Professional Documents
Culture Documents
Oc PT 2 - Student Copy - (Eng)
Oc PT 2 - Student Copy - (Eng)
Uploaded by
Ramkumar SundaramOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Oc PT 2 - Student Copy - (Eng)
Oc PT 2 - Student Copy - (Eng)
Uploaded by
Ramkumar SundaramCopyright:
Available Formats
PRACTICE TEST -02 JEE (MAIN + ADVANCED) 2021
ALL BATCH
ORGANIC CHEMISTRY TIME : 45 MIN
SECTION–I : (Maximum Marks : 80)
This section contains TWENTY questions.
Each question has FOUR options (A), (B), (C) and (D). ONLY ONE of these four options
is correct.
For each question, darken the bubble corresponding to the correct option in the ORS.
For each question, marks will be awarded in one of the following categories :
Full Marks : +4 If only the bubble corresponding to the correct option is darkened.
Zero Marks : 0 If none of the bubbles is darkened.
Negative Marks : –1 In all other cases
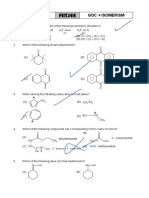
1. Which of the following do not represent resonating structure of
(Binphenyl)
(A) (B) – : +
(C) + :– (D)
2. Which of the following compounds are optically active ?
(A) Cl (B) Cl Cl
Cl
OH
Cl
(C) H2C=C=C (D)
Br OH
3. Compound which shows highest rate of electrophilic substitution :
CH3
H3C CH3
CH 3 CH3 CH3 C
(A) (B) (C) (D)
H3C
CH3 C C
H3C CH3 H3C CH3
CH3 CH3
1/6 1 ORGANIC CHEM. / PT # 2
PRACTICE TEST -02 JEE (MAIN + ADVANCED) 2021
ALL BATCH
4. Which of the nitrogen containing heterocyclic compound (BASE) is not present in DNA :
NH2 O NH2 O
C C C C
N NH
C N C NH HC N HC N–H
(A) HC (B) HC (C) (D)
C CH C C HC C HC C
N N N NH2 N O O
N N
H H Guanine
Adenine H H
Cytosine Uracil
5. Rate of reaction of following alkenes with H3OÅ :
CH=CH2 CH=CH2 CH=CH2 CH=CH2
O NO2 CH3
I II III IV
(A) IV > I > II > III (B) IV > II > I > III (C) II > IV > I > III (D) III > II > I > IV
O
H3CO
'n' RMgX
6. O
Cl
O
Value of 'n' is -
(A) 3 (B) 4 (C) 5 (D) 6
Mg 1. CO2
7. Br A + B
THF 1. H
LiAlH4
C
Correct statement about C.
(A) It gives turbidity in 5 min with lucas reagent (B) It is Ph—CHO
(C) It is Ph—CH2—OH (D) It gives racimisation with PCl3
(1) NaOH
8. Succinic acid (2) Electrolysis ?
The major organic product formed in above reaction is :
(A) CH3—CH3 (B) CH2=CH2 (C) (D) HCºCH
9. Formation of alkyl iodide from alkyl halide (R–Cl, R–Br) can be best synthesis by reaction
(A) Darzen process (B) Swarts reaction
(C) Williamson ether synthesis (D) Finkelstein reaction
10. A compound having molecular formula C5H10O gives red-orange ppt with 2, 4, DNP but gives
negative response to NaHSO3. Then compound is :
O OH
O
(A) (B) (C) (D)
