Professional Documents
Culture Documents
Lana Aboulaban - Atomic Math
Lana Aboulaban - Atomic Math
Uploaded by
ID16031Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lana Aboulaban - Atomic Math
Lana Aboulaban - Atomic Math
Uploaded by
ID16031Copyright:
Available Formats
Name: ________________________
Date/Period: __________________
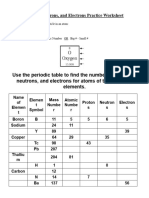
Atomic Number and Mass Number
Complete the following chart and answer the questions below.
Element Atomic Number of Number of
Mass Number
Name Number Protons Neutrons
carbon 6 6 6 12
Oxygen 8 8 8 16
hydrogen 1 1 0 1
Helium 2 2 2 4
hydrogen 1 1 2 3
nitrogen 7 7 7 14
Hydrogen 1 1 1 2
Uranium 92 92 146 238
Potassium 19 19 20 39
Sodium 11 11 12 23
Silver 47 47 61 108
Xenon 54 54 77 131
Bromine 35 35 45 80
Chromium 24 24 28 52
Sulfur 16 16 16 32
Gold 79 79 118 197
Osmium 76 76 114 190
How are the atomic number and the number of protons related to each other?
-The atomic number, and number of protons and electrons are the same
How do the number of protons, number of neutrons, and the mass number relate to each other?
-The atomic mass can be used to determine how many neutrons and protons are in an element. By subtracting the
number of protons from the atomic mass, the number of neutrons can be found. Similarly, by subtracting the number of
neutrons from the atomic mass, the number of protons can be determined. When the number of protons and neutrons are
added together you should get the atomic mass.
What is the one thing that determines the identity of an atom?
-The number of protons
What sub atomic parts are equal to make a neutral atom?
-When the number of protons and electrons are equal, then it makes a neutral atom.
Explain in your words how to calculate the mass of a sodium atom.
-Add the number of protons/electrons and the number of neutrons. If sodium has 11 electrons, then when multiplied by 2,
or when doubled, the atomic mass should be found. So, the atomic mass of sodium is 22.
Draw and label three element symbols that are representative of the periodic table. It should have atomic #,
mass #, name and element symbol.
You might also like
- Tadashi Okuyama, Mark Maskill - Organic Chemistry - A Mechanistic Approach-Oxford University Press (2013)Document681 pagesTadashi Okuyama, Mark Maskill - Organic Chemistry - A Mechanistic Approach-Oxford University Press (2013)Sooraj Srinivasan100% (14)
- Protons, Neutrons, and Electrons Practice Worksheet For 8th Grade AnswersDocument2 pagesProtons, Neutrons, and Electrons Practice Worksheet For 8th Grade AnswersDrama Music92% (13)
- Protons, Neutrons, and Electrons Practice Worksheet For 8th GradeDocument2 pagesProtons, Neutrons, and Electrons Practice Worksheet For 8th GradeDrama Music67% (3)
- Subatomic Particles WsDocument1 pageSubatomic Particles WsYhena ChanNo ratings yet
- Name Class Date: Twenty Electronic ConfigurationsDocument5 pagesName Class Date: Twenty Electronic ConfigurationsLeslie Vanessa CarrilloNo ratings yet
- 4 1aatomicsymbolworksheetday1Document2 pages4 1aatomicsymbolworksheetday1Anonymous DTO0TpapoNo ratings yet
