Professional Documents
Culture Documents
Metal Dispersion Bimetallic PT Re
Metal Dispersion Bimetallic PT Re
Uploaded by
marviOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Metal Dispersion Bimetallic PT Re
Metal Dispersion Bimetallic PT Re
Uploaded by
marviCopyright:
Available Formats
JOURNAL OF CATALYSW 85, 1-7 (1984)
Surface Area Measurement of Platinum/Rhenium/Alumina
I. Stoichiometry of Hydrogen-Oxygen Chemisorptions and Titrations
BRUCE H. ISAACS AND EUGENE E. PETERSEN
Department of Chemical Engineering, University of California, Berkeley, California 947.20
Received March 5, 1982; revised July 19, 1983
The stoichiometry of H2 and O2 adsorption by Pt/AI,O,, Re/A1203, and PtlRe/AlzO, has been
studied. The gas uptakes of the monometallic catalysts were used to predict the expected uptakes
by the Pt/Re/AIZOx catalyst assuming no interaction between the two metals. A significant decrease
in HZ chemisorption and an increase in titratable O2in the bimetallic, compared to that expected for
no metal interaction, suggest alloy formation in the Pt/Re/AlzO, catalyst. A stoichiometry for Hz
and O2 chemisorption and titration by Pt/ReiAlz03 consistent with these observations is proposed.
INTRODUCTION ferences in the results of these various
groups, particularly with respect to H2 che-
Metal surface area measurement by misorption by Pt and titration of O2 chemi-
selective gas chemisorption is commonly sorbed on Re. In this paper, we present fur-
utilized for characterization of reforming ther results on the uptake of H2 and O2 by
catalysts. In order to use selective gas Pt/Re/Alz03 catalysts and suggest a stoichi-
chemisorption for metal surface area mea- ometry for utilizing the measured gas up-
surement, the stoichiometry of the surface takes for surface area measurement.
reactions must be determined to relate the
experimentally measured gas uptakes to the EXPERIMENTAL
quantity of surface metal. The pulse ffow surface area apparatus
The uptake of H2 and O2 by Pt/Re/Alz03 used in this study is similar to that de-
catalysts was first examined by Free1 (Z), scribed by Free1 (1). All gases, argon, hy-
who did not suggest a stoichiometry to re- drogen, oxygen, 20% hydrogen in argon,
late his measured uptakes to surface metal. and 15% oxygen in argon, were of ultrahigh
A method for separate determination of Pt purity (Matheson). The argon, hydrogen,
and Re in the bimetallic catalyst was pro- and hydrogen in argon were further purified
posed by Menon et al. (2). These authors by passage through a fresh oxygen trap (All-
did not determine a stoichiometry for ad- tech), followed by a fresh 13x molecular
sorption by Re and later stated that they sieve (Matheson). The concentration of ox-
were not able to determine a reliable chemi- ygen in the carrier gas, argon, at the cata-
sorption value for Re (3). Bolivar eCal. (4, lyst was less than 0.1 ppm (6).
5) have also measured the uptake of H2 and The catalysts contained about 0.3 wt% Pt
O2 by Pt/Re/A1203 catalysts and proposed a and/or 0.3 wt% Re supported on r-A&O,,
stoichiometry to relate their measured up- obtained from Chevron Research Com-
takes to the quantity of surface metal. pany. They were calcined by the manufac-
The uptake of H2 and O2 by Pt/Re/Alz03 turer, probably at about 500°C (7). During
catalysts has been examined by several re- storage, the catalysts adsorbed water (7).
search groups; one group has proposed a Thus, the first step of the pretreatment was
stoichiometry for surface area measure- to dry the catalyst, in this case at 500°C for
ment. There are, however, significant dif- 3 h in flowing argon, 50 ml/min. The tem-
1
002l-95 17/84 $3.00
Copyright 0 1984 by Academic Press, Inc.
All rights of reproduction in any form reserved.
2 ISAACS AND PETERSEN
TABLE 1 tallic Pt dissociatively chemisorbs both Hz
Stoichiometry and Dispersion of Pt/A120, and O2 and each gas will titrate the other.
Also found in Table 1 is the dispersion, de-
Stoichiometry Dispersion fined as atoms surface metal/atoms total
metal (PtJPt), of the Pt catalyst calculated
Hz Chemisorption 0.54
according to each of the stoichiometric
Pt, t sH2 --, Pt,H
equations. The dispersion values, other
O2 Chemisorption 0.43 than the one determined by H2 chemisorp-
Pt, + to, -+ pt,o
tion, are in good agreement; the average is
1st O2 Titration 0.47 about 0.46 with an average percentage devi-
Pt,H + $0, + Pt,O t JH20
ation of about 4%. The dispersion deter-
Hz Titration 0.44 mined by H2 chemisorption is higher at
Pt,O + BHZ+ Pt,H t Hz0 0.54. Similar results have been obtained
2nd O2 Titration 0.48 previously: our ratio of (dispersion from H2
P&H + 20, + Pt,O + fHz0 titration/dispersion from H2 chemisorption)
= 0.82 is in very good agreement with the
perature was then lowered to about lOO”C, value of 0.81 found by Kobayishi et al. (9)
at which time the gas flow was switched to for a Pt/Al,O, catalyst having a similar dis-
hydrogen. After raising the temperature, at persion. Similarly, our value of (0*/H*) =
6”C/min, to 500°C the catalyst was reduced 0.80 is also in agreement with the results
for 15 h. The catalyst was then purged with summarized by Free1 (20).
argon at 500°C for 1 h, then cooled to room The dispersion of the Pt catalyst, as de-
temperature, which took about 1 h. termined by HZ. chemisorption, was also
The surface area measurements were measured in a static volumetric apparatus
made at room temperature with argon as a (11). The dispersion, in this case, was found
carrier gas, 50 ml/min. Sixty microliter to be 0.57, about 6% higher than that found
pulses of hydrogen, oxygen, 20% hydrogen in our pulse flow apparatus.
