Professional Documents
Culture Documents
Calculate The Number of Unit Cells in 8.1 G of Aluminium If It Crystallizes in A FCC Structure. - Sarthaks Econnect Largest On
Calculate The Number of Unit Cells in 8.1 G of Aluminium If It Crystallizes in A FCC Structure. - Sarthaks Econnect Largest On
Uploaded by
abhim270Copyright:
Available Formats
You might also like
- En 14582Document33 pagesEn 14582Vladimir Krzalic100% (1)
- Chemistry Problem JEE PDFDocument1,071 pagesChemistry Problem JEE PDFSaurav Dutt100% (5)
- How Are Dominance, Codominance and Incomplete Dominance Patterns of Inheritance Different From Each Other - Sarthaks EConnectDocument1 pageHow Are Dominance, Codominance and Incomplete Dominance Patterns of Inheritance Different From Each Other - Sarthaks EConnectPaarth GoyalNo ratings yet
- (A) State How The Constant Internal Environment Is Beneficial To Organisms. - Sarthaks Econnect Largest Online Education CommuDocument1 page(A) State How The Constant Internal Environment Is Beneficial To Organisms. - Sarthaks Econnect Largest Online Education CommuPaarth GoyalNo ratings yet
- A Convex Lens Has A Focal Length of 12 Cm. at What Distance From The Lens Should An Object of Height 6 CM Be Placed - Sarthaks eDocument1 pageA Convex Lens Has A Focal Length of 12 Cm. at What Distance From The Lens Should An Object of Height 6 CM Be Placed - Sarthaks elateral0thinkerNo ratings yet
- JEE NEET Physics Question Bank For ElectrostaticsDocument50 pagesJEE NEET Physics Question Bank For ElectrostaticsMBVIBES INSTIPHYNo ratings yet
- Home Random NotesDocument10 pagesHome Random NotesAnvi RoseNo ratings yet
- NTSE Syllabus - Complete NTSE Exam SyllabusDocument2 pagesNTSE Syllabus - Complete NTSE Exam Syllabusteresa castellanNo ratings yet
- 1st Year Physics Pairing Scheme 2020 - 11th Class - Ratta - PK PDFDocument19 pages1st Year Physics Pairing Scheme 2020 - 11th Class - Ratta - PK PDFCH M Ahmed0% (1)
- What Are The Expectedproducts of Hydrolysis of LactoseDocument1 pageWhat Are The Expectedproducts of Hydrolysis of Lactosebalajiharish75No ratings yet
- BITSAT Exam Pattern 2020Document3 pagesBITSAT Exam Pattern 2020Udaya BhaskarNo ratings yet
- Principles of Geotechnical Engineering 7th Edition Textbook Solutions Chegg - Com 9Document1 pagePrinciples of Geotechnical Engineering 7th Edition Textbook Solutions Chegg - Com 9Cristian A. GarridoNo ratings yet
- Which of The Following Compounds Will Show Cis Trans IsomerismDocument1 pageWhich of The Following Compounds Will Show Cis Trans IsomerismaliyaraseebNo ratings yet
- Screenshot 2023-11-28 at 1.47.22 PMDocument1 pageScreenshot 2023-11-28 at 1.47.22 PMAshish KumarNo ratings yet
- NTSE Syllabus - National Talent SearchDocument1 pageNTSE Syllabus - National Talent SearchSatyaranjan mahapatraNo ratings yet
- How Should I Prepare Electromagnetic Theory For Gate and IES ECE Both - QuoraDocument3 pagesHow Should I Prepare Electromagnetic Theory For Gate and IES ECE Both - Quoravigneshwaran50No ratings yet
- Phyllotaxy - NEET Biology NotesDocument1 pagePhyllotaxy - NEET Biology NotesReyansh JainNo ratings yet
- Chapter 2 2-003 (Basis)Document6 pagesChapter 2 2-003 (Basis)Jamiel CatapangNo ratings yet
- Anthe Syllabus and Sample PapersDocument24 pagesAnthe Syllabus and Sample PapersKishan kumarNo ratings yet
- NTSE Stage 1 Analysis Report Rajasthan PDFDocument7 pagesNTSE Stage 1 Analysis Report Rajasthan PDFShyama RanjanNo ratings yet