- Atomic Number Practice Short AssDocument1 pageAtomic Number Practice Short AssjoseNo ratings yet
- Chemistry - Mass Number and Atomic NumberDocument1 pageChemistry - Mass Number and Atomic Numberwemedo7083No ratings yet
- Atomic Structure Questions 9ZDocument2 pagesAtomic Structure Questions 9ZsfjjNo ratings yet
- Melody Rosales Exercise AtomsDocument2 pagesMelody Rosales Exercise AtomsB2 RosalesNo ratings yet
- Atomic Mass and Atomic Number WorksheetDocument1 pageAtomic Mass and Atomic Number WorksheetGuayNo ratings yet
- Atomic-number-and-Atomic-Mass - SheetDocument1 pageAtomic-number-and-Atomic-Mass - SheetnidhiNo ratings yet
- Subatomic Particles WsDocument1 pageSubatomic Particles WsJessa FerrerNo ratings yet
- Ch1 - Atoms and Molecules - P1 - L1 - WS1Document2 pagesCh1 - Atoms and Molecules - P1 - L1 - WS1Aminul IslamNo ratings yet
- Chapter 02 ISM Chang 14eDocument7 pagesChapter 02 ISM Chang 14elsytb2000No ratings yet
- All About The Periodic Table - Home Laboratory WorksheetDocument4 pagesAll About The Periodic Table - Home Laboratory WorksheetFrank Ed SerranoNo ratings yet
- KCSE Form 2 NotesDocument139 pagesKCSE Form 2 NotesN KatanaNo ratings yet
- Ionic Radius - Wikipedia PDFDocument29 pagesIonic Radius - Wikipedia PDFடேவிட் ஸ்No ratings yet
- Chemistry Atomic Mass and Atomic Number WorksheetDocument1 pageChemistry Atomic Mass and Atomic Number Worksheetanon-579447No ratings yet
- 1 Grade 11 Review AnswersDocument9 pages1 Grade 11 Review Answersapi-363234558No ratings yet
- Periodic Table Scavenger HuntDocument2 pagesPeriodic Table Scavenger HuntShawana AhmadNo ratings yet
- Isotopes WorksheetDocument1 pageIsotopes WorksheetBrentRJones100% (1)
- Atomic Structure and Periodic Table PDFDocument51 pagesAtomic Structure and Periodic Table PDFKevin NdanyiNo ratings yet
- Atomic Structure Practice Name - : (Atomic Mass-Atomic Number) (Same As Number of Protons)Document1 pageAtomic Structure Practice Name - : (Atomic Mass-Atomic Number) (Same As Number of Protons);No ratings yet
- Atomic Struct 2ADocument2 pagesAtomic Struct 2AVictoria FuenmayorNo ratings yet
- Atomic Radius Graphing ActivityDocument2 pagesAtomic Radius Graphing ActivitytweetyaarushiNo ratings yet
- Periodic Table ActivityDocument3 pagesPeriodic Table ActivityJanine Aytria SaleNo ratings yet
- Atomic Number and Atomic MassDocument2 pagesAtomic Number and Atomic MassKayra KamberogluNo ratings yet
- 1 Atomic Structure and Ions AnswersDocument2 pages1 Atomic Structure and Ions AnswersAwais NaeemNo ratings yet
- The Periodic Table of Elements: Daniel LundbergDocument2 pagesThe Periodic Table of Elements: Daniel LundbergEZLYEN AZLINNo ratings yet
- The Periodic Table of Elements 2018 ColorDocument2 pagesThe Periodic Table of Elements 2018 ColorCawf KgfNo ratings yet
- Subatomic StructuresDocument18 pagesSubatomic StructuresArlyn RoblesNo ratings yet
- Number of Protons WorksheetDocument4 pagesNumber of Protons WorksheetIrene SanchezNo ratings yet
- Periodic Table PDFDocument1 pagePeriodic Table PDFNfhjfj GhjkgjkNo ratings yet
- Table of IonsDocument2 pagesTable of IonsLucia Jimenez AlvarezNo ratings yet
- Atomic MassDocument1 pageAtomic MassDeepti JainNo ratings yet