in argon, or 15% oxygen in argon were uti-
Rhenium-Alumina Catalyst
lized for catalyst samples weighing about
1 tit- The stoichiometry for H2 and O2 chemi-
sorptions and titrations on Re/A1203 is not
RESULTS AND DISCUSSION
well established. Yates and Sinfelt (12)
The surface area measurements were found good agreement between crystallite
made in the order: Hz chemisorption, O2 size as measured by X-ray line broadening
titration, H2 titration, and second O2 titra- and H2 chemisorption, assuming HJRe, =
tion. HA designates H2 taken up in the H2 1, for a 10% Re/Si02 catalyst. In contrast,
chemisorption. OT, is O2 taken up in the Kubicka (13) found that metallic Re only
first O2 titration, HT is Hz in the Hz titra- adsorbs approximately 0.5 atoms H/atom
tion, and OTz is O2 taken up in the second Re,. Using this stoichiometry, she found
O2 titration. OA, the atoms of O2 chemi- good agreement between H2 chemisorption
sorbed, was calculated from the expres- and X-ray line broadening measurements
sion: OA = OTr - 4 HA (I). HA, OT, , HT, for 1% Re/SiO,; however, the agreement
OTz, and OA are all expressed in units of was less satisfactory if the Re was sup-
atoms. ported on r-A&O,. For a 1.33% Rely-A1203
catalyst, Free1 (I) found that the amount of
Platinum-Alumina Catalyst chemisorbed H2 increased with increasing
The stoichiometry generally accepted for chemisorption temperature and that only a
H2 and O2 chemisorptions and titrations on trace amount was chemisorbed at room
PtiA1203 is shown in Table 1 (8). Monome- temperature. He also found that his catalyst
SURFACE AREA MEASUREMENT OF PLATINUM/RHENIUM/ALUMINA, I 3
TABLE 2 lyst. There was also no uptake in a second
Stoichiometry for Re/AlzOr 02 titration, The catalyst did chemisorb 02,
with an O*/Re ratio of 0.34, which we take
Hz Chemisorption: No reaction to be equal to the Re dispersion. A stoichi-
Oz Chemisorption: Re, + to, -P Re,O ometry for Re/A1203 consistent with the
1st 0: Titration: Re, + 40, --f Re,O experimental measurements is shown in
(i.e., is actually an O2 chemisorption)
Table 2.
Hz Titration: No reaction
2nd O2 Titration: No reaction
Platinum-Rhenium-Alumina Catalyst
If it is assumed that there is no interac-
would chemisorb O2 and that the chemi- tion of the two metals of the bimetallic cata-
sorbed O2 could not be titrated with Hz. His lyst, a stoichiometry for Pt/Re/A1203 can be
ratio of OA/Re was about 0.35 to 0.5 de- obtained by merely adding the stoichiome-
pending on the length of reduction. Yao and tries of the two monometallic catalysts.
Shelef (14) found that 1.21% Rely-AlzOj This stoichiometry is shown in Table 3. It
chemisorbs 0.33 molecules CO/atom Re, suggests two possible strategies for deter-
which they took to be descriptive of the mining the dispersion of the Pt/Re/AlzOj
catalyst dispersion. They found OA/Re ra- catalyst:
tios larger than 1, which led them to suggest 1. Measure Pt, from H,; measure (Pt, +
that O2 chemisorption on Re may not be Re,) from 0,; calculate Re, by difference.
dissociative. These authors also found evi- 2. Measure (Pt, + Re,) from 0,; measure
dence of little H2 chemisorption by Re: Re, from OT, - OT,; calculate Pt, by differ-
their ratio of (H atoms adsorbed/CO mole- ence.
cules adsorbed) was only about 0.1 to 0.2. This second strategy is essentially that pro-
Bolivar et al. (4) assumed that the chemi- posed by Menon et al. (2).
sorption and titration stoichiometry for a Gas uptakes for the bimetallic Pt/Re/
2% Rely-A1203 catalyst was the same as A1203catalyst were calculated from the ex-
that for Pt/Al,O,. They found good agree- perimentally measured uptakes of the
ment between their chemisorption values monometallic catalysts assuming no inter-
and particle size measured by electron mi- action between the metals. These calcu-
croscopy if the H2 chemisorption and Hz lated values are compared with those ex-
titration were done at elevated tempera- perimentally determined for the Pt/Re/
ture. If the chemisorption and titration A1203 catalyst in Table 4. The terms
were run at room temperature, the H2 up- Oa (0, = OA - ONR)and ONR(ONR = OT,
takes were much lower. They found disper- - OTJ are descriptive of the ease of Hz
sion values of about 0.21 to 0.25. Thus, the
literature suggests that the chemisorption
stoichiometry of supported Re varies with TABLE 3
the support, the metal weight loading, and Stoichiometry for PtlRe/A120z Assuming no
the chemisorption temperature. Interaction of Metals
Our Re catalyst was supported on y-
Hz Chemisorption: Pt, + :H, + Pt,H
A1203, had a very low metal loading of 0.33
wt% and our chemisorption measurements O? Chemisorption: Pt, + $0, + pt,o
were made at room temperature. All of 1st O2 Titration: Pt,H + $0, + P&O + dHzO
these factors should result in little H2 che- Re, + to, -+ Re,O
misorption or H2 titration. Experimentally, Hz Titration: Pt,O + QH, --* Pt,H + HZ0
we found no H2 chemisorption or H2 titra- 2nd O2 Titration: P&H + $0, --, Pt,O + :HZO
tion by our monometallic Re/A1203 cata-
ISAACS AND PETERSEN
TABLE 4 further evidence of the reduction of the
Comparison of Gas Uptakes Calculated Assuming no number of pairs of Pt atoms necessary for
Metal Interaction and Those Experimentally dissociative H2 chemisorption and alloy
Determined for Pt&e/AlzOs formation.