- Fill in The Blanks The Formula Unit Mass of Ca3 (PO4) 2 Is...................... Chemistry Q&ADocument1 pageFill in The Blanks The Formula Unit Mass of Ca3 (PO4) 2 Is...................... Chemistry Q&Ashauryadiwan5No ratings yet
- Jr-JEE - NEET Weekly Test - Syllabus at 05-12-2022Document1 pageJr-JEE - NEET Weekly Test - Syllabus at 05-12-2022danishquadri4001No ratings yet
- Campus Placement at Cognizant Technology Solutions and Worked For 1month - 2012 Nov (Resigned Due To Pregnancy & Relocation To Korea)Document2 pagesCampus Placement at Cognizant Technology Solutions and Worked For 1month - 2012 Nov (Resigned Due To Pregnancy & Relocation To Korea)naveen kumar pendaNo ratings yet
- Detailed Analysis v2Document9 pagesDetailed Analysis v2Madhukar DakoreNo ratings yet
- Home - JIGSSOLANKIDocument9 pagesHome - JIGSSOLANKIAnvi RoseNo ratings yet
- Lakshya Major Test Series LeafDocument2 pagesLakshya Major Test Series LeafDivyanshu Gautam0% (1)
- Physics Revision Notes For Class 11Document13 pagesPhysics Revision Notes For Class 11Gurunikesh S0% (1)
- Schedule & Syllabus of ONLINE TEST SERIESDocument2 pagesSchedule & Syllabus of ONLINE TEST SERIESRomyNo ratings yet
- Leader Online Test Series For Jee Main Plus Advanced 2018Document2 pagesLeader Online Test Series For Jee Main Plus Advanced 2018Ayush SharmaNo ratings yet
- Thermodynamics: Message Boards Test Prep Centers SparkcollegeDocument23 pagesThermodynamics: Message Boards Test Prep Centers SparkcollegeMercy NunezNo ratings yet
- Safari - May 21, 2023 at 1:22 AMDocument1 pageSafari - May 21, 2023 at 1:22 AMgayauaiaiNo ratings yet
- Principles of Geotechnical Engineering 7th Edition Textbook Solutions Chegg - Com 8Document1 pagePrinciples of Geotechnical Engineering 7th Edition Textbook Solutions Chegg - Com 8Cristian A. GarridoNo ratings yet
- Sem1AY201718 LifelongLearningforNUSAlumni ListofModules PDFDocument7 pagesSem1AY201718 LifelongLearningforNUSAlumni ListofModules PDFManoj GuptaNo ratings yet
- Q3 Explain Why The Bulb Would Not Glow in The Arr PDFDocument1 pageQ3 Explain Why The Bulb Would Not Glow in The Arr PDFDhyansh MalhotraNo ratings yet
- Abstract FORM - NEW - 2023 - Abstract-1Document1 pageAbstract FORM - NEW - 2023 - Abstract-1sid.chilesNo ratings yet
- JEE Main 2023 April Session 2 Shift-1 (DT 08-04-2023) Detailed AnalysisDocument13 pagesJEE Main 2023 April Session 2 Shift-1 (DT 08-04-2023) Detailed AnalysisResonance EduventuresNo ratings yet
- UT & TE Planner - AY 2023-24 - Phase-01Document1 pageUT & TE Planner - AY 2023-24 - Phase-01Atharv KumarNo ratings yet
- Jee Main and Advanced Nurture Schedule and SyllabusDocument2 pagesJee Main and Advanced Nurture Schedule and SyllabuseGamingMafiaNo ratings yet
- Study PLANNER XI (First Step) - JEE Main & Advanced 2021-23 (Phase-3) - May'21 - February'22..Document28 pagesStudy PLANNER XI (First Step) - JEE Main & Advanced 2021-23 (Phase-3) - May'21 - February'22..ZzoNo ratings yet
- Principles of Geotechnical Engineering 7th Edition Textbook Solutions Chegg - Com 10Document1 pagePrinciples of Geotechnical Engineering 7th Edition Textbook Solutions Chegg - Com 10Cristian A. GarridoNo ratings yet
- JEE Main 2023 January Session 1 Shift-1 (DT 29-01-2023) Detailed AnalysisDocument12 pagesJEE Main 2023 January Session 1 Shift-1 (DT 29-01-2023) Detailed AnalysisResonance EduventuresNo ratings yet
- MCQ in Age, Work, Mixture, Digit, Motion Problems Part 3 - ECE Board ExamDocument21 pagesMCQ in Age, Work, Mixture, Digit, Motion Problems Part 3 - ECE Board Examsam labineNo ratings yet
- Test Planner - Yakeen NEET 2025Document3 pagesTest Planner - Yakeen NEET 2025AditiNo ratings yet
