Professional Documents
Culture Documents
Acids, Bases and Salts PDF
Acids, Bases and Salts PDF
Uploaded by
Zeyad OsamaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Acids, Bases and Salts PDF
Acids, Bases and Salts PDF
Uploaded by
Zeyad OsamaCopyright:
Available Formats
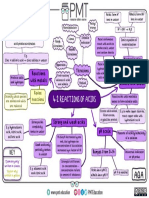
methyl orange
Indicators litmus
Bases+ammonium salts=ammonia thylmophthalein
Reactions
Acids+ammonia=ammonium salts
Acid: a substance producing H+ while
Proton donor
dissolved in water
Cu(NH3)4
Base: a substance that neutralizes an acid to
Proton acceptor
自由主题 form a salt and water
Cr(OH)4
Alkali: a base which is soluble in water,
Al(OH)4 Cations Definitions producing OH-
Zn(OH)4
Acids, Bases and
Acid oxides: oxides which react with bases,
Zn(NH3)4
Zn2+
Salts producing salt and water only
Basic oxides:oxides which react with acids,
potassium: lilac Oxides
producing salt and water only
calcium: orange-red Atmospheric oxides: oxides which can
behave as both basic and acid oxides
sodium: yellow
Flame test
lithium: red
Ion-testing Metal+acid E.g. Zinc+hydrochloric acid
barium: light green
Insoluble base+acid E.g. Copper(Ⅱ) oxide+Sulfuric acid
copper: blue-green Making salts aking salts of GroupⅠelements&
M .g. Ammonia+sulfuric acid=ammonium
E
Acid-base Titration
Ammonium salts sulfate
AgCl: white ppt
Precipitation reaction E.g. Producing barium sulfate
AgBr: Creamy ppt Cl-, Br-, I- Crystallization
AgI: yellow ppt
1. Add NaOH and aluminium foil
filtration
2. Warm gently Nitrate ions Anions
3. Pungent smelled gas turns damp litmus paper blue
Acidified nitric acid(to remove carbonates)+(BaNO3)2=BaSO4 Sulfate ions
Acidified potassium magnate turns colorless Sulfite ions
You might also like
- Business English Leaving A MessageDocument2 pagesBusiness English Leaving A MessageZeyad OsamaNo ratings yet
- Acid, Base and Salt - C-AA - DoneDocument14 pagesAcid, Base and Salt - C-AA - DoneVinod AgrawalNo ratings yet
- Partially Ionised in Water andDocument5 pagesPartially Ionised in Water andHikmaNo ratings yet
- Acids and BasesDocument2 pagesAcids and BasesfeliciaNo ratings yet
- E5 Lewis Acids and Bases (Session 1) November 5 - 11Document8 pagesE5 Lewis Acids and Bases (Session 1) November 5 - 11KIMIA Ronald Ivan WijayaNo ratings yet
- Acids and Bases MenuDocument32 pagesAcids and Bases MenuNovah GurulooNo ratings yet
- 4 Acids, Bases and SaltsDocument1 page4 Acids, Bases and SaltsAhnt htoo aungNo ratings yet
- Acids - For MergeDocument5 pagesAcids - For Mergeseolux13No ratings yet
- Fongrsy - Acids Bases and AlkalisDocument2 pagesFongrsy - Acids Bases and AlkalisDinangaNo ratings yet
- Today's Plan: Salts, Acids and BasesDocument5 pagesToday's Plan: Salts, Acids and Basesvinnie0905No ratings yet
- Chapter 7: Acids & Bases: Asid, Bes & AlkaliDocument11 pagesChapter 7: Acids & Bases: Asid, Bes & AlkaliAmin Kamarun ZamanNo ratings yet