O
2/6 2 ORGANIC CHEM. / PT # 2
PRACTICE TEST -02 JEE (MAIN + ADVANCED) 2021
ALL BATCH
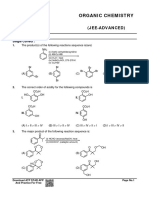
11. In which of the following reaction, one of the product obtained will give isocynide test ?
O
O
Br2 NH2 LAH
(A) CH3–C–NH2 ¾¾¾
KOH
® (B) ¾¾¾®
O
– + (i) CH - Br
N K ¾¾¾¾¾ ®
3
(C) (ii) H O+ (D) All of these
3
O
12. Which of the following is not correct ?
– –
(A) I > Br (Leaving group ability)
–
(B) HCºC– > OH (Nucleophilic strength)
(C) CH2 = CH2 > HC º CH (Electrophilic addition)
(D) + < + (Carbocation stability)
13. Markownikoff's rule is not applicable on which of the following alkene :
(A) (B) (C) (D)
14. Reaction correctly matched with their major product is/are -
Electrolysis
(A) CH3–CH2–COOK ¾¾¾¾¾ ® CH3–CH3
O Me O
(i) Mg
C Me— C— C—Me
(B) H C (ii) H2O
3 CH3 +
(iii) H / Me
R
(i)LiAlH 4
(C) R C Cl ¾¾¾¾
(ii)H 2O
® R C OH
O R
–
(D) Me3C–Cl + CH3O ¾® Me3C–OCH3
15. Major product of reaction is :
OH
(1) NaOH
+ CHCl3
(2) H +
OH OH OH OH
OHC CHO CHO
(A) (B) (C) (D)
CHO
CHO
3/6 3 ORGANIC CHEM. / PT # 2
PRACTICE TEST -02 JEE (MAIN + ADVANCED) 2021
ALL BATCH
16. Considering C2–C3 bond rotation, the most stable conformation for the compound CH3–CH–CH–CH 3
OH F
among following :
Me Me Me Me
HO H Me H H OH H OH
(A) (B) (C) (D)
H F H F H F Me F
Me OH Me H
17. Which of the following reaction sequence would be the best for synthesizing the compound,
1-bromo-3-propylbenzene ?
Br
1-Bromo-3-propylbenzene
O O
Br2 Cl Zn(Hg) Cl Br2 Zn(Hg)
(A) (B)
FeBr3 AlCl3 HCl AlCl3 FeBr3 HCl
O
Br2 Br2 Cl
Cl Zn(Hg)
(C) (D) FeBr3 AlCl 3
AlCl3 HCl FeBr3
18. Give the final major product from the following reaction sequence :
O
Ethylene glycol Collin's EtMgBr H+
OH + reagent H2O
H
HO O O O
(A) OH (B) (C) (D)
H
O HO
19. Identify major product for the following reaction :
Cl
Cl
NaOMe / MeOH
O2N NO2
OMe Cl Cl OMe
MeO MeO MeO Cl
(A) (B) (C) (D)
O2N NO2 O2N NH2 O2N NO2 O2 N NO2
20. Among the following compounds, which will react with acetone to give a product containing
C=N – NH–
(A) C6H5NHNH2 (B) (CH3)3N (C) C6H5NHC6H5 (D) None of these
4/6 4 ORGANIC CHEM. / PT # 2
PRACTICE TEST -02 JEE (MAIN + ADVANCED) 2021
ALL BATCH
SECTION-II : (Maximum Marks: 20)
This section contains FIVE questions.
The answer to each question is a NUMERICAL VALUE.
For each question, enter the correct numerical value (If the numerical value has more
than two decimal places, truncate/round-off the value to TWO decimal places; e.g. 6.25,
7.00, –0.33, –.30, 30.27, –127.30, if answer is 11.36777..... then both 11.36 and 11.37 will
be correct) by darken the corresponding bubbles in the ORS.
For Example : If answer is –77.25, 5.2 then fill the bubbles as follows.
+ – + –
0 0 0 0 • 0 0 0 0 0 0 • 0 0
1 1 1 1 • 1 1 1 1 1 1 • 1 1
2 2 2 2 • 2 2 2 2 2 2 • 2 2
3 3 3 3 • 3 3 3 3 3 3 • 3 3
4 4 4 4 • 4 4 4 4 4 4 • 4 4
5 5 5 5 • 5 5 5 5 5 5 • 5 5
6 6 6 6 • 6 6 6 6 6 6 • 6 6
7 7 7 7 • 7 7 7 7 7 7 • 7 7
8 8 8 8 • 8 8 8 8 8 8 • 8 8
9 9 9 9 • 9 9 9 9 9 9 • 9 9
Answer to each question will be evaluated according to the following marking scheme:
Full Marks : +4 If ONLY the correct numerical value is entered as answer.
Zero Marks : 0 In all other cases.