- Sem1AY201718 LifelongLearningforNUSAlumni ListofModulesDocument7 pagesSem1AY201718 LifelongLearningforNUSAlumni ListofModulesVance KangNo ratings yet
- Bak Li Wal FoundationDocument2 pagesBak Li Wal FoundationshaileshvcNo ratings yet
- OctoberDocument1 pageOctoberapi-371827688No ratings yet
- BYJU'S Test Series AITS (2019 - 2020) JEE/NEET Paper Dates: Schedule For Class 11Document2 pagesBYJU'S Test Series AITS (2019 - 2020) JEE/NEET Paper Dates: Schedule For Class 11Akash.SNo ratings yet
- UT, TE & ST Planner - First Step Group 1 - AY 2021-22 Updated On15.04.2021 Version 3.0Document2 pagesUT, TE & ST Planner - First Step Group 1 - AY 2021-22 Updated On15.04.2021 Version 3.0kjsc ncsdNo ratings yet
- Physics Group Subjects UpdatedDocument14 pagesPhysics Group Subjects Updatedvasavik.eeeNo ratings yet
- Sample PDF of Neet Ug Maths Book Vol 1Document14 pagesSample PDF of Neet Ug Maths Book Vol 1Mohamed radwanNo ratings yet
- B TechDocument12 pagesB TechProf. Anurag KumarNo ratings yet
- 1st Year Chemistry Pairing Scheme 2021 - 11th Class - Ratta - PKDocument2 pages1st Year Chemistry Pairing Scheme 2021 - 11th Class - Ratta - PKazeemNo ratings yet
- NTSE Detailed Analysis Jharkhand 2016 17Document8 pagesNTSE Detailed Analysis Jharkhand 2016 17Rishav KumarNo ratings yet
- Define Parasite and Total Parasite and Partial Parasites - Biology Q&ADocument1 pageDefine Parasite and Total Parasite and Partial Parasites - Biology Q&APrapti PatelNo ratings yet
- Ramzan Timetable Grade IX-ADocument1 pageRamzan Timetable Grade IX-AMBJNo ratings yet
- Question: Calculate The Density and Speci C Weight of Methane at 300 KDocument2 pagesQuestion: Calculate The Density and Speci C Weight of Methane at 300 KJamiel CatapangNo ratings yet
- 2021 Bchs Buea CBC Mock Results Al General RealDocument2 pages2021 Bchs Buea CBC Mock Results Al General RealSimonNo ratings yet
- What Can You Study With Your Subject Combinations?Document2 pagesWhat Can You Study With Your Subject Combinations?Boogy GrimNo ratings yet
- Paper Analysis 2015-2019 NSEJS PDFDocument43 pagesPaper Analysis 2015-2019 NSEJS PDFSrijan AgrawalNo ratings yet
- Cell and TissueDocument1 pageCell and TissueKanishk With pythonNo ratings yet
- Grease Resistance of Paper: Standard Test Method ForDocument3 pagesGrease Resistance of Paper: Standard Test Method ForDoicielNo ratings yet
- Unit 1 - Foundations of BiochemistryDocument28 pagesUnit 1 - Foundations of BiochemistryJoselitz Reyes TumulakNo ratings yet
- Percentage Composition and Empirical FormulaDocument36 pagesPercentage Composition and Empirical FormulaNeeta PandeyNo ratings yet
- Agar Diffusion TestDocument9 pagesAgar Diffusion TestAref DahabrahNo ratings yet
- S355 To Hardox 450Document1 pageS355 To Hardox 450Bien NguyenDuyNo ratings yet
- Coagulation and Filtration: Caroline S. Fitzpatrick and John GregoryDocument23 pagesCoagulation and Filtration: Caroline S. Fitzpatrick and John Gregoryjesse MouandzaNo ratings yet
- Unit 3 - Chemical BondingDocument56 pagesUnit 3 - Chemical BondingAchini SheharaNo ratings yet
- Chapter 7 Review: Vocabulary Section 7.3Document4 pagesChapter 7 Review: Vocabulary Section 7.3Christopher HurtNo ratings yet
- Molar Ratio Practice Problems: Assignment: ADocument2 pagesMolar Ratio Practice Problems: Assignment: ABLEUVANTAENo ratings yet
- Z PE Langer Nov2010 Tech RPTDocument271 pagesZ PE Langer Nov2010 Tech RPTHerbert ZugerNo ratings yet
- SP7021M00U24 000 A PDFDocument3 pagesSP7021M00U24 000 A PDFPedro Casimiro GámizNo ratings yet