- History and Subatomic Particle Review Take Two KEYDocument5 pagesHistory and Subatomic Particle Review Take Two KEYAlliya DaymonNo ratings yet
- Color Coding the Periodic TableDocument2 pagesColor Coding the Periodic TableMelva GuerraNo ratings yet
- The Periodic Table of Elements: Daniel LundbergDocument2 pagesThe Periodic Table of Elements: Daniel LundbergAHNAF AJMAINNo ratings yet
- The Periodic Table of Elements: Daniel LundbergDocument2 pagesThe Periodic Table of Elements: Daniel LundbergOgunbowale Olatayo BodunrinNo ratings yet
- The Periodic Table of Elements 2018 ColorDocument2 pagesThe Periodic Table of Elements 2018 ColorAayush GuptaNo ratings yet
- Group Activity1Document1 pageGroup Activity1ansalerochNo ratings yet
- Periodic TableDocument1 pagePeriodic TableAndrea MontalvanNo ratings yet
- AQA Phys Worksheet4.1.3Document1 pageAQA Phys Worksheet4.1.3CT ONo ratings yet
- Jadual Berkala UnsurDocument1 pageJadual Berkala Unsurkhadijah madhadzirNo ratings yet
- FORM 2 CHEMISTRY NOTEzS (2023 - 11 - 13 08 - 17 - 14 UTC)Document254 pagesFORM 2 CHEMISTRY NOTEzS (2023 - 11 - 13 08 - 17 - 14 UTC)joshuamumo588No ratings yet
- Subatomic Particles - FILLDocument2 pagesSubatomic Particles - FILLALMERA SHELLA CABOGONo ratings yet
- Elements and The Periodic Table WorksheetDocument4 pagesElements and The Periodic Table WorksheetVictoria StewartsonNo ratings yet
- Kami Export - Arabella McCarthy - Unit 3 Atom Packet - 10!04!2017!12!20 - 25 - 057Document4 pagesKami Export - Arabella McCarthy - Unit 3 Atom Packet - 10!04!2017!12!20 - 25 - 057Arabella McCarthyNo ratings yet
- Basic Atomic Structure WorksheetDocument4 pagesBasic Atomic Structure WorksheetTrisha GolesNo ratings yet
- Atomic Number and Mass NumberDocument2 pagesAtomic Number and Mass NumbergideonNo ratings yet
- (Download PDF) Chemistry Chemical Reactivity 11E 11Th Edition John C Kotz Full Chapter PDFDocument69 pages(Download PDF) Chemistry Chemical Reactivity 11E 11Th Edition John C Kotz Full Chapter PDFmarajnmiad100% (9)
- Full download Chemistry & Chemical Reactivity, 11e 11th Edition John C. Kotz file pdf all chapter on 2024Document44 pagesFull download Chemistry & Chemical Reactivity, 11e 11th Edition John C. Kotz file pdf all chapter on 2024gueneizvori100% (1)
- Chemistry Chemical Reactivity 11E 11Th Edition John C Kotz Full Chapter PDF ScribdDocument67 pagesChemistry Chemical Reactivity 11E 11Th Edition John C Kotz Full Chapter PDF Scribdjessica.carter247100% (11)
- Periodic TableDocument1 pagePeriodic TablesopheeyuhNo ratings yet
- Atomic Structure WorksheetsDocument3 pagesAtomic Structure WorksheetsJohnaire RowellNo ratings yet
- Atomic Structure WorksheetDocument2 pagesAtomic Structure Worksheetrangerblue94% (17)
- ElementDocument1 pageElementjasrille binbinonNo ratings yet
- Writing Formulae, Naming Compounds and Balancing EquationsDocument23 pagesWriting Formulae, Naming Compounds and Balancing Equationskadynnaidoo7No ratings yet
- Unusual Structures and Physical Properties in Organometallic ChemistryFrom EverandUnusual Structures and Physical Properties in Organometallic ChemistryNo ratings yet