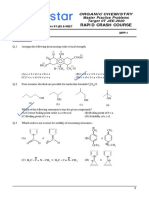
1. Number of moles of CO2 released when following molecule oxidised by O3/H2O followed by heating
2. How many reagents can be used for the given conversion.
OH O
(1) Cu, 300ºC (2) KMnO4 /HÅ (3) PCC /CH2Cl2 (4) K2Cr2O7 /HÅ
(5) MnO2 (6) Al(OCMe3)3/Acetone (7) NBS (8) [Ag(NH3)2]OH
(9) (i) TsCl (ii) DMSO (iii) NaHCO3 (10) (i) DIBAL-H (ii) H2O
3. Number of H exchanged by deuterium when following compound is treated with NaOD/D2O for
a long time :
O
5/6 5 ORGANIC CHEM. / PT # 2
PRACTICE TEST -02 JEE (MAIN + ADVANCED) 2021
ALL BATCH
4. Number of compound give effervescence of CO2() on reacting with NaHCO3 :
OH COOH
CH3 HO O
(i) (ii) (iii) (iv) HCl (v)
COOH HO O
OH
NH2
(vi) (vii) CH3–SO3H (viii) (ix)
COOH
5. Total number 1,2-shift taking place during rearrangment of the given compound.
6/6 6 ORGANIC CHEM. / PT # 2
You might also like
- (NEWS) Everything You Need To Ace Science in One Big Fat Notebook - The Complete Middle School Study Guide (Big Fat Notebooks) by UnlimitedDocument5 pages(NEWS) Everything You Need To Ace Science in One Big Fat Notebook - The Complete Middle School Study Guide (Big Fat Notebooks) by UnlimitedRamkumar Sundaram13% (15)
- FOUNDATION - MATHS - STD7 - 8 - M92 - FLOW - PLANNER - SAMPLE - PAPERS of RAJESH SIR 2022-23Document23 pagesFOUNDATION - MATHS - STD7 - 8 - M92 - FLOW - PLANNER - SAMPLE - PAPERS of RAJESH SIR 2022-23Ramkumar SundaramNo ratings yet
- Operating Manual: CentrifugeDocument75 pagesOperating Manual: CentrifugeCeleynes RTNo ratings yet
- M9 - Simulataneous Linear Equations - Test - 1598698260044 - DsaubDocument1 pageM9 - Simulataneous Linear Equations - Test - 1598698260044 - DsaubRamkumar SundaramNo ratings yet
- The Bogey BeastDocument5 pagesThe Bogey BeastBrayan ChiribogaNo ratings yet
- Alkyl Halides and Aryl Halides - QBDocument23 pagesAlkyl Halides and Aryl Halides - QBNETHAKANI SUJATHA100% (1)
- 2024 03 10 JM 25A ONLINE - PMD 1328930 2024 03 12 09 16Document17 pages2024 03 10 JM 25A ONLINE - PMD 1328930 2024 03 12 09 16gakot74126No ratings yet
- Isomerism DPPDocument4 pagesIsomerism DPPRAGHUL MNo ratings yet
- Practice Question EliminationDocument7 pagesPractice Question EliminationSarveshsinghNo ratings yet
- Goc 1Document3 pagesGoc 1Twisha ViraniNo ratings yet
- PB 003 Alkyl HalideDocument12 pagesPB 003 Alkyl HalideEklavya GoyalNo ratings yet
- Chemistry Max. Marks: 61 Section - I (Single Correct Choice Type)Document6 pagesChemistry Max. Marks: 61 Section - I (Single Correct Choice Type)VENKATA SRI RAM KOTIPALLI iitjkkdNo ratings yet
- Organic Chemistry Test-1 On Total Syllabus: Single CorrectDocument5 pagesOrganic Chemistry Test-1 On Total Syllabus: Single CorrectVanshaj GuptaNo ratings yet
- Black Board Problems For JEE Advanced Set-7Document8 pagesBlack Board Problems For JEE Advanced Set-7DikshantNo ratings yet
- Amine - DPPDocument9 pagesAmine - DPPIs IsNo ratings yet
- qUIZ CavDocument6 pagesqUIZ Cavsumit parasharNo ratings yet
- Mixed DPP 8 - 20 (Isomerism)Document37 pagesMixed DPP 8 - 20 (Isomerism)shresthgaur19No ratings yet
- A - 1 (Isomerism, Reaction Mechanism) - Question PaperDocument11 pagesA - 1 (Isomerism, Reaction Mechanism) - Question PaperSachin DedhiaNo ratings yet
- Goc + IsomerismDocument5 pagesGoc + IsomerismRohail HussainNo ratings yet
- Complete Course Organic ChemistrDocument11 pagesComplete Course Organic Chemistrmanash-12No ratings yet