- USP-NF AlbuterolDocument2 pagesUSP-NF AlbuterolWillian SilvaNo ratings yet
- Determination of Intrinsic Viscosity and MW OF PolystyreneDocument19 pagesDetermination of Intrinsic Viscosity and MW OF PolystyreneNouran ShedidNo ratings yet
- SNC1P Chemistry Practice WorksheetDocument3 pagesSNC1P Chemistry Practice WorksheetJosee GuindonNo ratings yet
- Zinc Oxide and Salicylic Acid PasteDocument1 pageZinc Oxide and Salicylic Acid PasteVu AnNo ratings yet
- IISER Syllabus: MathematicsDocument3 pagesIISER Syllabus: MathematicsIIT aspNo ratings yet
- Geostats CRM ListDocument27 pagesGeostats CRM ListAnonymous mawIPUNo ratings yet
- Carbochlorination of TiO2Document9 pagesCarbochlorination of TiO2Margarita CaceresNo ratings yet
- AS 1627.1-2003 Metal Finishing - Preparation and PretreatmenDocument21 pagesAS 1627.1-2003 Metal Finishing - Preparation and PretreatmenDang Thanh TuanNo ratings yet
- Coating Failures - Causes, Identification, and AnalysisDocument36 pagesCoating Failures - Causes, Identification, and AnalysisSUBODH100% (1)
- BS Iso 20280-2007Document22 pagesBS Iso 20280-2007ngobaochanNo ratings yet
- 9th Class Test - CambridgeDocument3 pages9th Class Test - CambridgeMuhammad Tanzeel Qaisar DogarNo ratings yet
- 07 - Ajie Manggala Putra (TML) - 26in BOC - BOD Pipeline ReplacementDocument8 pages07 - Ajie Manggala Putra (TML) - 26in BOC - BOD Pipeline ReplacementSamuel JohnNo ratings yet
- Progress 2 - POD Emergency TeamDocument65 pagesProgress 2 - POD Emergency TeamGh4n113 IdNo ratings yet
- ChemistryDocument4 pagesChemistryPrecious AjiboyeNo ratings yet
- HCU Chemistry 2011-2017 - Career EndeavourDocument78 pagesHCU Chemistry 2011-2017 - Career EndeavourSankar AdhikariNo ratings yet
- Chemistry II Lab 7Document2 pagesChemistry II Lab 7Jake OxleyNo ratings yet
- c3.4 Exam QuestionsDocument26 pagesc3.4 Exam Questionshaquangminh haiNo ratings yet
- ASTM C813-90 Standard Test Method For Hydrophobic Contamination On Glass by Contact Angle MeasurementDocument3 pagesASTM C813-90 Standard Test Method For Hydrophobic Contamination On Glass by Contact Angle MeasurementgustavoesanchezNo ratings yet
Calculate The Number of Unit Cells in 8.1 G of Aluminium If It Crystallizes in A FCC Structure. - Sarthaks Econnect Largest On
Calculate The Number of Unit Cells in 8.1 G of Aluminium If It Crystallizes in A FCC Structure. - Sarthaks Econnect Largest On
Uploaded by
abhim270Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Calculate The Number of Unit Cells in 8.1 G of Aluminium If It Crystallizes in A FCC Structure. - Sarthaks Econnect Largest On
Calculate The Number of Unit Cells in 8.1 G of Aluminium If It Crystallizes in A FCC Structure. - Sarthaks Econnect Largest On
Uploaded by
abhim270Copyright:
Available Formats
Test JEE NEET Home Q&A Unanswered Ask a Question Learn SWOS Quizard Login
Search...
Welcome to Sarthaks eConnect:
A unique platform where
students can interact with
teachers/experts/students to
Calculate the number of unit cells in 8.1 g of aluminium if it get solutions to their queries.
crystallizes in a fcc structure. Students (upto class 10+2)
preparing for All Government
Exams, CBSE Board Exam, ICSE
← Prev Question Next Question → Board Exam, State Board Exam,
JEE (Mains+Advance) and NEET
asked Nov 1, 2018 in Chemistry by Richa (61.9k points)
0 can ask questions from any
votes subject and get quick answers by
Calculate the number of unit cells in 8.1 g of aluminium if it crystallizes in a
subject teachers/
57.9k views fcc structure.
experts/mentors/students.
