Professional Documents
Culture Documents
Corrosion Types - Real Pictures
Corrosion Types - Real Pictures
Uploaded by
Jaswant Singh ChauhanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Corrosion Types - Real Pictures
Corrosion Types - Real Pictures
Uploaded by
Jaswant Singh ChauhanCopyright:
Available Formats
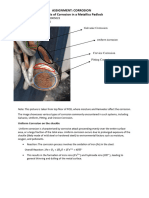
Type : Pitting
Reason : Pitting corrosion occurs due to local damage to the protective oxide layer
on the steel surface. Factors such as exposure to extreme weather conditions,
uneven distribution of cover material, or presence of contaminants can lead to small
pits. Once initiated, pitting corrosion can progress rapidly, causing severe and
widespread damage to the metal.
Mechanism: Pitting corrosion is a detrimental process that occurs when the
protective oxide layer on a metal surface is compromised. This deterioration usually
begins as tiny flaws or blemishes in the oxide layer, which enable destructive
particles to infiltrate. As these particles react with the metal, they trigger localized
chemical reactions that result in the release of metal ions within a limited space,
creating small pits. These pits serve as hotspots for ongoing corrosion, ultimately
causing significant damage in these concentrated, narrow areas.
Prevention : the application of protective coatings or paints that act as a barrier
against corrosive environments. Additionally, selecting corrosion-resistant materials,
such as stainless steel or titanium, for applications in harsh conditions can inherently
reduce susceptibility to pitting corrosion. Controlling environmental factors, such as
exposure to saltwater, acids, or pollutants, is essential in maintaining proper
conditions.
Type : Erosion Corrosion
Mechanism : Erosion corrosion occurs when fluid flow, carrying particles or corrosive
agents, abrades the metal surface, accelerating wear. This mechanical wear disrupts
the protective oxide layer, heightening the metal's vulnerability to chemical corrosion.
The synergy of mechanical erosion and chemical attack results in an increased rate of
material loss.
Reason : It typically occurs in environments where there is a combination of flowing
fluids and corrosive elements.
Prevention : crucial aspect in preventing erosion and corrosion is carefully selecting
materials, such as specialized alloys or coatings, that possess inherent resistance.
Another key measure is manipulating fluid conditions, like regulating flow rates, to
minimize abrasive damage. Furthermore, employing efficient filtration systems to
eliminate suspended particles in the fluid can significantly reduce the destructive
impact on metal surfaces.
Type : Uniform Corrosion
Mechanism : The electrochemical mechanism of uniform corrosion
involves oxidation and reduction reactions at the metal's surface. In an
aqueous environment, metal atoms undergo oxidation, releasing
electrons. These electrons flow through the metal, creating a flow of
electrical current. At the same time, oxygen or other oxidizing agents in
the environment gain electrons through reduction reactions. The overall
process is a series of electrochemical reactions, with metal dissolution
and the formation of metal ions in solution.
Reason : Factors influencing uniform corrosion include the chemical
composition of the environment, temperature, and the electrochemical
properties of the metal involved.
Prevention : Preventing uniform corrosion involves using corrosion-
resistant materials, applying protective coatings, controlling
environmental factors, and employing cathodic protection methods.
Regular inspections and maintenance are essential to detect and
address corrosion early on.
Type : Filiform corrosion
Mechanism : Filiform corrosion initiates when a flaw in a metal's
protective coating allows moisture to reach the surface. In the
presence of oxygen, an electrochemical reaction forms a localized
anodic region, leading to corrosion. The resulting metal ions react
with moisture, forming corrosion products.
These products create a pH gradient in the damaged coating,
prompting ion migration along the surface. This migration results in
thread-like corrosion product filaments under the intact coating,
manifesting as worm-like structures.
Accelerating factors include high humidity, salts, and contaminants.
Choosing the right coating and regular maintenance are crucial for
preventing filiform corrosion.
Reason : Improper coating or very thick coating which can easily be
wear off.
Prevention: Control the relative humidity. Use brittle coatings.
Type : Crevice corrosion
Mechanism : Crevice corrosion emerges due to disparities in ion
concentrations within an electrolyte solution and between distinct
regions of a single metal component. In the context of this concentration
cell, corrosion takes place in the specific area with a lower ion
concentration. This type of deterioration is commonly linked to the
existence of limited volumes of stagnant solution in enclosed spaces,
under deposits and seals, or within crevices, such as those found at
nuts and rivet heads.
Reason : Crevice corrosion is like when metal gets stuck in a tight spot,
and because it can't interact much with the surroundings, it becomes a
hotspot for corrosion due to trapped corrosive substances.
Prevention : To prevent crevice corrosion, use materials that resist
corrosion, design structures to avoid tight spots, ensure good drainage
to prevent stagnant areas, and conduct regular inspections with proper
sealing.