- 02 - Alkyl Halide - Aryl Halide (Questions)Document48 pages02 - Alkyl Halide - Aryl Halide (Questions)Sidhiprada PradhanNo ratings yet
- Biomoleculer and PolymerDocument10 pagesBiomoleculer and Polymerjiknown6No ratings yet
- (02-12-14) AlkenesDocument4 pages(02-12-14) Alkenessasi.curieNo ratings yet
- Quiz-General Organic Chemistry & Isomerism-Snd - SNDDocument4 pagesQuiz-General Organic Chemistry & Isomerism-Snd - SNDayesha sheikhNo ratings yet
- DPP-14 Text Solution 20230717100029Document3 pagesDPP-14 Text Solution 20230717100029ansarinaved8920No ratings yet
- Alkyl and Halide Ex. NeetDocument39 pagesAlkyl and Halide Ex. NeetashishNo ratings yet
- Goc Stereo PDFDocument32 pagesGoc Stereo PDFDeepak GargNo ratings yet
- JEE (Advanced) - 2018 TEST PAPER With Solution: (Exam Date: 20-05-2018) Part-1: ChemistryDocument13 pagesJEE (Advanced) - 2018 TEST PAPER With Solution: (Exam Date: 20-05-2018) Part-1: Chemistrysaravanaajani2012No ratings yet
- General Organic Chemistry: H NH NH N A)Document10 pagesGeneral Organic Chemistry: H NH NH N A)kamalNo ratings yet
- Chemistry Paper - I - Question PaperDocument6 pagesChemistry Paper - I - Question PaperKUNALNo ratings yet
- Halogen Derivatives PDFDocument38 pagesHalogen Derivatives PDFastha100% (1)
- Subject: Chemistry DATE: 19-03-2024: StereoisomerismDocument3 pagesSubject: Chemistry DATE: 19-03-2024: Stereoisomerism10 Million SubscribersNo ratings yet
- Sheet-5-Hydro CarbonDocument9 pagesSheet-5-Hydro CarbonZooper lNo ratings yet
- 2008Document7 pages2008prakhar vishwakarmaNo ratings yet
- Guided Revision: Sot Type 4 (-1) 1Document3 pagesGuided Revision: Sot Type 4 (-1) 1Shubham RajNo ratings yet
- Chapter 24. Amines and HeterocyclesDocument19 pagesChapter 24. Amines and Heterocycles張湧浩No ratings yet
- Spotlight Phase 2 2021 22 Day 1 in Class Assingement Chemistry OnlyDocument8 pagesSpotlight Phase 2 2021 22 Day 1 in Class Assingement Chemistry Onlysnohkmr04136No ratings yet
- Part - I: Subjective Questions: Section (A) : Electrophile, Nucleophile, Nucleophilicity, Leaving Group Ability SolventDocument18 pagesPart - I: Subjective Questions: Section (A) : Electrophile, Nucleophile, Nucleophilicity, Leaving Group Ability SolventRavi kumarNo ratings yet
- Isomerism Sheet - by NJ Sir PDFDocument42 pagesIsomerism Sheet - by NJ Sir PDFVikas Rana100% (2)
- Carbenes and NitrenesDocument13 pagesCarbenes and NitrenesdevendraNo ratings yet
- Alkyl HalidesDocument14 pagesAlkyl HalidesR.SaivigneshNo ratings yet
- Organic Chemistry Guided Revision Plan-Score AdvancedDocument4 pagesOrganic Chemistry Guided Revision Plan-Score AdvancedNamchrahsiNo ratings yet
- Alcohol Phenol Either and Probability 12Document23 pagesAlcohol Phenol Either and Probability 12jiknown6No ratings yet
- FIITJEE Kukatpally Jee Mains Test PDFDocument16 pagesFIITJEE Kukatpally Jee Mains Test PDFmadhavNo ratings yet
- Oc Taas Quiz 1 StudentDocument2 pagesOc Taas Quiz 1 Studentattackerasp1234No ratings yet
- Chemistry Test 24orgDocument24 pagesChemistry Test 24orgVineeth V TNo ratings yet
- Alcohols IIT PACEDocument19 pagesAlcohols IIT PACEDushyant GuptaNo ratings yet
- Ape Assignment 3Document7 pagesApe Assignment 3Atharva KulkarniNo ratings yet
- DPP-8 Text Solution 20230717095820Document4 pagesDPP-8 Text Solution 20230717095820ansarinaved8920No ratings yet