(Atomic mass of Al = 27 g mol-1)
solid state defects in solids theory of metals cbse class-12
Categories
All categories
Share It On
JEE
Facebook Twitter Email
NEET
Science
Physics
comment
Chemistry
Biology
Mathematics
Statistics
Environmental Science
Biotechnology
Social Science
Commerce
Electronics
Computer
Artificial Intelligence (AI)
Information Technology
Programming
Play Quiz Game > Political Science
Home Science
Psychology
1 Answer Sociology
English
answered Nov 1, 2018 by ShrutiBharti (34.7k points) Hindi
+1 selected Nov 1, 2018 by Vikash Kumar Aptitude
vote
Reasoning
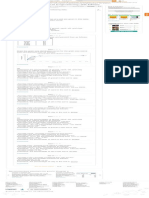
n = given mass/molar mass
GK
= 8.1/27 mol Olympiad
Skill Tips
Best answer
CBSE
RBSE
General
MSBSHSE
Tamilnadu Board
Kerala Board
Detailed Answer :
comment
← Prev Question Next Question →
Find MCQs & Mock Test
1 JEE Main 2024 Test Series
2 NEET Test Series
3 Class 12 Chapterwise MCQ Test
4 Class 11 Chapterwise Practice Test
5 Class 10 Chapterwise MCQ Test
6 Class 9 Chapterwise MCQ Test
7 Class 8 Chapterwise MCQ Test
8 Class 7 Chapterwise MCQ Test
Related questions
The edge length of unit cell of a metal, having molecular weight 75
+1 1 g/mol is 5Å, which crystallizes in cubic lattice.
vote answer
asked Nov 1, 2018 in Chemistry by Richa (61.9k points)
solid state defects in solids theory of metals cbse class-12
The metal calcium crystallises in a face-centred cubic unit cell with a =
0 1 0.556 nm. Calculate the density of the metal if :
votes answer
asked Nov 1, 2018 in Chemistry by Richa (61.9k points)
solid state defects in solids theory of metals cbse class-12
The radius of anion in an ionic solid is 100 pm. Find the radius of cation
0 1 which just fits in its
votes answer
asked Nov 1, 2018 in Chemistry by Richa (61.9k points)
solid state defects in solids theory of metals cbse class-12
What is the total volume of atoms in a face centred cubic unit cell of a
0 1 metal? (r is atomic radius)
votes answer
asked Nov 1, 2018 in Chemistry by Richa (61.9k points)
solid state defects in solids theory of metals cbse class-12
Solid A is a very hard electrical insulator in solid as well as molten
0 1 state and melts at an extremely high temperature.
votes answer
asked Nov 1, 2018 in Chemistry by Richa (61.9k points) Powered By
solid state defects in solids theory of metals cbse class-12
Send feedback About Us Privacy Policy Terms of Use Copyright Contact Us FAQ Advertise Careers
Managed by Sarthaks Digitizing India User Contribution to Sarthaks eConnect
You might also like
- En 14582Document33 pagesEn 14582Vladimir Krzalic100% (1)
- Chemistry Problem JEE PDFDocument1,071 pagesChemistry Problem JEE PDFSaurav Dutt100% (5)
- How Are Dominance, Codominance and Incomplete Dominance Patterns of Inheritance Different From Each Other - Sarthaks EConnectDocument1 pageHow Are Dominance, Codominance and Incomplete Dominance Patterns of Inheritance Different From Each Other - Sarthaks EConnectPaarth GoyalNo ratings yet
- (A) State How The Constant Internal Environment Is Beneficial To Organisms. - Sarthaks Econnect Largest Online Education CommuDocument1 page(A) State How The Constant Internal Environment Is Beneficial To Organisms. - Sarthaks Econnect Largest Online Education CommuPaarth GoyalNo ratings yet
- A Convex Lens Has A Focal Length of 12 Cm. at What Distance From The Lens Should An Object of Height 6 CM Be Placed - Sarthaks eDocument1 pageA Convex Lens Has A Focal Length of 12 Cm. at What Distance From The Lens Should An Object of Height 6 CM Be Placed - Sarthaks elateral0thinkerNo ratings yet
- JEE NEET Physics Question Bank For ElectrostaticsDocument50 pagesJEE NEET Physics Question Bank For ElectrostaticsMBVIBES INSTIPHYNo ratings yet