You might also like
- 201: Health & Safety: Sample Questions Answer GuideDocument7 pages201: Health & Safety: Sample Questions Answer GuideGheorghe Ciubotaru78% (9)
- Bobcat S160Document949 pagesBobcat S160David Solis71% (14)
- R-30iA Cimplicity Operator Manual (B-82604EN 01)Document90 pagesR-30iA Cimplicity Operator Manual (B-82604EN 01)YoganandhK100% (1)
- TPMS 77Document1 pageTPMS 77Miguel Andres PERSONALNo ratings yet
- Dennis Wambua Corrosion in BruceDocument30 pagesDennis Wambua Corrosion in Brucedenniswambua10669No ratings yet
- Corrosion Minimizing ProceduresDocument5 pagesCorrosion Minimizing ProceduresCh. Muhammad UsamaNo ratings yet
- CorrosionDocument31 pagesCorrosionLyle Joseph Legaspi100% (1)
- Corrosion Prevention For MetalsDocument17 pagesCorrosion Prevention For Metalsabdul100% (1)
- SodaPDF-converted-Laboratory 2 - Corrosion and Degradation of MaterialsDocument4 pagesSodaPDF-converted-Laboratory 2 - Corrosion and Degradation of MaterialsRyan Ryan RyanNo ratings yet
- Fundamentals of Corrosion and Corrosion Control: Reaction With Its EnvironmentDocument8 pagesFundamentals of Corrosion and Corrosion Control: Reaction With Its EnvironmentvicedaNo ratings yet
- CorrosionnnnDocument3 pagesCorrosionnnnJoannah claire AlforqueNo ratings yet
- CorrosionDocument33 pagesCorrosionSherina Mae GonzalesNo ratings yet
- CorrosionDocument8 pagesCorrosionGM VillaneaNo ratings yet
- Corrosion-Dikonversi-DikompresiDocument56 pagesCorrosion-Dikonversi-DikompresiMariaNo ratings yet
- CorrosionDocument4 pagesCorrosionpramod.bNo ratings yet
- Welding CorrosionDocument36 pagesWelding CorrosionKe HalimunNo ratings yet
- Corrosion Prevention & ControlDocument16 pagesCorrosion Prevention & ControlAl KefahNo ratings yet
- 14jvPjlOD7ixx 1zB m0G1AkJruGesGszDocument8 pages14jvPjlOD7ixx 1zB m0G1AkJruGesGszbrian kiruiNo ratings yet
- Corrosion and Its Prevention: Presented by B.Chandaneswar Kumar 15001A0839 Iv Btech Chemical EngineeringDocument8 pagesCorrosion and Its Prevention: Presented by B.Chandaneswar Kumar 15001A0839 Iv Btech Chemical EngineeringChandaneswarkumar BoddaniNo ratings yet
- 3B4 - Tutorial 8Document6 pages3B4 - Tutorial 8katehughes332No ratings yet
- Corrosion Inhibition by Acacia ConcinnaDocument25 pagesCorrosion Inhibition by Acacia ConcinnaHarish KumarNo ratings yet
- Corrosion, Prevention and ControlDocument60 pagesCorrosion, Prevention and ControlCherry Obias100% (1)
- Activity 2Document7 pagesActivity 2Ren RenNo ratings yet
- Chem ProjectDocument12 pagesChem ProjectAnanya AgrawalNo ratings yet
- Corrosion - JUDocument32 pagesCorrosion - JUSWAGATAM BAZNo ratings yet
- Corrosion Prevention and ControlDocument10 pagesCorrosion Prevention and ControlBensoyNo ratings yet
- Corrosion Prevention and Control: By: Patick James Pallo Ram Jun RegaladoDocument10 pagesCorrosion Prevention and Control: By: Patick James Pallo Ram Jun RegaladoBensoyNo ratings yet
- Environmental Degradation PDFDocument10 pagesEnvironmental Degradation PDFMuhammad UsmanNo ratings yet
- CorrosionDocument16 pagesCorrosionRadu GenisNo ratings yet
- Home Assignment ChemistryDocument2 pagesHome Assignment ChemistryAdithya GullaNo ratings yet
- Design For Corrosion Resistance: Uniform AttackDocument11 pagesDesign For Corrosion Resistance: Uniform AttackIndra PradanaNo ratings yet
- Corrosion and Its Types: Engineering Material AssignmentDocument6 pagesCorrosion and Its Types: Engineering Material AssignmentHasieb Alam KhanNo ratings yet
- Waleeed CorrosionDocument6 pagesWaleeed CorrosionWaleed EmaraNo ratings yet
- Corrosion: Corrosion Fatigue. Classification of Corrosion ProcessesDocument6 pagesCorrosion: Corrosion Fatigue. Classification of Corrosion ProcessesAhmed AymanNo ratings yet
