Professional Documents
Culture Documents
Di-Phenyl Methane
Di-Phenyl Methane
Uploaded by
nurjahan.shaheen.78Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Di-Phenyl Methane
Di-Phenyl Methane
Uploaded by
nurjahan.shaheen.78Copyright:
Available Formats
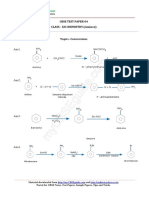
UNIT- IV
DI-PHENYLMETHANE
Synthesis:
1. By Friedel crafts synthesis:
CH2Cl
AlCl3 H2
+ C
Benyl chloride Benzene Diphenyl methane
2 AlCl3 H2
+ CH2Cl2 C
Dichloromethane
Benzene Diphenyl methane
2. From benzophenone:
O
HI/P H2
C heat C
Benzophenone Diphenyl methane
Chemical reactions:
1. Oxidation:
O
H2 K2Cr2O7
C H2SO4 C
Diphenyl methane Benzophenone
2. Reduction:
H2 red-hot
C
Diphenyl methane Fluorene
3. Nitration:
H2 HNO3 H2
C H2SO4 C NO2
Diphenyl methane Nitro-diphenyl methane
4. Bromination:
Br
H2 Br2
C CH
Diphenyl methane Diphenyl methyl bromide
Structure:
1. It is a solid polycyclic aromatic hydrocarbon (PAH) compound.
2. It is a white solid
3. It is consisting of two isolated benzene rings.
4. Molecular of formula C13H12
5. Molecular weight 168.234 gm/mole
6. IUPAC name 1,1'-Methylenedibenzene
7. Hybridization of C is sp2 & sp3
Medicinal Uses:
1. Used as antispasmodic
2. Used as antiulcer agent
3. Used as antihistamine
4. Used as antianxiety
5. Used as synthetic steroid
Derivatives of diphenylmethane:
O OH
C CH
Benzophenone Benzhydrol
You might also like
- Physical Science p2 Gr12 LAST PUSHDocument119 pagesPhysical Science p2 Gr12 LAST PUSHZwavhudi100% (2)
- Organic Reactions Worksheet AnswersDocument5 pagesOrganic Reactions Worksheet Answersdrix lou100% (1)
- Experiment 9 Alkenes From Alcohols Analysis of A Mixture by Gas ChromatographyDocument8 pagesExperiment 9 Alkenes From Alcohols Analysis of A Mixture by Gas ChromatographyBbbbb100% (1)
- Sion CalculationDocument387 pagesSion CalculationHena AgrawalNo ratings yet
- Poly Nuclear FinalDocument118 pagesPoly Nuclear Finaljasmeet ghumanNo ratings yet
- BenzeneDocument39 pagesBenzenesar34ws100% (1)
- Polynuclear CompoundsDocument102 pagesPolynuclear CompoundsFrancis Adu-marfoNo ratings yet
- Chapter 17 - Organic Chemistry PDFDocument18 pagesChapter 17 - Organic Chemistry PDFAarush SharmaNo ratings yet
- Set 4 PSPM Dk024Document7 pagesSet 4 PSPM Dk024anis fazilaNo ratings yet
- Heter 0Document22 pagesHeter 0Lot AdewumilotNo ratings yet
- Triphenyl MethaneDocument9 pagesTriphenyl MethaneHanan AliNo ratings yet
- 12th Chemistry Quick ReferenceDocument7 pages12th Chemistry Quick ReferenceSPCET.FY.24No ratings yet
- CHAPTER 6.0 BENZENE AND ITS DERIVATIVESDocument12 pagesCHAPTER 6.0 BENZENE AND ITS DERIVATIVESSuhaila ArzimiNo ratings yet
- Set 2 PSPM Dk024Document7 pagesSet 2 PSPM Dk024anis fazilaNo ratings yet
- NOMENCLATURE AROMATIC COMPOUNDS (Answer)Document4 pagesNOMENCLATURE AROMATIC COMPOUNDS (Answer)Nurain azmanNo ratings yet
- Aromatic HydrocarbonsDocument7 pagesAromatic HydrocarbonskailashNo ratings yet
- Arene: Chimie Organica Anul I/ FSIA/Rodica DinicaDocument22 pagesArene: Chimie Organica Anul I/ FSIA/Rodica DinicaAlexandra Ioan100% (1)
- OC04 Arenes Exercise AnswersDocument18 pagesOC04 Arenes Exercise Answersjavierheng314No ratings yet
- Carbonyl Compounds NotesDocument5 pagesCarbonyl Compounds NotesCBIT CIVIL A1No ratings yet
- M7 - Check-In Activity 1Document1 pageM7 - Check-In Activity 1Nathaniel ComboNo ratings yet
- Addition Reactions of AlkenesDocument18 pagesAddition Reactions of AlkenesPinaNo ratings yet
- 009 ????? Amines and Diazonium Salts DPP 03 Yakeen 20 2023Document3 pages009 ????? Amines and Diazonium Salts DPP 03 Yakeen 20 2023iampriyatiwarii890No ratings yet