- Hyrdogen Storage TechnologiesFrom EverandHyrdogen Storage TechnologiesMehmet SankirNo ratings yet
- The Evolution of MatterDocument482 pagesThe Evolution of Matteranakin68100% (8)
- Chemistry Fun FactsDocument2 pagesChemistry Fun FactsBabitha MonteiroNo ratings yet
- Chemistry Form 6 Sem 2 05 WebDocument56 pagesChemistry Form 6 Sem 2 05 WebNg Swee Loong StevenNo ratings yet
- Chapter 10 Radioactivity Teacher Guide1Document29 pagesChapter 10 Radioactivity Teacher Guide1Mohd Nurul Hafiz AlawiNo ratings yet
- Chem 121Document16 pagesChem 121VAILA OBYNo ratings yet
- Chemistry Core g9Document62 pagesChemistry Core g9YahyaNo ratings yet
- Curriculum Implementation MatrixDocument11 pagesCurriculum Implementation MatrixReymart VillapeñaNo ratings yet
- 2086 02 SP 6RP AfpDocument12 pages2086 02 SP 6RP AfpahmedNo ratings yet
- Science Unit Test Grade: 8 Unit 1: Matter: Do Not Turn The Page Until Instructed To Do SoDocument13 pagesScience Unit Test Grade: 8 Unit 1: Matter: Do Not Turn The Page Until Instructed To Do Soapi-238949685No ratings yet
- Summative Test IDocument2 pagesSummative Test IRanie EsponillaNo ratings yet
- The Periodic Table of The Elements (LP)Document3 pagesThe Periodic Table of The Elements (LP)A99519No ratings yet
- Basic Prenciples of Radiation Protection For RpoDocument94 pagesBasic Prenciples of Radiation Protection For RpoMohammed Al-leswasNo ratings yet
- Radioactive IsotopeDocument1 pageRadioactive IsotopeAvon Glorane MirandaNo ratings yet
- Department of Education: Name: Score: Grade: 7 - EMERALDDocument10 pagesDepartment of Education: Name: Score: Grade: 7 - EMERALDEmmanz CaballeroNo ratings yet
- Physical and Chemical Principles CompiledDocument75 pagesPhysical and Chemical Principles Compiledsiams fadnierhsaNo ratings yet
- General Chemistry 1 Week 2 Activity SheetsDocument4 pagesGeneral Chemistry 1 Week 2 Activity SheetslkNo ratings yet
- Chapter 2 Atoms, Molecules and StoichiometryDocument8 pagesChapter 2 Atoms, Molecules and StoichiometryTilak K C100% (1)
- CHEM 101 Chapter 1Document118 pagesCHEM 101 Chapter 1mikayla sirovatkaNo ratings yet
- Sci8 Q3 Mod4 PeriodicTableofElements v3Document41 pagesSci8 Q3 Mod4 PeriodicTableofElements v3Cirille AgpaoaNo ratings yet
- Chapter 7. Covalent and Metallic BondingDocument23 pagesChapter 7. Covalent and Metallic Bondingnacha meyyNo ratings yet
- Chapter 1Document19 pagesChapter 1Kyrie IrvingNo ratings yet
- General Chemistry - DocsDocument17 pagesGeneral Chemistry - DocsJohn leeNo ratings yet
- 2005 Syllabus PDFDocument30 pages2005 Syllabus PDFPhiri AgnesNo ratings yet
- Aqa 7404 7405 Collins SampleDocument33 pagesAqa 7404 7405 Collins SampleJustin HadinataNo ratings yet
- S No Unit Portion To Be Reduced: CHEMISTRY (043) Class XIDocument2 pagesS No Unit Portion To Be Reduced: CHEMISTRY (043) Class XIDivyansh BishtNo ratings yet
- Chang Problems Chapter 2Document10 pagesChang Problems Chapter 2ChaNo ratings yet
- Werner Heisenberg, Physics and BeyondDocument203 pagesWerner Heisenberg, Physics and Beyondma.vicenteserrano68No ratings yet
- STSE ClassDocument29 pagesSTSE ClassNikeNo ratings yet
- Elements and CompoundsDocument9 pagesElements and CompoundsPaulNo ratings yet