- Unit VI Corrosion Science: Course Instructor-Dr. Shailesh DhokeDocument58 pagesUnit VI Corrosion Science: Course Instructor-Dr. Shailesh DhokeLadliNo ratings yet
- Materials and Processes For Agricultural and Biosystems EngineeringDocument35 pagesMaterials and Processes For Agricultural and Biosystems EngineeringMelanie D. Aquino BaguioNo ratings yet
- Project P 1 CLGG AhsbhDocument16 pagesProject P 1 CLGG Ahsbhpranav mahajanNo ratings yet
- Two Corrosion Protection MethodsDocument5 pagesTwo Corrosion Protection MethodsShukry AmiryNo ratings yet
- Corrosion PresentationDocument16 pagesCorrosion PresentationDjoudNo ratings yet
- Corrosion and RustDocument9 pagesCorrosion and RustahmedNo ratings yet
- Corrosion Types: Direct Chemical Attack Is Also FundamentallyDocument5 pagesCorrosion Types: Direct Chemical Attack Is Also FundamentallyKemal ToprakNo ratings yet
- Corrosion Protection of SteelDocument3 pagesCorrosion Protection of Steelbenandy2018No ratings yet
- Lecture # 5Document5 pagesLecture # 5Ch. Muhammad UsamaNo ratings yet
- Corrosion Problems and Alloy SolutionsDocument61 pagesCorrosion Problems and Alloy Solutionszuudee100% (1)
- Taboada Chapter 17Document3 pagesTaboada Chapter 17Carl Vincent TaboadaNo ratings yet
- ChemistryDocument25 pagesChemistryHarish KumarNo ratings yet
- Forms of CorrosionDocument18 pagesForms of Corrosionjoker princeNo ratings yet
- Chemistry Unit - 2 NotesDocument13 pagesChemistry Unit - 2 Notesjoshinihar19No ratings yet
- Corrosion of Metals (Aluminium) in Hydrocarbons (Kerosene)Document22 pagesCorrosion of Metals (Aluminium) in Hydrocarbons (Kerosene)Ajibola AjiboyeNo ratings yet
- Corrossion: It's Impact and PreventionDocument5 pagesCorrossion: It's Impact and PreventionEditor IJTSRDNo ratings yet
- Corrosion BasicsDocument35 pagesCorrosion BasicsAbdul Wajid AliNo ratings yet
- Types of Corrosion 1Document30 pagesTypes of Corrosion 1Lion ManabatNo ratings yet
- Corrosioninpiping 210425063500Document29 pagesCorrosioninpiping 210425063500MekineNo ratings yet
- Corrosion: Food Process Equipment Design Module-2Document20 pagesCorrosion: Food Process Equipment Design Module-2ssfoodtechNo ratings yet
- L O-3 4Document3 pagesL O-3 4KALOY SANTOSNo ratings yet
- Presentation On CorrosionDocument7 pagesPresentation On CorrosionBemgbaNo ratings yet
- MetallurgyDocument36 pagesMetallurgyOneil Prettyboyswagg LeitchNo ratings yet
- Ways To Control CorrosionDocument37 pagesWays To Control CorrosionKyle DugayoNo ratings yet
- Causes of CorrosionDocument3 pagesCauses of Corrosionamit100% (1)
- Corrosion Is The Gradual Destruction of Materials (Usually: MetalsDocument4 pagesCorrosion Is The Gradual Destruction of Materials (Usually: MetalsJonathan KingNo ratings yet
- Seam 213 Final Week 2Document40 pagesSeam 213 Final Week 2Jeycule BerendezNo ratings yet
- Types of CorrosionDocument8 pagesTypes of CorrosionNiranjana SivaNo ratings yet
- Why Do Metals Rust? An Easy Read Chemistry Book for Kids | Children's Chemistry BooksFrom EverandWhy Do Metals Rust? An Easy Read Chemistry Book for Kids | Children's Chemistry BooksNo ratings yet
- Resistance Spot WeldingDocument29 pagesResistance Spot WeldingJaswant Singh ChauhanNo ratings yet
- Corrosion Assignment - Three Type of CorrosionDocument2 pagesCorrosion Assignment - Three Type of CorrosionJaswant Singh ChauhanNo ratings yet
- Refractories - ClassificationDocument7 pagesRefractories - ClassificationJaswant Singh ChauhanNo ratings yet
- Electric Enclosure Material Presentation (Read-Only)Document2 pagesElectric Enclosure Material Presentation (Read-Only)Jaswant Singh ChauhanNo ratings yet
- LCR CircuitDocument9 pagesLCR CircuitJaswant Singh ChauhanNo ratings yet