- Chart 2-1 - 231222 - 164319Document1 pageChart 2-1 - 231222 - 164319mahatosandip1888No ratings yet
- Organic Compounds Containing NitrogentDocument8 pagesOrganic Compounds Containing NitrogentMonjurul LaskarNo ratings yet
- Reactions of Hydrocarbon NotesDocument3 pagesReactions of Hydrocarbon NotesAngelika KrisNo ratings yet
- 23 - Nitrogen Compounds CORNELLDocument9 pages23 - Nitrogen Compounds CORNELLGeorge SolomouNo ratings yet
- Desconexión de Grupo Parte 2Document29 pagesDesconexión de Grupo Parte 2Johanna GalanNo ratings yet
- Phenanthrene 1Document24 pagesPhenanthrene 1Basanta Rajkhowa100% (1)
- More Exam Review solutions (2)Document8 pagesMore Exam Review solutions (2)ututoringunion1No ratings yet
- TM 2 Fundamentals of Organic Chemistry 7th Edition by John McMurryDocument15 pagesTM 2 Fundamentals of Organic Chemistry 7th Edition by John McMurrysukma AsaNo ratings yet
- Addition Reactions of Alkenes (1) Mark PaulDocument32 pagesAddition Reactions of Alkenes (1) Mark PaulMark Paul Lipata BenitezNo ratings yet
- Benzene and Its Derivatives: NAME: - GROUPDocument3 pagesBenzene and Its Derivatives: NAME: - GROUPHaiyi GohNo ratings yet
- POC II Unit 1Document29 pagesPOC II Unit 1adapasivagangaNo ratings yet
- Cyclisations - Solutions 2013Document7 pagesCyclisations - Solutions 2013Ngọc HảiNo ratings yet
- Problemas QuímicaDocument18 pagesProblemas Químicamarizaperu16No ratings yet
- Cbse Test Paper-04 Class - Xii Chemistry (Amines) : NH NhcochDocument3 pagesCbse Test Paper-04 Class - Xii Chemistry (Amines) : NH NhcochShreyash KolekarNo ratings yet
- OCI Lecture6-7Document18 pagesOCI Lecture6-7Baga DagaNo ratings yet
- AlkenesDocument27 pagesAlkenesWasajja NajibNo ratings yet
- Bài Tập Chuỗi Chuyển Hóa Hữu Cơ 2Document10 pagesBài Tập Chuỗi Chuyển Hóa Hữu Cơ 2A4K74 HUP100% (1)
- CBSE Class 12 Chemistry Chapter 13 - Amines Important Questions 2023-24Document18 pagesCBSE Class 12 Chemistry Chapter 13 - Amines Important Questions 2023-24Afzal MohamedNo ratings yet
- 0910 4 Abs PDFDocument9 pages0910 4 Abs PDFLAURA LUC�A ATENCIA CASTILLONo ratings yet
- 0910 4 AbsDocument9 pages0910 4 AbsEngr Muhammad AqibNo ratings yet
- 21 (H2) Nitro Cpds (QNS)Document26 pages21 (H2) Nitro Cpds (QNS)Amelia WongNo ratings yet
- Aromatic CompoundsDocument26 pagesAromatic CompoundsnathasyaNo ratings yet
- Topic 3: Major Bulk Organic Major Bulk Organic Chemicals From PropyleneDocument9 pagesTopic 3: Major Bulk Organic Major Bulk Organic Chemicals From PropyleneYong LiNo ratings yet
- Addition Reactions of AlkenesDocument32 pagesAddition Reactions of AlkenesLalitha Kurumanghat100% (1)
- Medicinal Chemistry-Ii: 1.anti-Infective Agents: FDocument14 pagesMedicinal Chemistry-Ii: 1.anti-Infective Agents: FAnonymous ionOPaqlkNo ratings yet
- Exercise C7 - Ans SchemeDocument3 pagesExercise C7 - Ans Schemeknn233610437No ratings yet
- 1227 AppDDocument7 pages1227 AppDAlfredo BarcenasNo ratings yet
- Polunuclear Hydrocarbon: Napthalene: As Per PCI Curriculum Pharmaceutical Chemistry-II Second Year B. Pharmacy (Sem-III)Document22 pagesPolunuclear Hydrocarbon: Napthalene: As Per PCI Curriculum Pharmaceutical Chemistry-II Second Year B. Pharmacy (Sem-III)Ronak ModiNo ratings yet
- 10 Halogen Derivatives of Alkanes and ArenesDocument3 pages10 Halogen Derivatives of Alkanes and Arenesfedbit2020No ratings yet
- STUDY164@126162Document5 pagesSTUDY164@126162Dr-Muhammad YaseenNo ratings yet
- Organic Chemistry Mock Exam (ANSWER KEY)Document7 pagesOrganic Chemistry Mock Exam (ANSWER KEY)k.talle039No ratings yet
- Lab 09 A Reduction Reaction - Benzil To HydrobenzoinDocument7 pagesLab 09 A Reduction Reaction - Benzil To HydrobenzoinkrlinzNo ratings yet