- 15 Lecture PPTDocument7 pages15 Lecture PPTDavid Hoktua Siregar siregarNo ratings yet
- Redox Cheat SheetDocument1 pageRedox Cheat SheetJonathan ChunNo ratings yet
- Acids and Bases: MD - Safwat X Riesaf HossainDocument9 pagesAcids and Bases: MD - Safwat X Riesaf HossainMd SafwatNo ratings yet
- Chem NotesDocument4 pagesChem Noteshossainariq20No ratings yet
- MHRN P BLOCK Group 15 Nitrogen FamilyDocument23 pagesMHRN P BLOCK Group 15 Nitrogen Familysriyabehera11No ratings yet
- Reviewer SemDocument14 pagesReviewer SemJoyce M.No ratings yet
- Acid and AlkaliDocument6 pagesAcid and AlkaliSNo ratings yet
- Chapter 6 Acids, Bases and SaltsDocument32 pagesChapter 6 Acids, Bases and SaltsAnne Marie Ya Jie GOHNo ratings yet
- CHAPTER 5 Acids, Bases and Salts - Mind MapDocument4 pagesCHAPTER 5 Acids, Bases and Salts - Mind MapHari Krishna KommiNo ratings yet
- Annotated-Acid-base EquilibriaDocument11 pagesAnnotated-Acid-base EquilibriaVECNANo ratings yet
- Acid and Bases 2Document5 pagesAcid and Bases 2liyasariNo ratings yet
- 12 Chemistry Imp Ch4 3Document2 pages12 Chemistry Imp Ch4 3Gopal PenjarlaNo ratings yet
- ChemistryDocument6 pagesChemistrygaming3x3x3No ratings yet
- 4 Acids&BasesDocument3 pages4 Acids&BasesVon Joby RomeroNo ratings yet
- 0620 - 04 Acids, Bases and SaltsDocument214 pages0620 - 04 Acids, Bases and SaltsShivamNo ratings yet
- 05a Qualitative Analysis For Organic CompoundsDocument5 pages05a Qualitative Analysis For Organic CompoundsReyo VillanuevaNo ratings yet
- 14.1 Properties of Acids & BasesDocument10 pages14.1 Properties of Acids & BasesOmar AlwaerNo ratings yet
- Acids Bases and SaltsDocument22 pagesAcids Bases and SaltsARNAV DEYNo ratings yet
- 4.2. Reactions of AcidsDocument1 page4.2. Reactions of AcidsAnais BegueNo ratings yet
- Acid, Bases and Salts.Document14 pagesAcid, Bases and Salts.lucy.murrayNo ratings yet
- E5 Lewis Acids and Bases Acids: Bronsted: Acids Are Proton DonorsDocument9 pagesE5 Lewis Acids and Bases Acids: Bronsted: Acids Are Proton DonorsJohn HenricksNo ratings yet
- Intro SummaryDocument1 pageIntro SummaryChastine CruzNo ratings yet
- Name - Class - : Q: No1. Choose The Correct Answer. 10Document2 pagesName - Class - : Q: No1. Choose The Correct Answer. 10Syed Salman SaeedNo ratings yet
- Topic 7 NotesDocument2 pagesTopic 7 Notesmarin tamNo ratings yet
- Module 6 Acid Base ReactionsDocument7 pagesModule 6 Acid Base Reactionsisaheqq12No ratings yet
- This Is A Good FileDocument12 pagesThis Is A Good FileTanushka YadavNo ratings yet
- 3 Acids and BasesDocument11 pages3 Acids and BasesNashoNo ratings yet
- Aromatic CompoundsDocument99 pagesAromatic CompoundsMukesh BaskaranNo ratings yet
- Acid, Bases and Salts Class 10Document7 pagesAcid, Bases and Salts Class 10Gowtham LNo ratings yet
- 4 Supplementary Exercise Acids and BasesDocument113 pages4 Supplementary Exercise Acids and Bases云吸仓鼠吉尼斯保持者No ratings yet