TPR studies (1.5) have also shown a sup-
Ratio Calculated Experimental pression of H2 adsorption-desorption in the
value value
Pt/Re/Alz03 catalyst which was attributed
HJF’t 0.54 0.22 to fewer Pt pairs due to alloy formation. An
OA/(Pt + Re) 0.38 0.57 IR study (4) of CO chemisorption on Pt/Re/
OR/pt 0.43 0.72 A&O3 has shown that the intensity of
Q-de 0.34 0.43 bridged CO decreases much more rapidly
than the intensity of linear CO as the % Re
increases. This finding was also attributed
titration. For monometallic Pt/A1203, ONR to a lowering of Pt pairs. Biloen et al. (27)
= 0 and OR = OA, and for monometallic also found a suppression of dissociative
Re/A1203, ONR= OA and OR = 0. The poor chemisorption, relative to nondissociative
agreement between the values calculated chemisorption, in Pt/Re/SiO* catalysts
assuming no interaction and those obtained which they attributed to alloy formation.
experimentally suggests an interaction be- Bolivar et al. (4), however, assumed no
tween the two metals of the Pt/Re/A1203 suppression of H2 chemisorption by Pt due
catalyst.’ to the presence of Re, based on their TPD
Of particular note is the large suppres- studies (18). Menon et al. (2) also assumed
sion of H2 chemisorption in the bimetallic no suppression of H2 chemisorption on Pt/
catalyst relative to the expected chemisorp- Re/A1203 catalysts.
tion assuming no interaction of the Pt and It is also shown in Table 4 that the ratio
the Re. The quantity of H2 actually ad- Oa/Pt experimentally measured for the Pt/
sorbed by the Pt/Re/Alz03 catalyst is only Re/A1203catalyst is almost twice as large as
about 40% of that which would be chemi- what would be predicted assuming no metal
sorbed if no Re was present. interaction. OR is indicative of the amount
A suppression of HI chemisorption of of chemisorbed O2 atoms which can be ti-
similar magnitude to that shown in Table 4 trated by HZ. Since monometallic ReO is
was first observed by Free1 (I) who sug- not titratable by HZ, this enhancement of
gested that this finding was consistent with titratable O2 also suggests alloy formation
alloy formation. Various other authors (7, in the Pt/Re/Alz03 catalyst. Probably, al-
25, 26) have also suggested that during re- loyed Pt supplies atomic H which is capable
duction of a Pt/Re/A120s catalyst an alloy is of titrating ReO in the alloy even though
formed. An alloy of Pt and Re would be molecular H2 is not. Free1 (I) also found an
expected to have fewer contiguous Pt at- increase in titratable O2 over what would be
oms than a normal Pt crystallite; i.e., one expected with no metal interaction. He, in
would expect fewer Pt pairs in a Pt-Re al- fact, found titratable O2to total Pt ratios for
loy than in pure Pt. Monometallic Re does his Pt/Re/A1203 catalysts as high as 1.64.
not chemisorb H2 while monometallic Pt Bolivar et al. (4) also found an increase in
dissociatively chemisorbs H2 which re- titratable O2 in their Pt/Re/A1203 catalysts
quires a pair of Pt atoms. Our observation which they attributed to alloy formation.
of the suppression of Hz chemisorption is Menon et al. (2), however, assumed that
the titration of the ReO of the Pt/Re/Alz03
’ We shall refer to this interaction as alloy formation
for convenience, the exact nature of the interaction is catalyst was not affected by the presence of
not known. The term bimetallic cluster may be prefer- Pt.
red by some readers. Since both monometallic Pt and Re are
SURFACE AREA MEASUREMENT OF PLATINUM/RHENIUM/ALUMINA, I 5
TABLE 5 est neighbor. Thus, the quantity PtSRis only
Proposed Stoichiometry for Chemisorption and a semiquantitative measure of the extent of
Titration by PtlRe/A120, alloying. Two types of Re are also found,
alloyed and unalloyed.
Hz Chemlsorption: P&R t 1H2 -+ Pi,%
The stoichiometry shown in Table 5 sug-
02 Chemisorption: Pt,R + 102 -f Pt,RO
pt,“R + 102 + Pt,NRO
gests the following procedure for determin-
Re, alloyed t 102 -+ Re, alloyed 0 ing the dispersion of the Pt/Re/AlZ03 cata-
Re, unalloyed + 102 -t Re, unalloyed 0
lyst:
1st 02 Titration: PtsRH + 202 + PtsRO + .+HZO
PtsNR + 102 + PtqNRO
1. Measure (Pt, + Re,) from OA.
Re, alloyed + 102 -+ Re, alloyed 0 2. Measure Re, unalloyed from OTl
Re, unalloyed + 102 - Re, unalloyed 0
OT2.
H2 Titration: PtsRO t #H* -f PtsRH t Hz0
Pi I NRO + Hz- PtSNR + Hz0
3. Calculate (Pt, + Re, alloyed) by differ-
Re, alloyed 0 + Hz -) Re, alloyed + Hz0 ence.
2nd 02 Titration: PtqRH t Woz + FY,~O + &1>0 4. Measure PtSRfrom HA.
