Professional Documents
Culture Documents
If You Play-WPS Office
If You Play-WPS Office
Uploaded by
awitg031Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
If You Play-WPS Office
If You Play-WPS Office
Uploaded by
awitg031Copyright:
Available Formats
1.
If you play ping pong, chances are you've come across the occasionally dented
ball. Restore its roundness by popping it in a pan of water. Warm the water gently
while stirring and the air inside the ball will expand as it heats up. The expanding
air will push out the dent and restore the ball's roundness.
2. When we read the warnings mentioned on deodorant bottles, we realise why it
indicates keeping the bottle away from sunlight and high temperature. As Charles
Law states, under high temperatures, the air molecules inside the bottle will
expand, which can burst the deodorant bottle; cause an explosion.
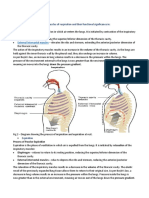
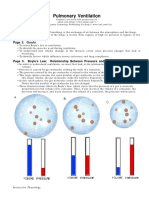
3. Boyle's law has application in human breathing. As the lungs expands, the
volume inside the lungs increases and the pressure inside decreases (it follows
Boyle's law). As the pressure is in lower concentration inside the body, the air
moves inside the lungs from outsides. When you inhale, muscles increase the size
of your thoracic (chest) cavity and expand your lungs. This increases their volume,
so pressure inside the lungs decreases.
4. As the bubbles move upwards, the pressure on them decreases. This causes an
increase in volume. So, as the bubbles move upwards, their size increases. The
pressure under a liquid surface varies with depth. ... Thus, when a bubble rises
from below the surface it encounters less pressure. This causes the volume to
increase and the bubble rises in size as it rises from a depth
5. As a hot air balloons rise pressure decreases. Since the pressure is decreasing
as you rise through the sky, the temperature will also decrease, this
demonstrating Gay Lussac's Law. However, the temperature of the air inside the
hot air balloon is able to stay hot.
You might also like
- ExamplesDocument12 pagesExamplesMoldir SerikNo ratings yet
- Balloon Lung ExperimentDocument4 pagesBalloon Lung ExperimentdyanenfalhadapNo ratings yet
- Applications of Gas LawsDocument3 pagesApplications of Gas LawsCharm GorospeNo ratings yet
- Chem Law LawDocument2 pagesChem Law LawFaye Lianne MaderoNo ratings yet
- Just Breathe Activity-Lungs WooksheetDocument1 pageJust Breathe Activity-Lungs WooksheetJhade Rio GadinganNo ratings yet
- Applications of Boyle's LaawDocument2 pagesApplications of Boyle's Laawcherrymaeregalario2001No ratings yet
- Gas Laws Form 4 Exercise and ExamplesDocument6 pagesGas Laws Form 4 Exercise and ExampleszahidahoseinNo ratings yet
- Science 10 LungsDocument1 pageScience 10 LungsJheanna JaypeNo ratings yet
- Definition of Boyle's Law: What Is An Ideal Gas?Document4 pagesDefinition of Boyle's Law: What Is An Ideal Gas?حسين كاظم ياسينNo ratings yet
- Science ScriptDocument2 pagesScience ScriptTrixie San DiegoNo ratings yet
- Boyles LawDocument6 pagesBoyles LawMara TillesNo ratings yet
- Gideon Elisha A. Ballesteros 10-WisdomDocument1 pageGideon Elisha A. Ballesteros 10-WisdomRandomStuffNo ratings yet
- BoyleDocument1 pageBoylePASTEURSYNCH GELBOLINGO, ERNIE IINo ratings yet
- 3rd Activity Guide QuestionsDocument5 pages3rd Activity Guide QuestionsAcademic ServicesNo ratings yet
- Real Life Application of Boyles LawDocument12 pagesReal Life Application of Boyles LawChelsie Rose GalangcoNo ratings yet
- How Can I Make My NSTP Engagement Meaningful Sec39 DagcutanDocument1 pageHow Can I Make My NSTP Engagement Meaningful Sec39 DagcutanMaxine TaeyeonNo ratings yet
- Applications of Charles LawDocument2 pagesApplications of Charles LawHelmaNo ratings yet
- Handouts For Week 1 and 2Document3 pagesHandouts For Week 1 and 2Angel rose OrbeNo ratings yet
- Boyle's LawDocument24 pagesBoyle's LawRalph Jasil Esparagoza EscanillasNo ratings yet
- SciDocument1 pageSciJasmine BarquillaNo ratings yet
