings yet
- Methodology For Research I.6Document6 pagesMethodology For Research I.6Estilita BazánNo ratings yet
- Drug RDocument8 pagesDrug Rwww.santhoshvjd123No ratings yet
- Drug Recommendation System Based On Sentiment Analysis of Drug Reviews Using Machine LearningDocument8 pagesDrug Recommendation System Based On Sentiment Analysis of Drug Reviews Using Machine LearningtafNo ratings yet
- Locating Credible Databases and ResearchDocument7 pagesLocating Credible Databases and ResearchTrex WritersNo ratings yet
- Page 2012 RESEARCH DESIGNS IN SPORTS PHYSICAL THERAPY PDFDocument11 pagesPage 2012 RESEARCH DESIGNS IN SPORTS PHYSICAL THERAPY PDFValeRichardNo ratings yet
- 2010 The Informationist - Building Evidence For An EmergingDocument10 pages2010 The Informationist - Building Evidence For An EmergingxaviergeorgeNo ratings yet
- Principles of Drug Literature Evaluation For Observational Study DesignsDocument13 pagesPrinciples of Drug Literature Evaluation For Observational Study Designs李姵瑩No ratings yet
- Journal of Clinical Nursing - 2022 - Burke - Factors That Influence Hospital Nurses Escalation of Patient Care in ResponseDocument50 pagesJournal of Clinical Nursing - 2022 - Burke - Factors That Influence Hospital Nurses Escalation of Patient Care in ResponsePaulaNo ratings yet
- Evidence-Based Nursing (Qualitative Research)Document6 pagesEvidence-Based Nursing (Qualitative Research)focus16hoursgmailcom100% (1)
- SJNHC 55 105-106 ewJDdEJDocument2 pagesSJNHC 55 105-106 ewJDdEJhassan mahmoodNo ratings yet
- Clinical Research Study Designs: The EssentialsDocument8 pagesClinical Research Study Designs: The EssentialsKanar MahmoodNo ratings yet
- Ictm111 Week 15Document2 pagesIctm111 Week 15KATHREEN CRYSTALLE SALVADORNo ratings yet
- Measuring A Caring Culture in Hospitals: A Systematic Review of InstrumentsDocument10 pagesMeasuring A Caring Culture in Hospitals: A Systematic Review of InstrumentsWinataria YantiNo ratings yet
- Drug Recommendation SystemDocument7 pagesDrug Recommendation SystemRichard WaniNo ratings yet
- DOCUMENTATIONDocument9 pagesDOCUMENTATIONNashleyah AnayatinNo ratings yet
- Sample Size Calculation in Clinical Trials: MedicineDocument5 pagesSample Size Calculation in Clinical Trials: MedicineKarolina PolskaNo ratings yet
- Statistics V: Introduction To Clinical Trials and Systematic ReviewsDocument4 pagesStatistics V: Introduction To Clinical Trials and Systematic Reviewsnabil mansourNo ratings yet
- Evidence Based MedicineDocument4 pagesEvidence Based MedicineCarlos Antonio Párraga GómezNo ratings yet
- Marco Abuaitah DPDMSDocument21 pagesMarco Abuaitah DPDMSAdnan RafiNo ratings yet
- Persepsi Caring Case-JurnalDocument7 pagesPersepsi Caring Case-JurnalYuni PurwatiNo ratings yet
- Clinical Examination of The ShoulderDocument195 pagesClinical Examination of The ShouldernikithaNo ratings yet
- Research Papers Evidence Based PracticeDocument7 pagesResearch Papers Evidence Based Practicegz3ezhjc100% (1)
- Writing A Clinically Appraised Topic Is A Team SpoDocument2 pagesWriting A Clinically Appraised Topic Is A Team Spo大敏No ratings yet
- Virtual Trials and Real-World Evidence Data CollectionDocument4 pagesVirtual Trials and Real-World Evidence Data CollectionRoopali AggarwalNo ratings yet
- Tyra Jackson Workshop Draft 2 Research PapeDocument13 pagesTyra Jackson Workshop Draft 2 Research Papeapi-457810629No ratings yet