Pt,NR + $02 - pt,“R0 According to the proposed stoichiometry, it
Re, alloyed + $02 - Re, alloyed 0
is not possible to separate the quantity (Pt,
+ Re, alloyed) since only a percentage of
capable of chemisorbing 02, the ratio OA/ the Pt, can be measured by Hz chemisorp-
(Pt + Re) should be indicative of the overall tion and Re, alloyed 0 is titrated identically
dispersion of the Pt/Re/A1203 catalyst (1, to PtSNRO. Thus, a totally separate disper-
19). It appears that the overall dispersion of sion value for each metal cannot be ob-
the Pt/Re/Alz03 catalyst is significantly tained by this method.
larger than would be expected with no The proposed stoichiometry differs from
metal interaction. that suggested by Bolivar et al. (4, 5) pri-
If alloyed ReO is titratable, the quantity marily in its description of H2 chemisorp-
ONRwhich is related to the amount of nonti- tion by the Pt of the Pt/Re/A1203 catalyst.
tratable 02, should be descriptive of the Bolivar et al. assume normal H2 chemisorp-
quantity of unalloyed Re,. It appears that tion. For confirmation of their proposed
the amount of unalloyed surface Re in the stoichiometry, Bolivar et al. showed good
Pt/Re/A120, catalyst is similar to that in the agreement between their experimentally
monometallic Re/A1203 catalyst for this measured ratio HT/OT, and that predicted
particular pretreatment. by their stoichiometry, HT/OTl = 0.125 g/
g. It should be noted that our proposed stoi-
chiometry also predicts that HT/OTl =
Proposed Stoichiometry for a Supported 0.125 g/g; this ratio is independent of the
Platinum-Rhenium Catalyst amount of H2 chemisorbed by Pt. In their
The comparison shown in Table 4 be- most recent work (20), this research group
tween the actual gas uptake by a Pt/Re/ also found evidence that “could reflect a
A1203 catalyst and that expected assuming somewhat lower hydrogen coverage of Pt,
no interaction of the two metals suggests in the platinum-rhenium alloys compared
alloy formation in the reduced Pt/Re/Alz03 to Pt, in pure platinum.”
catalyst. This information leads us to sug- TPR studies (7, 15) have shown that Pt
gest the stoichiometry shown in Table 5 for and Re oxides are immiscible leading to
Pt/Re/AlzOX catalysts. Two types of surface segregation of alloyed Pt-Re upon oxida-
Pt are found in the Pt/Re/AlzOj catalyst, tion. Bolivar et al. (4) found that the ratio
that which is able to chemisorb Hz, PtSR, OT,/OTl continually decreased as n, the
and that which is not, PtSNR.Pt which che- number of the titration, increased. They
misorbs H2 may either be unalloyed, or be suggested that the room temperature O2 ti-
alloyed and have another Pt atom as a near- trations may themselves cause some segre-
ISAACS AND PETERSEN
consistent with our TPR results (7). It is
not, however, in agreement with the results
of Bolivar et al. (4). They found that a phys-
ical mixture of Pt and Re has an OTZ/OT1
ratio more than twice as large as that pre-
dicted by the sum of the monometallics,
which suggests metal interaction. The ex-
perimental OT2/OT1 ratio for our physical
mixture is .66, which is identical to the
value predicted by the sum of the monome-
tallies. As discussed previously (7), the
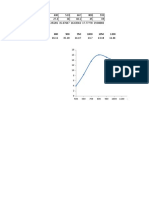
Titration Number n findings of Bolivar et al. may be due to their
use of uncalcined catalysts.
FIG. 1. Variation of gas uptake in successive titra-
tions of Pt/Re/A1203.
CONCLUSIONS
Surface area measurements indicate that
gation of the alloyed Pt-Re. A similar sug-
the Pt/AI,O, catalyst has a dispersion of ap-
gestion has also been made for bimetallic
proximately 0.5 and that the stoichiometry
Pt-Pd catalysts (21) based on IR studies.
for H2 and O2 chemisorptions and titrations
We also find a continual decrease in OTJ
on this catalyst is in agreement with that
OT1 as n increases, as shown in Fig. 1.
commonly accepted. The Re/A1203catalyst
Thus, it appears that the structure of the Pt/
does not chemisorb HZ, it dissociatively
Re/A1203 catalyst is altered by the surface
chemisorbs 02, but the chemisorbed O2
area measurement and that another reac-
cannot be titrated by Hz. This proposed
tion needs to be added to the stoichiometry
stoichiometry is consistent with the avail-
presented in Table 5:
able information on H2 and O2 uptake by
supported Re catalysts. The O2 chemisorp-
Re, alloyed 4 Re, unalloyed
tion measurement indicates that the disper-
The extent of this reaction can be quantified sion of the Re/A1203 catalyst is about 0.34.
by assuming that there is no Re, unalloyed Comparison of the gas uptakes expected
in the Pt/Re/Alz03 catalyst after drying at based upon the sum of the monometallic
100°C (22). catalysts with those experimentally found
for the Pt/RelAlzOJ catalyst suggests alloy
Physical Mixture of Supported Platinum formation in the reduced bimetallic cata-
and Rhenium Catalysts lyst. The Pt/Re/A1203 catalyst exhibits a
The experimentally determined gas up- suppression of H2 chemisorption and an in-
takes of a physical mixture of Pt/A1203 and
Re/A1203 are compared to those calculated TABLE 6
from the sum of the monometallics in Table Comparison of Gas Uptakes Calculated Assuming no
6. Physical mixtures are of interest as it has Metal Interaction and Those Experimentally
been proposed (23) that physical mixtures Determined for Physical Mixture
of Pt and Re and bimetallic Pt/Re/AlzOj Ratio Calculated Experimental
have identical stability for reforming. The value value
measured uptakes for the physical mixture
are essentially identical to the sum of the HA/Pt 0.54 0.56
monometallics, which suggests no metal in- O,/(Pt + Re) 0.38 0.43
0.43 0.48
teraction. The same result has been found ORm
0.34 0.39
ONR/Re
previously by Free1 (I). This result is also
SURFACE AREA MEASUREMENT OF PLATINUM/RHENIUM/ALUMINA, I 7
crease in titratable 02, both of which are 5. Tournayan, L., Charcosset, H., Frety, R., Le-
consistent with alloy formation. The gas clercq, C., Turlier, P., Barbier, J., and Leclercq,
G., Thermochim. Acta 27, 95 (1978).
uptakes also suggest that the overall disper- 6 Isaacs,B. H., PhD thesis-Chap. 3, Dept. of
sion of the bimetallic catalyst is larger than Chemical Engineering, University of California,
one would expect with no metal interac- Berkeley, California. Submitted.
tion, while the quantity of unalloyed sur- 7 Isaacs, B. H., and Petersen, E. E., J. Catal. 77,43
face Re is about the same as in the Re/A1203 (1982).