- Chemistry 1302: CHAPTER 3.3 - Acid Base ChemistryDocument15 pagesChemistry 1302: CHAPTER 3.3 - Acid Base Chemistryzak mahmoudNo ratings yet
- Acids, Bases and SaltsDocument6 pagesAcids, Bases and SaltsTajiriMollelNo ratings yet
- Acids BasesDocument39 pagesAcids BasesMarek JacečkoNo ratings yet
- Acids, Bases & SaltsDocument31 pagesAcids, Bases & SaltsPradipjha JhaNo ratings yet
- Acids, Bases and SaltsDocument15 pagesAcids, Bases and SaltsSarah MariaNo ratings yet
- FI-Medicinal Chemistry 1-Smsrt Genap - TerbaruDocument24 pagesFI-Medicinal Chemistry 1-Smsrt Genap - TerbaruSahabat BaljaiNo ratings yet
- Acid BaseDocument25 pagesAcid BaseyusmahanimNo ratings yet
- Chemistry CH 8Document11 pagesChemistry CH 8husnainraza0403No ratings yet
- ( (Chapter 8&9 - Acids and Bases, Salts) )Document8 pages( (Chapter 8&9 - Acids and Bases, Salts) )bharadiadishitaNo ratings yet
- Carboxylic AcidsDocument4 pagesCarboxylic AcidsAryan GovenderNo ratings yet
- Grade 10 Chemistry Week 12 Lesson 1Document4 pagesGrade 10 Chemistry Week 12 Lesson 1nesiaroberts903No ratings yet
- Acids,+Bases+&+Salts +vocabularyDocument19 pagesAcids,+Bases+&+Salts +vocabularyunknown33No ratings yet
- Acids and BasesDocument8 pagesAcids and BasesMohamed MamdouhNo ratings yet
- Acids and BasesDocument18 pagesAcids and BasesMohammad Fahim NurNo ratings yet
- Edited - Acids Bases (Part 2) 6092 TeacherDocument27 pagesEdited - Acids Bases (Part 2) 6092 TeachersamrobbiesingcabarreraNo ratings yet
- Cac Phan Ung Cua AncolDocument1 pageCac Phan Ung Cua AncolVy Na Ngo100% (1)
- Acids, Bases and SaltsDocument34 pagesAcids, Bases and SaltsLakshan FernandoNo ratings yet
- Surfing Chemistry Y12 Mod 6Document70 pagesSurfing Chemistry Y12 Mod 6claire.zqmNo ratings yet
- Chemical Formula - Common Compunds - Typs of Chemical FormulaDocument7 pagesChemical Formula - Common Compunds - Typs of Chemical FormulaSHAMS QUAMARNo ratings yet
- LKLKLDocument1 pageLKLKLZeyad OsamaNo ratings yet
- Quadratic Equations Exercises MSDocument9 pagesQuadratic Equations Exercises MSZeyad OsamaNo ratings yet
- Chapter 8Document4 pagesChapter 8Zeyad OsamaNo ratings yet
- Novel 1Document13 pagesNovel 1Zeyad OsamaNo ratings yet
- Chapter 9Document5 pagesChapter 9Zeyad OsamaNo ratings yet
- Main Words: Tags CreatedDocument12 pagesMain Words: Tags CreatedZeyad OsamaNo ratings yet
- Book Review2Document4 pagesBook Review2Zeyad OsamaNo ratings yet
- HackingDocument5 pagesHackingZeyad OsamaNo ratings yet
- 5 Worksheet: Mole Concept and Stoichiometric Calculations: Junior Tukkie Winter School 1 Dr. S. Swanepoel (2020)Document2 pages5 Worksheet: Mole Concept and Stoichiometric Calculations: Junior Tukkie Winter School 1 Dr. S. Swanepoel (2020)Dina Anggraini PramitasariNo ratings yet
- China Steel SpecificationDocument45 pagesChina Steel SpecificationMohit KohliNo ratings yet
- Gmail - SN 500 Base OilDocument3 pagesGmail - SN 500 Base Oilstalin fernandesNo ratings yet
- Joyce Exp7 ChemlabDocument7 pagesJoyce Exp7 ChemlabKristine Joyce CaloNo ratings yet