- Home Random NotesDocument10 pagesHome Random NotesAnvi RoseNo ratings yet
- NTSE Syllabus - Complete NTSE Exam SyllabusDocument2 pagesNTSE Syllabus - Complete NTSE Exam Syllabusteresa castellanNo ratings yet
- 1st Year Physics Pairing Scheme 2020 - 11th Class - Ratta - PK PDFDocument19 pages1st Year Physics Pairing Scheme 2020 - 11th Class - Ratta - PK PDFCH M Ahmed0% (1)
- What Are The Expectedproducts of Hydrolysis of LactoseDocument1 pageWhat Are The Expectedproducts of Hydrolysis of Lactosebalajiharish75No ratings yet
- BITSAT Exam Pattern 2020Document3 pagesBITSAT Exam Pattern 2020Udaya BhaskarNo ratings yet
- Principles of Geotechnical Engineering 7th Edition Textbook Solutions Chegg - Com 9Document1 pagePrinciples of Geotechnical Engineering 7th Edition Textbook Solutions Chegg - Com 9Cristian A. GarridoNo ratings yet
- Which of The Following Compounds Will Show Cis Trans IsomerismDocument1 pageWhich of The Following Compounds Will Show Cis Trans IsomerismaliyaraseebNo ratings yet
- Screenshot 2023-11-28 at 1.47.22 PMDocument1 pageScreenshot 2023-11-28 at 1.47.22 PMAshish KumarNo ratings yet
- NTSE Syllabus - National Talent SearchDocument1 pageNTSE Syllabus - National Talent SearchSatyaranjan mahapatraNo ratings yet
- How Should I Prepare Electromagnetic Theory For Gate and IES ECE Both - QuoraDocument3 pagesHow Should I Prepare Electromagnetic Theory For Gate and IES ECE Both - Quoravigneshwaran50No ratings yet
- Phyllotaxy - NEET Biology NotesDocument1 pagePhyllotaxy - NEET Biology NotesReyansh JainNo ratings yet
- Chapter 2 2-003 (Basis)Document6 pagesChapter 2 2-003 (Basis)Jamiel CatapangNo ratings yet
- Anthe Syllabus and Sample PapersDocument24 pagesAnthe Syllabus and Sample PapersKishan kumarNo ratings yet
- NTSE Stage 1 Analysis Report Rajasthan PDFDocument7 pagesNTSE Stage 1 Analysis Report Rajasthan PDFShyama RanjanNo ratings yet
- Fill in The Blanks The Formula Unit Mass of Ca3 (PO4) 2 Is...................... Chemistry Q&ADocument1 pageFill in The Blanks The Formula Unit Mass of Ca3 (PO4) 2 Is...................... Chemistry Q&Ashauryadiwan5No ratings yet
- Jr-JEE - NEET Weekly Test - Syllabus at 05-12-2022Document1 pageJr-JEE - NEET Weekly Test - Syllabus at 05-12-2022danishquadri4001No ratings yet
- Campus Placement at Cognizant Technology Solutions and Worked For 1month - 2012 Nov (Resigned Due To Pregnancy & Relocation To Korea)Document2 pagesCampus Placement at Cognizant Technology Solutions and Worked For 1month - 2012 Nov (Resigned Due To Pregnancy & Relocation To Korea)naveen kumar pendaNo ratings yet
- Detailed Analysis v2Document9 pagesDetailed Analysis v2Madhukar DakoreNo ratings yet
- Home - JIGSSOLANKIDocument9 pagesHome - JIGSSOLANKIAnvi RoseNo ratings yet
- Lakshya Major Test Series LeafDocument2 pagesLakshya Major Test Series LeafDivyanshu Gautam0% (1)
- Physics Revision Notes For Class 11Document13 pagesPhysics Revision Notes For Class 11Gurunikesh S0% (1)
- Schedule & Syllabus of ONLINE TEST SERIESDocument2 pagesSchedule & Syllabus of ONLINE TEST SERIESRomyNo ratings yet
- Leader Online Test Series For Jee Main Plus Advanced 2018Document2 pagesLeader Online Test Series For Jee Main Plus Advanced 2018Ayush SharmaNo ratings yet
- Thermodynamics: Message Boards Test Prep Centers SparkcollegeDocument23 pagesThermodynamics: Message Boards Test Prep Centers SparkcollegeMercy NunezNo ratings yet
- Safari - May 21, 2023 at 1:22 AMDocument1 pageSafari - May 21, 2023 at 1:22 AMgayauaiaiNo ratings yet
- Principles of Geotechnical Engineering 7th Edition Textbook Solutions Chegg - Com 8Document1 pagePrinciples of Geotechnical Engineering 7th Edition Textbook Solutions Chegg - Com 8Cristian A. GarridoNo ratings yet