8. Benson, J. E., and Boudart, M., J. Catal. 4, 704
catalyst for this particular pretreatment. (1965).
Unlike the Pt/Re/A1203 catalyst, a physical 9. Kobayashi, M., Inoue, Y., Takahashi, N.,
mixture of Pt/Al,O, and Re/A1203 is found Burwell, R. L., Butt, J. B., and Cohen, J. B., /.
to behave as the sum of the monometallics. Catal. 64, 74 (1980).
A stoichiometry consistent with the ex- 10. Freel, J., Catalysis 25, 149 (1972).
II. Kellner, C. S., Ph.D. thesis, Dept. of Chemical
perimental observations for Hz and O2 che- Engineering, University of California, Berkeley,
misorption and titration by Pt/RelA1203 is Calif., 1981.
suggested that allows the quantities (Pt, + 12. Yates, D. J. C., and Sinfelt, J. H., J. Catal. 14, 182
Re,), (Pt, + Re, alloyed), Re, unalloyed, (1969).
and PtsRto be calculated from the measured 13. Kubicka, H., J. Catal. 12, 223 (1968).
14. Yao, H. C., and Shelef, M., J. Catal. 44, 392
gas uptakes. Separate determination of the (1976).
dispersion of the Pt and the Re of the Pt/Re/ IS. Wagstaff, N., and Prins. R., J. Catal. 59, 434
AllO catalyst cannot be obtained with this (1979).
method since Re, alloyed and PtsNRreact 16. Bolivar, C., Charcosset, H., Frety, R., Primet,
identically. M., Tournayan, L., Betizeau, C., Leclercq, G.,
and Maurel, R., J. Catal. 39, 249 (1975).
ACKNOWLEDGMENTS 17. Biloen, P., Helle, J. N., Verbeek, H., Dautzeen-
berg, F. M., and Sachtler, W. M. H., J. Catal. 63,
This work was supported in part by the National 112 (1980).
Science Foundation, Grant Eng. 7910412. We also 18. Bolivar, C., Charcosset, H., Frety, R., Leclercq,
thank Dr. Walt Buss of the Chevron Research Com- G., and Neff, B., “Proceedings, 1st International
pany, Richmond, California, for supplying the cata- Symposium Thermal Analysis, Salford, Sept.
lysts and Dr. C. Stephen Kellner for helpful discus- 1976.”
sions. 19. Savostin, Yu. A., Zaidman, N. W., Kozhevni-
kova, N. G. Milova, L. P., Kolomiichuk, V. N.,
REFERENCES and Maslova, T. A., React. Kinet. Catal. Lett. 3,
1. Freel, J., Prepr. Amer. Chem. Sot. Div. Petrol. 271 (1975).
Chem. 18, 10 (1973). 20. Barbier, J., Charcosset, H., Periera, G., and Ri-
2. Menon, P. G., Sieders, J., Streefkerk, F. J., and viere, J., Appl. Catal. 1, 71 (1981).
Van Keulen, G. J., J. Catal. 29, 188 (1973). 21. Grill, C. M., and Gonzalez, R. D., J. Catal. 64,
3. Menon, P. G., and Prasad, J., “Proceedings, 6th 487 (1980).
International Congress on Catalysis, London,” p. 33 Isaacs, B. H., and Petersen, E. E., J. Catal. 85, 8
-1.
1061. 1976. (1984).
4. Bolivar, C., Charcosset, H., Frety, R., Primet, 23. Bertolacini, R. J., and Pellet, R. J., “Proceedings
M., Toumayan, L., Betizeau, C., Leclercq, G., International Symposium on Catalyst Deactiva-
and Maurel, R., J. Catal. 45, 163 (1976). tion,” p. 73. Antwerp, 1980.
You might also like
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Qadri Group PDFDocument19 pagesQadri Group PDFAhmed NiazNo ratings yet
- Reyes1998 PDFDocument6 pagesReyes1998 PDFMateus PinheiroNo ratings yet
- Kinetics Acetone Hydrogenation Over Pt/A1203 Catalysts: F. Rositani, GalvagnoDocument7 pagesKinetics Acetone Hydrogenation Over Pt/A1203 Catalysts: F. Rositani, GalvagnoNaufal AdityasNo ratings yet
- Au-Pt/Co O Catalyst For Methane Combustion: LetterDocument4 pagesAu-Pt/Co O Catalyst For Methane Combustion: LetterfafaporkNo ratings yet
- RoleofReinPtReTiO2WGS Seshan 2008Document12 pagesRoleofReinPtReTiO2WGS Seshan 2008leonardoNo ratings yet
- Ozone Decomposition in Water Kinetic StudyDocument5 pagesOzone Decomposition in Water Kinetic StudyJESUS PLAZAS SALDAÑANo ratings yet
- Reaction of Hydroxyl Radical With Acetone. 2. Products and Reaction MechanismDocument12 pagesReaction of Hydroxyl Radical With Acetone. 2. Products and Reaction MechanismdamfukNo ratings yet
- Ethylene OxideDocument14 pagesEthylene OxidegkarakasNo ratings yet
- Ethylene Oxide Kinetics and MechanismDocument10 pagesEthylene Oxide Kinetics and MechanismjohnNo ratings yet
- Liebhafsky1932 PDFDocument15 pagesLiebhafsky1932 PDFK K LoachNo ratings yet
- Kinetics of Arsenopyrite Oxidative Dissolution by Oxygen: Forest P. Walker, Madeline E. Schreiber, J. Donald RimstidtDocument9 pagesKinetics of Arsenopyrite Oxidative Dissolution by Oxygen: Forest P. Walker, Madeline E. Schreiber, J. Donald RimstidtBiSOPNo ratings yet
- Oxidation of Carbon Monoxide Over Nanoparticles of Cobalt OxidesDocument6 pagesOxidation of Carbon Monoxide Over Nanoparticles of Cobalt OxidesDario EberhardtNo ratings yet