- Evans Christian Rosal - Gen Chem FinalsDocument3 pagesEvans Christian Rosal - Gen Chem FinalsEvans Christian Velasquez RosalNo ratings yet
- Liah Pls Print Ty I Love You!Document2 pagesLiah Pls Print Ty I Love You!AndreaGabrielleNo ratings yet
- Charles' Law - Real Life Applications: Watch What Happens To A Helium Balloon On A Cold DayDocument2 pagesCharles' Law - Real Life Applications: Watch What Happens To A Helium Balloon On A Cold DayEnriquez JohnNo ratings yet
- SCRAPBOOKDocument19 pagesSCRAPBOOKJulius Michael Guinto100% (1)
- Biology HW:: Activities That Often Occur Outside of The Will and Is A Natural ProcessDocument2 pagesBiology HW:: Activities That Often Occur Outside of The Will and Is A Natural Processrose llarNo ratings yet
- LoveubabykoDocument2 pagesLoveubabykoSheila Mae NimNo ratings yet
- Boyles LawDocument1 pageBoyles LawRevilloNo ratings yet
- 4th Quarter Module 1Document2 pages4th Quarter Module 1jackielou trongcosoNo ratings yet
- Charles LawDocument12 pagesCharles LawLerr Real RelleNo ratings yet
- Day 3Document12 pagesDay 3Quila QuaboNo ratings yet
- Microsoft Word - Altitude PhysiologyDocument49 pagesMicrosoft Word - Altitude PhysiologyMetinee AngsutorntakNo ratings yet
- The Science/Mechanics of BreathingDocument1 pageThe Science/Mechanics of BreathingVan MichaelNo ratings yet
- Boyles Law CompleteDocument4 pagesBoyles Law CompleteLincoln PiaoanNo ratings yet
- Entropy 03 00116Document2 pagesEntropy 03 00116Ali MpkNo ratings yet
- DhfdjhfjdsDocument21 pagesDhfdjhfjdsMichael EscribaNo ratings yet
- Uses and Applications of The Different Gas LawsDocument8 pagesUses and Applications of The Different Gas LawsWin7Addict100% (30)
- Miguel, John Ryan, D.G Lab Act 7Document3 pagesMiguel, John Ryan, D.G Lab Act 7Simon DeleonNo ratings yet
- Lung Model ActivityDocument2 pagesLung Model ActivityAddy IletoNo ratings yet
- Chapter 1: Respiration: ObjectiveDocument3 pagesChapter 1: Respiration: ObjectiveYugenNo ratings yet
- Xperiment ChemDocument5 pagesXperiment ChemExynos NemeaNo ratings yet
- Roselyn 1Document5 pagesRoselyn 1Lecsos clyde Pang-agNo ratings yet
- End of Respiration, Beginning of Reproduction TranscriptionDocument7 pagesEnd of Respiration, Beginning of Reproduction TranscriptionmarthafaragNo ratings yet
- SciDocument2 pagesSciErica BiancaNo ratings yet
- Study Guide 4: Diaphragm External Intercostal MusclesDocument37 pagesStudy Guide 4: Diaphragm External Intercostal MusclesItadori YujiNo ratings yet
- Diaphragmatic Breathing vs. Belly BreathingDocument8 pagesDiaphragmatic Breathing vs. Belly BreathingTatiana JosanNo ratings yet
- Recall The Functions of The Organs in The Gas Exchange SystemDocument14 pagesRecall The Functions of The Organs in The Gas Exchange SystemAhmad AhmadNo ratings yet
- The Human Breathing System Part2Document23 pagesThe Human Breathing System Part2Delfin Jr UrbanoNo ratings yet
- Application of BoyleDocument10 pagesApplication of BoyleRuthcel BautistaNo ratings yet
- Properties of AirDocument6 pagesProperties of AirDaoud KhanNo ratings yet
- Effective BreathingDocument2 pagesEffective Breathingjosejazmine4No ratings yet
- Soda BottleDocument3 pagesSoda BottleBenedick CruzNo ratings yet
- Human Breathing MechanismDocument22 pagesHuman Breathing MechanismNisa TaniesaNo ratings yet
- Breathing, Respiration A Level BiologyDocument2 pagesBreathing, Respiration A Level Biologysna240839No ratings yet
- Boyles LawDocument2 pagesBoyles LawjaNo ratings yet
- Xperiment ChemDocument5 pagesXperiment ChemExynos NemeaNo ratings yet
- Breathe in Through The Nose, Out Through The Mouth Question: How Important Is Breathing To Your Health and Is There A Proper Way ToDocument2 pagesBreathe in Through The Nose, Out Through The Mouth Question: How Important Is Breathing To Your Health and Is There A Proper Way ToyuuNo ratings yet
- Breathing Mechanism in Humans: LessonDocument5 pagesBreathing Mechanism in Humans: LessonjoyNo ratings yet
- Pulmonary Ventilation PDFDocument11 pagesPulmonary Ventilation PDFSrsblackieNo ratings yet
- LawDocument6 pagesLawJaymart BobilesNo ratings yet