- Sem1AY201718 LifelongLearningforNUSAlumni ListofModules PDFDocument7 pagesSem1AY201718 LifelongLearningforNUSAlumni ListofModules PDFManoj GuptaNo ratings yet
- Q3 Explain Why The Bulb Would Not Glow in The Arr PDFDocument1 pageQ3 Explain Why The Bulb Would Not Glow in The Arr PDFDhyansh MalhotraNo ratings yet
- Abstract FORM - NEW - 2023 - Abstract-1Document1 pageAbstract FORM - NEW - 2023 - Abstract-1sid.chilesNo ratings yet
- JEE Main 2023 April Session 2 Shift-1 (DT 08-04-2023) Detailed AnalysisDocument13 pagesJEE Main 2023 April Session 2 Shift-1 (DT 08-04-2023) Detailed AnalysisResonance EduventuresNo ratings yet
- UT & TE Planner - AY 2023-24 - Phase-01Document1 pageUT & TE Planner - AY 2023-24 - Phase-01Atharv KumarNo ratings yet
- Jee Main and Advanced Nurture Schedule and SyllabusDocument2 pagesJee Main and Advanced Nurture Schedule and SyllabuseGamingMafiaNo ratings yet
- Study PLANNER XI (First Step) - JEE Main & Advanced 2021-23 (Phase-3) - May'21 - February'22..Document28 pagesStudy PLANNER XI (First Step) - JEE Main & Advanced 2021-23 (Phase-3) - May'21 - February'22..ZzoNo ratings yet
- Principles of Geotechnical Engineering 7th Edition Textbook Solutions Chegg - Com 10Document1 pagePrinciples of Geotechnical Engineering 7th Edition Textbook Solutions Chegg - Com 10Cristian A. GarridoNo ratings yet
- JEE Main 2023 January Session 1 Shift-1 (DT 29-01-2023) Detailed AnalysisDocument12 pagesJEE Main 2023 January Session 1 Shift-1 (DT 29-01-2023) Detailed AnalysisResonance EduventuresNo ratings yet
- MCQ in Age, Work, Mixture, Digit, Motion Problems Part 3 - ECE Board ExamDocument21 pagesMCQ in Age, Work, Mixture, Digit, Motion Problems Part 3 - ECE Board Examsam labineNo ratings yet
- Test Planner - Yakeen NEET 2025Document3 pagesTest Planner - Yakeen NEET 2025AditiNo ratings yet
- Sem1AY201718 LifelongLearningforNUSAlumni ListofModulesDocument7 pagesSem1AY201718 LifelongLearningforNUSAlumni ListofModulesVance KangNo ratings yet
- Bak Li Wal FoundationDocument2 pagesBak Li Wal FoundationshaileshvcNo ratings yet
- OctoberDocument1 pageOctoberapi-371827688No ratings yet
- BYJU'S Test Series AITS (2019 - 2020) JEE/NEET Paper Dates: Schedule For Class 11Document2 pagesBYJU'S Test Series AITS (2019 - 2020) JEE/NEET Paper Dates: Schedule For Class 11Akash.SNo ratings yet
- UT, TE & ST Planner - First Step Group 1 - AY 2021-22 Updated On15.04.2021 Version 3.0Document2 pagesUT, TE & ST Planner - First Step Group 1 - AY 2021-22 Updated On15.04.2021 Version 3.0kjsc ncsdNo ratings yet
- Physics Group Subjects UpdatedDocument14 pagesPhysics Group Subjects Updatedvasavik.eeeNo ratings yet
- Sample PDF of Neet Ug Maths Book Vol 1Document14 pagesSample PDF of Neet Ug Maths Book Vol 1Mohamed radwanNo ratings yet
- B TechDocument12 pagesB TechProf. Anurag KumarNo ratings yet
- 1st Year Chemistry Pairing Scheme 2021 - 11th Class - Ratta - PKDocument2 pages1st Year Chemistry Pairing Scheme 2021 - 11th Class - Ratta - PKazeemNo ratings yet
- NTSE Detailed Analysis Jharkhand 2016 17Document8 pagesNTSE Detailed Analysis Jharkhand 2016 17Rishav KumarNo ratings yet
- Define Parasite and Total Parasite and Partial Parasites - Biology Q&ADocument1 pageDefine Parasite and Total Parasite and Partial Parasites - Biology Q&APrapti PatelNo ratings yet
- Ramzan Timetable Grade IX-ADocument1 pageRamzan Timetable Grade IX-AMBJNo ratings yet
- Question: Calculate The Density and Speci C Weight of Methane at 300 KDocument2 pagesQuestion: Calculate The Density and Speci C Weight of Methane at 300 KJamiel CatapangNo ratings yet
- 2021 Bchs Buea CBC Mock Results Al General RealDocument2 pages2021 Bchs Buea CBC Mock Results Al General RealSimonNo ratings yet