- Comparative Studies of Low-Temperature Water-Gas Shift Reaction Over PT Ceo, Au Ceo, and Au Fe O CatalystsDocument7 pagesComparative Studies of Low-Temperature Water-Gas Shift Reaction Over PT Ceo, Au Ceo, and Au Fe O CatalystsLucas MarchiniNo ratings yet
- Investigations On The Defect Chemistry and The Sintering of Barium Titanate Ceramics by Oxygen CoulometryDocument7 pagesInvestigations On The Defect Chemistry and The Sintering of Barium Titanate Ceramics by Oxygen Coulometrykholid ristantoNo ratings yet
- Infrared Study of A Novel Acid-Base Site On ZrO2 by Adsorbed Probe Molecules.Document7 pagesInfrared Study of A Novel Acid-Base Site On ZrO2 by Adsorbed Probe Molecules.rafelNo ratings yet
- 2002-ENEA-Preliminary Studies On PbO Reduction in Liquid Pb-Bi Eutectic by Flowing HydrogenDocument4 pages2002-ENEA-Preliminary Studies On PbO Reduction in Liquid Pb-Bi Eutectic by Flowing Hydrogencqc2318273994No ratings yet
- Matsuhashi - Determination of Relative Acid StreDocument5 pagesMatsuhashi - Determination of Relative Acid StreSORIN AVRAMESCUNo ratings yet
- Kinetics of MethanationDocument12 pagesKinetics of MethanationGabriela Campos DávilaNo ratings yet
- Hydrogen PeroxideDocument12 pagesHydrogen PeroxideKautsar Ul HaqNo ratings yet
- Articles: Structural Investigation of The Hydrolysis-Condensation Process of Modified Titanium IsopropoxideDocument5 pagesArticles: Structural Investigation of The Hydrolysis-Condensation Process of Modified Titanium IsopropoxideDeddy Triyono Nugroho AdiNo ratings yet
- Improving Sulphur Tolerance of Methane Combustion Catalysts: R. Torbati, L. Lisi, S. Cimino, G. RussoDocument6 pagesImproving Sulphur Tolerance of Methane Combustion Catalysts: R. Torbati, L. Lisi, S. Cimino, G. RussoJohnny SaavedraNo ratings yet
- Hydration of Propylene Oxide by Hydrogen Peroxide Over Titania CatalystsDocument6 pagesHydration of Propylene Oxide by Hydrogen Peroxide Over Titania CatalystsA MahmoodNo ratings yet
- D2O NHA ManuscriptDocument18 pagesD2O NHA ManuscriptMkhagramNo ratings yet
- Coke Formation On Pt/Zro /al O Catalysts During CH Reforming With CoDocument5 pagesCoke Formation On Pt/Zro /al O Catalysts During CH Reforming With CoWassachol SumarasinghaNo ratings yet
- Reith Oxidation of Na2so3Document7 pagesReith Oxidation of Na2so3cmegmhiNo ratings yet
- Theoretical Studies On Aluminate and Sodium Aluminate Species in Models For Aqueous Solution: Al (Oh), Al (Oh), and Naal (Oh)Document9 pagesTheoretical Studies On Aluminate and Sodium Aluminate Species in Models For Aqueous Solution: Al (Oh), Al (Oh), and Naal (Oh)HoHo WindyantoNo ratings yet
- Spectroscopic Detection of CO Dissociation On Defect Sites On Ru (1 0 9) : Implications For Fischer-Tropsch Catalytic ChemistryDocument4 pagesSpectroscopic Detection of CO Dissociation On Defect Sites On Ru (1 0 9) : Implications For Fischer-Tropsch Catalytic Chemistryamirhosein saqafiNo ratings yet
- Acido Borico Con Ac TartäricoDocument6 pagesAcido Borico Con Ac TartäricoDonaldo HerreraNo ratings yet
- J. Electrochem. Soc. 1948 Lilliendahl 235 47Document13 pagesJ. Electrochem. Soc. 1948 Lilliendahl 235 47Jairo Silva CoreaNo ratings yet
- Linge and A. L. Jones : K,, of The Hydrogen Chromate Ion Has BeenDocument10 pagesLinge and A. L. Jones : K,, of The Hydrogen Chromate Ion Has BeenNicole HuertaNo ratings yet
- A Comparative Study of Water-Gas-Shift Reaction Over Ceria Supported Metallic CatalystsDocument8 pagesA Comparative Study of Water-Gas-Shift Reaction Over Ceria Supported Metallic CatalystsleonardoNo ratings yet
- CyC - Propuesta 1 de PIADocument11 pagesCyC - Propuesta 1 de PIAKike SalasNo ratings yet
- CP Na5p3o10Document6 pagesCP Na5p3o10agnarindra01_8550147No ratings yet
- AC Catalst PTDocument5 pagesAC Catalst PTJarretNo ratings yet
- Takahashi 1979Document6 pagesTakahashi 1979habbibrachmanNo ratings yet
- Analisis de RodiozonatoDocument13 pagesAnalisis de RodiozonatoDonaldo HerreraNo ratings yet
- Photocatalytic Reaction of H20+Co2 Over Pure and Doped Rh/Tio2Document5 pagesPhotocatalytic Reaction of H20+Co2 Over Pure and Doped Rh/Tio2sick_oneNo ratings yet
- PalmerDocument8 pagesPalmerotavioNo ratings yet
- Potassium Nitrate Decomposition Paper PURCHASED Fro Acs - Org Michaelstarr1969Document4 pagesPotassium Nitrate Decomposition Paper PURCHASED Fro Acs - Org Michaelstarr1969michaelstarr1969No ratings yet
- I Io. Chemical Physics Letters: Votr N CDocument5 pagesI Io. Chemical Physics Letters: Votr N CthucinorNo ratings yet