- South 24 ParganasDocument26 pagesSouth 24 ParganasDFBDHHNo ratings yet
- 01 Alchol, Phenol and Ether Theory Final EDocument19 pages01 Alchol, Phenol and Ether Theory Final Eummer farooqNo ratings yet
- Literature SurveyDocument7 pagesLiterature SurveyVikash Sepat0% (1)
- Application NoteDocument4 pagesApplication NoteAyoub GhabriNo ratings yet
- Zhuzhou UKO Carbide End MillDocument78 pagesZhuzhou UKO Carbide End MillTaylan KaraçelikNo ratings yet
- Model 2100bDocument4 pagesModel 2100bkeyur1109No ratings yet
- The Effect of Chemical Additives On The Strength, Stiffness and Elongation Potential of Paper - OPEN ACCESSDocument13 pagesThe Effect of Chemical Additives On The Strength, Stiffness and Elongation Potential of Paper - OPEN ACCESSHgagselim SelimNo ratings yet
- Eletrochemistry Previous Qns With AnswersDocument8 pagesEletrochemistry Previous Qns With AnswersAkshay SureshNo ratings yet
- Development of Natural Bio-Plantation Waste As Pulp For Paper MakingDocument6 pagesDevelopment of Natural Bio-Plantation Waste As Pulp For Paper MakingIngrid ContrerasNo ratings yet
- Ukk Soal 2019Document12 pagesUkk Soal 2019juliamaulanam.pd1989No ratings yet
- Best-Practises RO PlantDocument25 pagesBest-Practises RO PlantjdadhaNo ratings yet
- Coordination Compounds Final RevisionDocument3 pagesCoordination Compounds Final RevisionROWA new year CelebrationNo ratings yet
- Wear Behavior of Magnesium Alloy AZ91 Hybrid Composite MaterialsDocument10 pagesWear Behavior of Magnesium Alloy AZ91 Hybrid Composite MaterialstarasasankaNo ratings yet
- USMLE Normal Lab ValuesDocument2 pagesUSMLE Normal Lab Valuesmearbbear100% (1)
- KF Titration 1 PDFDocument8 pagesKF Titration 1 PDFFabianus Galih Ari WigunaNo ratings yet
- SOP For Nitrogen Determination of KjeldahlDocument5 pagesSOP For Nitrogen Determination of Kjeldahlroqhayya shaikNo ratings yet
- Us20030041935a1 PDFDocument10 pagesUs20030041935a1 PDFAlperen BozdemirNo ratings yet
- NUTR1R06w PDFDocument110 pagesNUTR1R06w PDFAli Rıza Üreten100% (1)
- 01 - Moles, Equations & Acids CORNELLDocument30 pages01 - Moles, Equations & Acids CORNELLGeorge SolomouNo ratings yet
- Aluminum Alloys 101 - The Aluminum AssociationDocument2 pagesAluminum Alloys 101 - The Aluminum Associationeric92647No ratings yet
- CC1 Lab Ass.Document4 pagesCC1 Lab Ass.CHRIS JOHN PAUL KEVIN BANTULODNo ratings yet
- STERISAFE Pro Disinfection Robot 1711Document8 pagesSTERISAFE Pro Disinfection Robot 1711Bach PhamNo ratings yet
- FAS Style 42Document2 pagesFAS Style 42JohnNo ratings yet
- Classification of FertilizersDocument4 pagesClassification of FertilizersSatyam WankhedeNo ratings yet
- CH - 03 - Prac - Test-Web RDocument8 pagesCH - 03 - Prac - Test-Web RMartria EhabNo ratings yet
- Tech Topic Reduced Shrinkage in Molding Compound ApplicationsDocument2 pagesTech Topic Reduced Shrinkage in Molding Compound ApplicationsAhmed ZamanNo ratings yet