- What Can You Study With Your Subject Combinations?Document2 pagesWhat Can You Study With Your Subject Combinations?Boogy GrimNo ratings yet
- Paper Analysis 2015-2019 NSEJS PDFDocument43 pagesPaper Analysis 2015-2019 NSEJS PDFSrijan AgrawalNo ratings yet
- Cell and TissueDocument1 pageCell and TissueKanishk With pythonNo ratings yet
- Grease Resistance of Paper: Standard Test Method ForDocument3 pagesGrease Resistance of Paper: Standard Test Method ForDoicielNo ratings yet
- Unit 1 - Foundations of BiochemistryDocument28 pagesUnit 1 - Foundations of BiochemistryJoselitz Reyes TumulakNo ratings yet
- Percentage Composition and Empirical FormulaDocument36 pagesPercentage Composition and Empirical FormulaNeeta PandeyNo ratings yet
- Agar Diffusion TestDocument9 pagesAgar Diffusion TestAref DahabrahNo ratings yet
- S355 To Hardox 450Document1 pageS355 To Hardox 450Bien NguyenDuyNo ratings yet
- Coagulation and Filtration: Caroline S. Fitzpatrick and John GregoryDocument23 pagesCoagulation and Filtration: Caroline S. Fitzpatrick and John Gregoryjesse MouandzaNo ratings yet
- Unit 3 - Chemical BondingDocument56 pagesUnit 3 - Chemical BondingAchini SheharaNo ratings yet
- Chapter 7 Review: Vocabulary Section 7.3Document4 pagesChapter 7 Review: Vocabulary Section 7.3Christopher HurtNo ratings yet
- Molar Ratio Practice Problems: Assignment: ADocument2 pagesMolar Ratio Practice Problems: Assignment: ABLEUVANTAENo ratings yet
- Z PE Langer Nov2010 Tech RPTDocument271 pagesZ PE Langer Nov2010 Tech RPTHerbert ZugerNo ratings yet
- SP7021M00U24 000 A PDFDocument3 pagesSP7021M00U24 000 A PDFPedro Casimiro GámizNo ratings yet
- USP-NF AlbuterolDocument2 pagesUSP-NF AlbuterolWillian SilvaNo ratings yet
- Determination of Intrinsic Viscosity and MW OF PolystyreneDocument19 pagesDetermination of Intrinsic Viscosity and MW OF PolystyreneNouran ShedidNo ratings yet
- SNC1P Chemistry Practice WorksheetDocument3 pagesSNC1P Chemistry Practice WorksheetJosee GuindonNo ratings yet
- Zinc Oxide and Salicylic Acid PasteDocument1 pageZinc Oxide and Salicylic Acid PasteVu AnNo ratings yet
- IISER Syllabus: MathematicsDocument3 pagesIISER Syllabus: MathematicsIIT aspNo ratings yet
- Geostats CRM ListDocument27 pagesGeostats CRM ListAnonymous mawIPUNo ratings yet
- Carbochlorination of TiO2Document9 pagesCarbochlorination of TiO2Margarita CaceresNo ratings yet
- AS 1627.1-2003 Metal Finishing - Preparation and PretreatmenDocument21 pagesAS 1627.1-2003 Metal Finishing - Preparation and PretreatmenDang Thanh TuanNo ratings yet
- Coating Failures - Causes, Identification, and AnalysisDocument36 pagesCoating Failures - Causes, Identification, and AnalysisSUBODH100% (1)
- BS Iso 20280-2007Document22 pagesBS Iso 20280-2007ngobaochanNo ratings yet
- 9th Class Test - CambridgeDocument3 pages9th Class Test - CambridgeMuhammad Tanzeel Qaisar DogarNo ratings yet
- 07 - Ajie Manggala Putra (TML) - 26in BOC - BOD Pipeline ReplacementDocument8 pages07 - Ajie Manggala Putra (TML) - 26in BOC - BOD Pipeline ReplacementSamuel JohnNo ratings yet
- Progress 2 - POD Emergency TeamDocument65 pagesProgress 2 - POD Emergency TeamGh4n113 IdNo ratings yet
- ChemistryDocument4 pagesChemistryPrecious AjiboyeNo ratings yet
- HCU Chemistry 2011-2017 - Career EndeavourDocument78 pagesHCU Chemistry 2011-2017 - Career EndeavourSankar AdhikariNo ratings yet
- Chemistry II Lab 7Document2 pagesChemistry II Lab 7Jake OxleyNo ratings yet
- c3.4 Exam QuestionsDocument26 pagesc3.4 Exam Questionshaquangminh haiNo ratings yet
- ASTM C813-90 Standard Test Method For Hydrophobic Contamination On Glass by Contact Angle MeasurementDocument3 pagesASTM C813-90 Standard Test Method For Hydrophobic Contamination On Glass by Contact Angle MeasurementgustavoesanchezNo ratings yet