- Au - Rutile Catalysts - Effect of The Activation Atmosphere On The Gold-Xim-2011Document7 pagesAu - Rutile Catalysts - Effect of The Activation Atmosphere On The Gold-Xim-2011leonardoNo ratings yet
- DeactivationMechanismWGS Wang 2002Document18 pagesDeactivationMechanismWGS Wang 2002leonardoNo ratings yet
- Ohtsuka 2010Document7 pagesOhtsuka 2010Rodrigo Regla MuñozNo ratings yet
- Effect of Alumina - Titania Supports On The Activity of PD, PT and Bimetallic PD - PT Catalysts For Hydrorefining ApplicationsDocument5 pagesEffect of Alumina - Titania Supports On The Activity of PD, PT and Bimetallic PD - PT Catalysts For Hydrorefining ApplicationssaraseeNo ratings yet
- Vol 47 - 2 0008 PDFDocument37 pagesVol 47 - 2 0008 PDFSahil DaradeNo ratings yet
- Carbon Dioxide Absorption Into Promoted Carbonate SolutionsDocument10 pagesCarbon Dioxide Absorption Into Promoted Carbonate SolutionsDunyu LiuNo ratings yet
- Cinetica AA FCDocument4 pagesCinetica AA FCRicardo MartinezNo ratings yet
- ' C - After Demineralization, The Sample Was DriedDocument10 pages' C - After Demineralization, The Sample Was Driedsarve1No ratings yet
- Hydrogenation of Aromatics On Modi®ed Platinum Alumina CatalystsDocument12 pagesHydrogenation of Aromatics On Modi®ed Platinum Alumina Catalystssj singhNo ratings yet
- TS. Trương Thái Giang - Hội thảo khoa học Đại học Thành ĐôDocument10 pagesTS. Trương Thái Giang - Hội thảo khoa học Đại học Thành ĐôLưu Thu HàNo ratings yet
- Transesterification Kinetics of Phenyl Salicylate 2Document20 pagesTransesterification Kinetics of Phenyl Salicylate 2Lucas de Lima e SousaNo ratings yet
- Acidity and Basicity of Metal Oxide Surfaces 1 II. Determination by Catalytic Decomposition of IsopropanolDocument9 pagesAcidity and Basicity of Metal Oxide Surfaces 1 II. Determination by Catalytic Decomposition of Isopropanolnguyennha1211No ratings yet
- TMP CAB1Document7 pagesTMP CAB1FrontiersNo ratings yet
- Effect of Ammonia On PT, Ru, RH, and Ni Cathodes During The Alkaline Hydrogen Evolution ReactionDocument13 pagesEffect of Ammonia On PT, Ru, RH, and Ni Cathodes During The Alkaline Hydrogen Evolution ReactionsamypalNo ratings yet
- Kinetic Study of The Catalytic Reforming of Methane With Carbon Dioxide To Synthesis Gas Over Ni - La2O3 Catalyst PDFDocument8 pagesKinetic Study of The Catalytic Reforming of Methane With Carbon Dioxide To Synthesis Gas Over Ni - La2O3 Catalyst PDFMarcus NguyễnNo ratings yet
- Ria PDFDocument23 pagesRia PDFria andrianiNo ratings yet
- Gas-Phase Hydroformylation of Propene Over Silica-Supported PPH - Modified Rhodium CatalystsDocument9 pagesGas-Phase Hydroformylation of Propene Over Silica-Supported PPH - Modified Rhodium CatalystsIlireaNo ratings yet
- Pyrite PaperDocument6 pagesPyrite PaperPham Thi HoaNo ratings yet
- A System of Instruction in the Practical Use of the BlowpipeFrom EverandA System of Instruction in the Practical Use of the BlowpipeNo ratings yet
- RPA Impact of Hydrogen in Trickle Bed Reactor OperationDocument4 pagesRPA Impact of Hydrogen in Trickle Bed Reactor OperationmarviNo ratings yet
- SBA-15 Support SynthesizingDocument13 pagesSBA-15 Support SynthesizingmarviNo ratings yet
- Nature - Hydrogen SpilloverDocument8 pagesNature - Hydrogen SpillovermarviNo ratings yet
- Preparation of Metallic Monolithic PtFeCrAl FiberDocument6 pagesPreparation of Metallic Monolithic PtFeCrAl FibermarviNo ratings yet
- 02-The Preparation of Catalytic MaterialsDocument25 pages02-The Preparation of Catalytic MaterialsmarviNo ratings yet
- H2 SCR To ReplicateDocument8 pagesH2 SCR To ReplicatemarviNo ratings yet
- Structure Sensitivity in HC SomorjaiDocument7 pagesStructure Sensitivity in HC SomorjaimarviNo ratings yet
- An Overview of Low Temperature NOx Storage and Reduction From Automotive ExhaustDocument5 pagesAn Overview of Low Temperature NOx Storage and Reduction From Automotive ExhaustmarviNo ratings yet
- Effects of Temperature On The Formation of Secondary Organic Aerosol From Amine PrecursorsDocument12 pagesEffects of Temperature On The Formation of Secondary Organic Aerosol From Amine PrecursorsmarviNo ratings yet
- Synergism Between The Lewis and BrÖ Nsted Acid Sites On HZSM-5 Zeolites in The Conversion of MethylcyclohexaneDocument7 pagesSynergism Between The Lewis and BrÖ Nsted Acid Sites On HZSM-5 Zeolites in The Conversion of MethylcyclohexanemarviNo ratings yet
- Brochure Ball Mills enDocument16 pagesBrochure Ball Mills enmarviNo ratings yet