- Scholarly Research Paper - Reagan ToddDocument6 pagesScholarly Research Paper - Reagan Toddapi-735430277No ratings yet
- Diya Sana K N - Research MethodologyDocument22 pagesDiya Sana K N - Research MethodologydiywritesanunknownwriterNo ratings yet
- Evidence-Based Nursing PracticeDocument14 pagesEvidence-Based Nursing PracticeRose TeodosioNo ratings yet
- A Practical Approach To Evidence-Based Dentistry: VIDocument12 pagesA Practical Approach To Evidence-Based Dentistry: VIDiego Pinto PatroniNo ratings yet
- Analisis Kuantitatif PDFDocument10 pagesAnalisis Kuantitatif PDFSiti QamariaNo ratings yet
- MBE Evaluacion de TrabajosDocument6 pagesMBE Evaluacion de TrabajosPaolo StrozziNo ratings yet
- Pi Is 0884217519300061Document2 pagesPi Is 0884217519300061PANAO, BABYLYN T.No ratings yet
- Cavite State University Don Severino de Las Alas CampusDocument7 pagesCavite State University Don Severino de Las Alas CampusCorrine IvyNo ratings yet
- Clinical Research Associate - The Comprehensive Guide: Vanguard ProfessionalsFrom EverandClinical Research Associate - The Comprehensive Guide: Vanguard ProfessionalsNo ratings yet
- Read How To InstallDocument1 pageRead How To Installlorien86No ratings yet
- I9 Acceptable Documents-2Document1 pageI9 Acceptable Documents-2lorien86No ratings yet
- South Carolina Department of Motor Vehicles Response To Insurance NoticeDocument1 pageSouth Carolina Department of Motor Vehicles Response To Insurance Noticelorien86No ratings yet
- Demystifying Buprenorphine Regulations For Pharmacists and CliniciansDocument10 pagesDemystifying Buprenorphine Regulations For Pharmacists and Clinicianslorien86No ratings yet
- 7yefxymwtsdd-RCE0848RSVPanel FinalHandoutforEnduring 7.27.22Document62 pages7yefxymwtsdd-RCE0848RSVPanel FinalHandoutforEnduring 7.27.22lorien86No ratings yet
- Document Medication Waste at The Time of RemovalDocument1 pageDocument Medication Waste at The Time of Removallorien86No ratings yet
- BD Pyxis™ Medstation™ Es: Device SettingsDocument1 pageBD Pyxis™ Medstation™ Es: Device Settingslorien86No ratings yet
- Assign and Load A MedicationDocument2 pagesAssign and Load A Medicationlorien86No ratings yet
- BD Pyxis™ Medstation™ Es: DiscrepanciesDocument1 pageBD Pyxis™ Medstation™ Es: Discrepancieslorien86No ratings yet
- Clinical Data Category (CDC)Document1 pageClinical Data Category (CDC)lorien86No ratings yet
- BD Pyxis™ Medstation™ Es: Block Storage SpaceDocument1 pageBD Pyxis™ Medstation™ Es: Block Storage Spacelorien86No ratings yet
- BD Pyxis™ Medstation™ Es: Destock A MedicationDocument1 pageBD Pyxis™ Medstation™ Es: Destock A Medicationlorien86No ratings yet
- Configure A Storage SpaceDocument4 pagesConfigure A Storage Spacelorien86No ratings yet
- Linzess Package InsertDocument21 pagesLinzess Package Insertlorien86No ratings yet
- Add A Temporary PatientDocument1 pageAdd A Temporary Patientlorien86No ratings yet
- 0PHRM 5202 Course SyllabusDocument7 pages0PHRM 5202 Course Syllabuslorien86No ratings yet
- Susy PPT - Road Map To AccreditationDocument86 pagesSusy PPT - Road Map To AccreditationSUSANTONo ratings yet
- Ehr Poster 1 1Document1 pageEhr Poster 1 1api-542441070No ratings yet
- Telepractica y Fonoaudiologia - Referencias (Retamal-Walter) PDFDocument2 pagesTelepractica y Fonoaudiologia - Referencias (Retamal-Walter) PDFAlejandro QuezadaNo ratings yet
- Kpi Aa Ip 2023Document10 pagesKpi Aa Ip 2023Poppy FitraNo ratings yet
- Cochrane LibraryDocument19 pagesCochrane LibraryBrankaPodgoricaNo ratings yet