- NEET Organic Chemistry - Some Basic Principles and Techniques Important QuestionsDocument18 pagesNEET Organic Chemistry - Some Basic Principles and Techniques Important QuestionsThamizharuvi. ANo ratings yet
- Chem 2016Document4 pagesChem 2016Shubhankar ChakrabortyNo ratings yet
- Organic Compounds Containing NitrogenDocument19 pagesOrganic Compounds Containing NitrogenPradyumnNo ratings yet
- Jumbo Home Test-1 - FinalDocument59 pagesJumbo Home Test-1 - FinalSayali SachinNo ratings yet
- Dr. Gurumeet C Wadhawa Department of Chemistry Karmaveer Bhaurao Patil, College Vashi, Navi MumbaiDocument44 pagesDr. Gurumeet C Wadhawa Department of Chemistry Karmaveer Bhaurao Patil, College Vashi, Navi MumbaiGurumeet WadhavaNo ratings yet
- ExerciseDocument50 pagesExerciseAbhiNo ratings yet
- DPP 3 Electronic EffectDocument3 pagesDPP 3 Electronic EffectUsha SherkhaneNo ratings yet
- C Ch-25 AminesDocument7 pagesC Ch-25 Aminesmysoftinfo.incNo ratings yet
- GoodgDocument19 pagesGoodgTusharNo ratings yet
- CAC Glorifire T2 Ques.+Key 18-05-2024Document9 pagesCAC Glorifire T2 Ques.+Key 18-05-2024jerupatianjaliNo ratings yet
- Rapid Crash Course: Single CorrectDocument8 pagesRapid Crash Course: Single CorrectHudsun HornetNo ratings yet
- RP RP CL CL CL RP CL PRDocument8 pagesRP RP CL CL CL RP CL PRJAIMIN PATELNo ratings yet
- GujCET - D26 Mar 2023Document34 pagesGujCET - D26 Mar 2023aadityabhagchandaniNo ratings yet
- Carbenes and Nitrenes by IIT CampusDocument13 pagesCarbenes and Nitrenes by IIT CampusHemant soni100% (1)
- Atp Mega RevDocument14 pagesAtp Mega RevSURAKSHA PATELNo ratings yet
- Class Test 05 EngDocument3 pagesClass Test 05 EngRamkumar SundaramNo ratings yet
- Rotational MotionDocument19 pagesRotational MotionRamkumar SundaramNo ratings yet
- Low Carb On Any BudgetDocument25 pagesLow Carb On Any BudgetRamkumar Sundaram100% (1)
- PCP Practicals 2021 - 22Document2 pagesPCP Practicals 2021 - 22Ramkumar SundaramNo ratings yet
- Fit Course DetailsDocument2 pagesFit Course DetailsRamkumar SundaramNo ratings yet
- Covid-19 Home Testing Using Rapid Antigen Tests (Rats)Document2 pagesCovid-19 Home Testing Using Rapid Antigen Tests (Rats)Ramkumar SundaramNo ratings yet
- Purusha Suktam PDFDocument13 pagesPurusha Suktam PDFRamkumar SundaramNo ratings yet
- S Chidambaram: Haematology Good Health Package Test Name Result Unit Bio Ref - Interval MethodDocument11 pagesS Chidambaram: Haematology Good Health Package Test Name Result Unit Bio Ref - Interval MethodRamkumar SundaramNo ratings yet
- Crompton Greaves (CG Power) Single Phase Motors Catalogue and Price List 2020 PDFDocument10 pagesCrompton Greaves (CG Power) Single Phase Motors Catalogue and Price List 2020 PDFRamkumar SundaramNo ratings yet
- Janmashtami SpecialDocument1 pageJanmashtami SpecialRamkumar SundaramNo ratings yet
- F, Rt-1-O .: To /lai °'Document9 pagesF, Rt-1-O .: To /lai °'Ramkumar SundaramNo ratings yet
- Naren ProbabilityDocument5 pagesNaren ProbabilityRamkumar SundaramNo ratings yet
- Macros FileDocument12 pagesMacros FileRamkumar SundaramNo ratings yet
- Abhyasa Pustakam PDFDocument76 pagesAbhyasa Pustakam PDFjoker883288% (8)
- (Nick Arnold Tony de Saulles) Nasty Nature PDFDocument81 pages(Nick Arnold Tony de Saulles) Nasty Nature PDFRamkumar SundaramNo ratings yet
- Aman Apparent ProjectDocument56 pagesAman Apparent ProjectAmensisa DugasaNo ratings yet