- What Is A Heat ExchangerDocument4 pagesWhat Is A Heat ExchangermarviNo ratings yet
- Palagin-2021-Mapping Vibrational Spectra To The - (Accepted Version)Document31 pagesPalagin-2021-Mapping Vibrational Spectra To The - (Accepted Version)marviNo ratings yet
- Catalysts Used For PNADocument3 pagesCatalysts Used For PNAmarviNo ratings yet
- Female Sex - Ratio in India - A Review: January 2012Document17 pagesFemale Sex - Ratio in India - A Review: January 2012marviNo ratings yet
- Supplementary Data: Lifeng Wang, Chengyang Yin and Ralph T. YangDocument3 pagesSupplementary Data: Lifeng Wang, Chengyang Yin and Ralph T. YangmarviNo ratings yet
- Book 1Document4 pagesBook 1marviNo ratings yet
- 21 DecDocument114 pages21 DecmarviNo ratings yet
- Simultaneous Removal of NO and Soot Particulate From Diesel Exhaust by in Situ Catalytic Generation and Utilisation of N O Catherine Davies (2018)Document3 pagesSimultaneous Removal of NO and Soot Particulate From Diesel Exhaust by in Situ Catalytic Generation and Utilisation of N O Catherine Davies (2018)marviNo ratings yet
- Finite Impulse Response (FIR) Filter: Dr. Dur-e-Shahwar Kundi Lec-7Document37 pagesFinite Impulse Response (FIR) Filter: Dr. Dur-e-Shahwar Kundi Lec-7UsamaKhalidNo ratings yet
- Murad MuminovDocument2 pagesMurad Muminovmurodmuminov1221No ratings yet
- 8 DMT Mix MN - Ryan (10!1!24) Ratio N ProportionDocument1 page8 DMT Mix MN - Ryan (10!1!24) Ratio N ProportionRohan MehtaNo ratings yet
- G9 TSC - Diary EntryDocument4 pagesG9 TSC - Diary EntryRoshani SamantNo ratings yet
- Ngo Writ 2 Reflective LetterDocument6 pagesNgo Writ 2 Reflective Letterapi-656316973No ratings yet
- Choose One of The Two Products We Created in ClassDocument3 pagesChoose One of The Two Products We Created in ClassIsmail AliNo ratings yet
- Household Services 4th Quarter Answer KeyDocument1 pageHousehold Services 4th Quarter Answer KeyJohn Matthew PrimaNo ratings yet
- Application Form For Non-Teaching PostsDocument3 pagesApplication Form For Non-Teaching PostsMrs. Dhwani Hakani ADF-PHDNo ratings yet
- Eigen Values of A Matrix by PowerDocument9 pagesEigen Values of A Matrix by PowerVarnika SinghNo ratings yet
- Week 7 Arta111 MidtermDocument5 pagesWeek 7 Arta111 MidtermCASTRO, ANDREI KARL Z.No ratings yet
- 2 Teak DeckingDocument25 pages2 Teak DeckingJoshua Swee100% (1)
- 27-07-2020 - SR - ICON ALL<-Prog-I&II, All - INDIA - e-TEST - SERIES - Jee-ADV (2018-P1&P2) - AFT-09 - FINAL KEYDocument1 page27-07-2020 - SR - ICON ALL<-Prog-I&II, All - INDIA - e-TEST - SERIES - Jee-ADV (2018-P1&P2) - AFT-09 - FINAL KEYSai GokulNo ratings yet
- Human Values and Gender Sensitisation PPT Presentation Assignment 1 Final - PPTMDocument10 pagesHuman Values and Gender Sensitisation PPT Presentation Assignment 1 Final - PPTMMubasshir AliNo ratings yet
- MIT18 05S14 Class5slides PDFDocument17 pagesMIT18 05S14 Class5slides PDFAftab SaadNo ratings yet
- ME 308 Machine Elements Ii: Spring Design - 1Document66 pagesME 308 Machine Elements Ii: Spring Design - 1xxxNo ratings yet
- Chapter 1: Introduction To Applied Economics Economic ResourcesDocument4 pagesChapter 1: Introduction To Applied Economics Economic ResourcesLudgi RuizNo ratings yet
- Blended Learning Environments That Work An Evidence BasedDocument12 pagesBlended Learning Environments That Work An Evidence Basedfirdamerdeka27No ratings yet
- Natural Disasters VocabularyDocument1 pageNatural Disasters VocabularySabrina Gabriela50% (2)
- (Prelim) Understanding Culture, Society, and PoliticsDocument92 pages(Prelim) Understanding Culture, Society, and PoliticsAdrian DionisioNo ratings yet
- Acadamic StressDocument23 pagesAcadamic Stressalene100% (1)
- Microeconomic Analysis NotesDocument23 pagesMicroeconomic Analysis NotesMinira JafarovaNo ratings yet
- Mod01 L02Document12 pagesMod01 L02Pankaj Kumar SainiNo ratings yet
- Self Evaluation 11 13Document3 pagesSelf Evaluation 11 13api-702593326No ratings yet
- Ikigai (Passion)Document10 pagesIkigai (Passion)Siti KhoirunnisaNo ratings yet
- Arenstorf OriginalDocument18 pagesArenstorf OriginalMister DumbledoreNo ratings yet
- MTESI004 Shaking Things Up PDFDocument56 pagesMTESI004 Shaking Things Up PDFAndre OuimetNo ratings yet
- Python-2 - Unit-6,7,8 - Game DevelopmentDocument38 pagesPython-2 - Unit-6,7,8 - Game DevelopmentPalak RathoreNo ratings yet
- Disaster Management ApproachDocument16 pagesDisaster Management ApproachSamir SitaulaNo ratings yet
- C-Reactive Protein (CRP)Document9 pagesC-Reactive Protein (CRP)Ibrahim Alturaiki100% (1)