- Case Based Question AminesDocument4 pagesCase Based Question AmineshaddinjohnjNo ratings yet
- Unit-12 Carbonyl Compounds 2023Document20 pagesUnit-12 Carbonyl Compounds 2023jagannathanNo ratings yet
- Sample DesignDocument2 pagesSample DesignAbe AlagNo ratings yet
- Research Article: Synthesis and Analgesic Activity of Novel Derivatives of 1,2-Substituted BenzimidazolesDocument7 pagesResearch Article: Synthesis and Analgesic Activity of Novel Derivatives of 1,2-Substituted BenzimidazolesRahul B SNo ratings yet
- OC04 Arenes Tutorial AnswersDocument21 pagesOC04 Arenes Tutorial Answersjavierheng314No ratings yet
- EAMCET QR Chemistry SR Chem 17.organic Chemistry Nitrogen Containing CompoundsDocument11 pagesEAMCET QR Chemistry SR Chem 17.organic Chemistry Nitrogen Containing CompoundsJagadeesh GoliNo ratings yet
- Mccombie, Stacey: SaundersDocument3 pagesMccombie, Stacey: SaundersLuciano PaoloNo ratings yet
- Chemistry With COCl2Document17 pagesChemistry With COCl2johann69009No ratings yet
- Named ReactionsDocument19 pagesNamed Reactionsscicws1133No ratings yet
- Alkenes and AlkynesDocument39 pagesAlkenes and Alkynesrajeevbansal1807No ratings yet
- PolymersDocument9 pagesPolymersChhavi SharmaNo ratings yet
- Chemistry: Worksheet - 53 (Lecture-01) Topic: Haloalkanes & HaloarenesDocument5 pagesChemistry: Worksheet - 53 (Lecture-01) Topic: Haloalkanes & Haloarenesakhil sNo ratings yet
- Test - D18 Dec 2022Document9 pagesTest - D18 Dec 2022PrinceNo ratings yet
- UntitledDocument8 pagesUntitledsam cuadraNo ratings yet
- Ie5b03475 Si 001Document361 pagesIe5b03475 Si 001Ricardo Jiménez florezNo ratings yet
- Alcohols Phenols and Ethers Anil HssliveDocument16 pagesAlcohols Phenols and Ethers Anil HsslivemartyNo ratings yet
- Reductions in Organic Chemistry (Hudlicky) 2Document322 pagesReductions in Organic Chemistry (Hudlicky) 2Dombedosbaffo50% (2)
- Asam Amino, Peptida Dan ProteinDocument9 pagesAsam Amino, Peptida Dan ProteinEugenia ShepanyNo ratings yet
- Acid-Catalyzed Dehydration of Cyclohexanol To Cyclohexene Lab - ReportDocument7 pagesAcid-Catalyzed Dehydration of Cyclohexanol To Cyclohexene Lab - ReportMcAdam TULAPI100% (1)
- Biosynthesis of LIpidsDocument21 pagesBiosynthesis of LIpidsJoyce Hanniel CastinoNo ratings yet
- Biomolecules MindmapDocument5 pagesBiomolecules MindmapOm SambheNo ratings yet
- Error Definition IMM 6.6 VersaCell 3.7.mdbDocument157 pagesError Definition IMM 6.6 VersaCell 3.7.mdbОлександрNo ratings yet
- Sanghani HG Thesis ChemistryDocument265 pagesSanghani HG Thesis ChemistryOleg PopusoiNo ratings yet
- Pyrazole Ring: University of Baghdad College of PharmacyDocument17 pagesPyrazole Ring: University of Baghdad College of Pharmacyشمس صبيح عبد الرحيمNo ratings yet
- Chemistry Chapter 20 LECDocument122 pagesChemistry Chapter 20 LECsaxman011No ratings yet
- Glicoles PDFDocument16 pagesGlicoles PDFalfredoNo ratings yet
- CHM 102 Past Test QuestionsDocument15 pagesCHM 102 Past Test QuestionsCharlie StonesNo ratings yet
- ChemDocument19 pagesChemmelissaaverinaNo ratings yet
- Process Production of PETDocument7 pagesProcess Production of PETDhiyyah MardhiyyahNo ratings yet
- Short Notes Class 12 Chemistry 2023Document37 pagesShort Notes Class 12 Chemistry 2023Aman singh TomarNo ratings yet
- The Effect of Substituents On ReactivityDocument30 pagesThe Effect of Substituents On ReactivityAbhimanyu GuptaNo ratings yet
- Chem Reviewer FinalsDocument120 pagesChem Reviewer FinalsJonathan SaydeNo ratings yet
- Nitrostyrene Reduction by Ordinary Baker's YeastDocument10 pagesNitrostyrene Reduction by Ordinary Baker's Yeastbanjo01100% (1)