- Class Ix Biology Assignment 6 The Fundamental Unit of LifeDocument4 pagesClass Ix Biology Assignment 6 The Fundamental Unit of LifeMadhusudan BanerjeeNo ratings yet
- En 1090 2 Ex3 PDFDocument1 pageEn 1090 2 Ex3 PDFlai nguyenNo ratings yet
- Retur PlanDocument6 pagesRetur PlanMuhammad RizkyNo ratings yet
- Scott and OllisDocument16 pagesScott and OllisReynaldo Caballero QuirozNo ratings yet
- Chapter - 15: Combustion of FuelsDocument25 pagesChapter - 15: Combustion of FuelsMuhammad AliNo ratings yet
- TECHNOLOGY4 Answer 2Document7 pagesTECHNOLOGY4 Answer 2ابومحمد الكنانيNo ratings yet
- Sab 2112 - L7 ConcreteDocument54 pagesSab 2112 - L7 Concreteapi-19705508No ratings yet
- ISO 7599 INTERNATIONAL STANDARD. Anodizing of Aluminium and Its Alloys General Specifications For Anodic Oxidation Coatings On AluminiumDocument28 pagesISO 7599 INTERNATIONAL STANDARD. Anodizing of Aluminium and Its Alloys General Specifications For Anodic Oxidation Coatings On Aluminium杜文欽No ratings yet
- Qapco TestDocument6 pagesQapco TesttheoNo ratings yet
- 2017 - Multiscale Polymer Composites A Review of The Interlaminar Fracture Toughness ImprovementDocument27 pages2017 - Multiscale Polymer Composites A Review of The Interlaminar Fracture Toughness ImprovementSubramani PichandiNo ratings yet
- Dom 24Document3 pagesDom 24Brandong Franco MejíaNo ratings yet
- CHEMISTRY 2 ModelDocument72 pagesCHEMISTRY 2 ModelJuvzNo ratings yet
- List of Customers Reference: FRP Storage Tank & Process TankDocument11 pagesList of Customers Reference: FRP Storage Tank & Process TankDyah Ayu widatiNo ratings yet
- 5.stabilisation and SolidificationDocument18 pages5.stabilisation and SolidificationFx NiubieNo ratings yet
- Electrodeposition Fer 02Document10 pagesElectrodeposition Fer 02MatteoNo ratings yet
- CAT15 - 6 - Pressure Zinga PDFDocument25 pagesCAT15 - 6 - Pressure Zinga PDFJonathan GiraldoNo ratings yet
- 1 s2.0 S0956053X19306579 MainDocument9 pages1 s2.0 S0956053X19306579 MainVanessa FerreiraNo ratings yet
- Using Mole Calculations To Solve Problems: Learning ObjectivesDocument5 pagesUsing Mole Calculations To Solve Problems: Learning ObjectivesMark John Paul CablingNo ratings yet
- Gupta Et Al 2016 EJSBDocument8 pagesGupta Et Al 2016 EJSBrg1326No ratings yet
- Estimation of Serum Creatinine by Routine Jaffes Method and by Dry Chemistry in Icteric and Hemolytic Serum SamplesDocument8 pagesEstimation of Serum Creatinine by Routine Jaffes Method and by Dry Chemistry in Icteric and Hemolytic Serum Samplessyedamasoomazahra9No ratings yet
- Swiss Tool Catalog - EN - Small PDFDocument338 pagesSwiss Tool Catalog - EN - Small PDFPalade LucianNo ratings yet
- C2 C3 C4 & Cam Variations On The Theme of PhotosynthesisDocument20 pagesC2 C3 C4 & Cam Variations On The Theme of PhotosynthesisKoons KoonsNo ratings yet
- Impregnation Fla EDocument16 pagesImpregnation Fla ENghia Phan TrungNo ratings yet
- S&T Module 3Document8 pagesS&T Module 3Pj LlagasNo ratings yet
- ASEAN Harmonized Tariff Nomenclature 2022Document5 pagesASEAN Harmonized Tariff Nomenclature 2022lito77No ratings yet
- DNA PCR DNA Degradation Solutions: Product Information SheetDocument2 pagesDNA PCR DNA Degradation Solutions: Product Information SheetchiralicNo ratings yet
- Physics Books - Navneet Practice Paper and Activity Sheets Multiple Choice QuestionsDocument50 pagesPhysics Books - Navneet Practice Paper and Activity Sheets Multiple Choice QuestionsAamir KhanNo ratings yet