- Tutorial - Strengthening MechanismsDocument2 pagesTutorial - Strengthening MechanismsJaswant Singh ChauhanNo ratings yet
- Mild Steel Uniaxial Aluminium EquibiaxialDocument8 pagesMild Steel Uniaxial Aluminium EquibiaxialJaswant Singh ChauhanNo ratings yet
- Complex AnalysisDocument1 pageComplex AnalysisJaswant Singh ChauhanNo ratings yet
- Material: ASTM A395 Ductile Iron or ASTM A105 Forged Steel: ID H TDocument1 pageMaterial: ASTM A395 Ductile Iron or ASTM A105 Forged Steel: ID H T1mmahoneyNo ratings yet
- Adolescent Development and Teaching Reflection - CDocument2 pagesAdolescent Development and Teaching Reflection - Capi-374467245No ratings yet
- Chest Pain.Document53 pagesChest Pain.Shimmering MoonNo ratings yet
- Correlational Relationship of Nursing Professional Subjects and Board Exam Performance of Isabela State University-Echague November 2022 Nursing Licensure Examination TakersDocument20 pagesCorrelational Relationship of Nursing Professional Subjects and Board Exam Performance of Isabela State University-Echague November 2022 Nursing Licensure Examination TakersInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Interpretation of Vane Shear Tests For Geotechnical Stability CalculationsDocument9 pagesInterpretation of Vane Shear Tests For Geotechnical Stability CalculationsiliavaNo ratings yet
- Maltego CEDocument4 pagesMaltego CEArief PrihantoroNo ratings yet
- 35kV AL 133% TRXLPE One-Third Neutral LLDPE Primary UD: SPEC 81252Document5 pages35kV AL 133% TRXLPE One-Third Neutral LLDPE Primary UD: SPEC 81252Anonymous hxyW3v5QqVNo ratings yet
- Tarun Sharma (2007EME18) Nikhil Sharma (2007EME33) Ishan Chib (2007EME30) Sumit Khajuria (2007EME37)Document20 pagesTarun Sharma (2007EME18) Nikhil Sharma (2007EME33) Ishan Chib (2007EME30) Sumit Khajuria (2007EME37)Tarun SharmaNo ratings yet
- Design Review Checklist For Road ProjectsDocument93 pagesDesign Review Checklist For Road ProjectsFaez El-NinoNo ratings yet
- Basic Data Kar Part 4Document11 pagesBasic Data Kar Part 4vpmohammedNo ratings yet
- Binocular VisionDocument49 pagesBinocular VisionekaNo ratings yet
- Function 4Document61 pagesFunction 4Jegatheesh EashwaranNo ratings yet
- General ConclusionDocument2 pagesGeneral ConclusionAdelNo ratings yet
- Evs - Final Unit 3 - Environment Management & Sustainable DevelopmentDocument105 pagesEvs - Final Unit 3 - Environment Management & Sustainable DevelopmentchhaviNo ratings yet
- 4 Lec AJ Culture Fall2023Document50 pages4 Lec AJ Culture Fall2023muhammad zohaibNo ratings yet
- Terrermix Ramadhan RecipesDocument50 pagesTerrermix Ramadhan Recipesfirdaus hadzwanNo ratings yet
- C Quick GuideDocument34 pagesC Quick Guidemmarin1982No ratings yet
- Please Quote This Reference Number in All Future CorrespondenceDocument2 pagesPlease Quote This Reference Number in All Future CorrespondenceSaipraveen PerumallaNo ratings yet
- OverviewDocument346 pagesOverviewarnaldospbrNo ratings yet
- Paper 5 Examiner's Script: 3 Minutes (5 Minutes For Groups of Three)Document5 pagesPaper 5 Examiner's Script: 3 Minutes (5 Minutes For Groups of Three)IsaMar NuñezNo ratings yet
- 099CYUT5512017 001卵礫石Document212 pages099CYUT5512017 001卵礫石ruizhou04 wuNo ratings yet
- St. Augustine As EducatorDocument7 pagesSt. Augustine As EducatorMaria Soledad TubayNo ratings yet
- Respuestas Ingles Modul 6Document16 pagesRespuestas Ingles Modul 6Ximena Silva CelyNo ratings yet
- Fema f2Document2 pagesFema f2santoshkumar86No ratings yet
- HRC - Forms - Employee Application - Rev2019 NewDocument3 pagesHRC - Forms - Employee Application - Rev2019 NewGanang CucuNo ratings yet
- Hitachi Datasheet Advanced Server Ds120Document2 pagesHitachi Datasheet Advanced Server Ds120adio77No ratings yet