- Citation:: Critical Appraisal of A Trial RCT WorksheetDocument4 pagesCitation:: Critical Appraisal of A Trial RCT WorksheetThai CheNo ratings yet
- Hand Hygiene Audit To Study Adherence To Hand Hygiene in A Tertiary Care HospitalDocument4 pagesHand Hygiene Audit To Study Adherence To Hand Hygiene in A Tertiary Care HospitalIJAR JOURNALNo ratings yet
- Stage 1 Meaningful Use - Attestation Worksheet - Core Measures PDFDocument7 pagesStage 1 Meaningful Use - Attestation Worksheet - Core Measures PDFSpit FireNo ratings yet
- June DTRDocument38 pagesJune DTRBrylle FuentesNo ratings yet
- An Interoperable Ehealth Reference Architecture For Primary CareDocument6 pagesAn Interoperable Ehealth Reference Architecture For Primary CareMaleja Camargo Vila100% (1)
- Chapter 15 - Word DocsDocument15 pagesChapter 15 - Word DocsAbigail Ann BinalayNo ratings yet
- Chapter Two EhealthDocument10 pagesChapter Two Ehealthaynu gebreNo ratings yet
- Ffiffl: Office of The Controller of ExaminationsDocument4 pagesFfiffl: Office of The Controller of Examinationssunny rahmanNo ratings yet
- NURS FPX 6030 Assessment 6 Final Project SubmissionDocument11 pagesNURS FPX 6030 Assessment 6 Final Project Submissionzadem5266No ratings yet
- Information Technology and Community HealthDocument18 pagesInformation Technology and Community HealthDj Gwyn MandigmaNo ratings yet
- Model Pengadaan Obat Dengan Metode ABC VEN Di RS X Semarang: Jurnal Manajemen Kesehatan IndonesiaDocument5 pagesModel Pengadaan Obat Dengan Metode ABC VEN Di RS X Semarang: Jurnal Manajemen Kesehatan IndonesiaMumu AFNo ratings yet
- Ni 2 WordDocument2 pagesNi 2 WordLeigh Angelika Dela CruzNo ratings yet
- Interventions To Improve The Appropriate Use ofDocument213 pagesInterventions To Improve The Appropriate Use ofFarmacia Clínica FES ZaragozaNo ratings yet
- NURS FPX 6412 Assessment 2 Presentation To The OrganizationDocument8 pagesNURS FPX 6412 Assessment 2 Presentation To The Organizationzadem5266No ratings yet
- Report Feb 7 - 11Document15 pagesReport Feb 7 - 11birhane gebreegziabiherNo ratings yet
- NURS FPX 6612 Assessment 2 Quality Improvement ProposalDocument4 pagesNURS FPX 6612 Assessment 2 Quality Improvement Proposaljoohnsmith070No ratings yet
- Nurs FPX 4020 Assessment 1 Enhancing Quality and SafetyDocument6 pagesNurs FPX 4020 Assessment 1 Enhancing Quality and Safetyjoohnsmith070No ratings yet
- NHS FPX 6008 Assessment 2 Needs Analysis For ChangeDocument8 pagesNHS FPX 6008 Assessment 2 Needs Analysis For Changejoohnsmith070No ratings yet
- Electronic Health Record (EHR) Work Group (WG) - Attendance: HL7 WG Meeting - May 2019 - Montréal, QCDocument44 pagesElectronic Health Record (EHR) Work Group (WG) - Attendance: HL7 WG Meeting - May 2019 - Montréal, QCSridhar AppasamyNo ratings yet
- NURS FPX 6416 Assessment 2 Technology Needs Assessment Summary and Implementation PlanDocument4 pagesNURS FPX 6416 Assessment 2 Technology Needs Assessment Summary and Implementation PlanEmma WatsonNo ratings yet
- JBI Levels of Evidence 2014 0Document5 pagesJBI Levels of Evidence 2014 0Rita MarquesNo ratings yet
- Nursing Informatics: "In Data We Trust"Document18 pagesNursing Informatics: "In Data We Trust"Melissa Marie Custodio100% (1)
- NURS FPX 6612 Assessment 1 Triple Aim Outcome MeasuresDocument8 pagesNURS FPX 6612 Assessment 1 Triple Aim Outcome MeasuresEmma WatsonNo ratings yet
- Pharmacy College ListDocument6 pagesPharmacy College ListHNo ratings yet