Professional Documents
Culture Documents
Teixidor 1987
Teixidor 1987
Uploaded by
agcfilesloverCopyright:
Available Formats
You might also like
- Method Statement For I&C Calibration and LaboratoryDocument20 pagesMethod Statement For I&C Calibration and LaboratoryUtku Can Kılıç100% (1)
- ACETANILIDEDocument10 pagesACETANILIDEDiego AlonsoNo ratings yet
- Low Cost, High Efficiency Solar Cell Based On Dye-Sensitized Colloidal TiO2 FilmDocument4 pagesLow Cost, High Efficiency Solar Cell Based On Dye-Sensitized Colloidal TiO2 FilmWildan MocholladNo ratings yet
- Ed 079 P 1092Document2 pagesEd 079 P 1092Júlia Comas BarcelóNo ratings yet
- Closed Paramagnetic Electron-Precise Cluster'Document2 pagesClosed Paramagnetic Electron-Precise Cluster'Nikhil BhoumikNo ratings yet
- Double Layer I. Qualitative Detection of Adsorption of Ions and Neutral Molecules On The Mercury Electrode and Excess Charge On The Electrode PDFDocument3 pagesDouble Layer I. Qualitative Detection of Adsorption of Ions and Neutral Molecules On The Mercury Electrode and Excess Charge On The Electrode PDFAndresQuesadaNo ratings yet
- Thermal Conductivity Superlattices: Revie%Document6 pagesThermal Conductivity Superlattices: Revie%venkatsrNo ratings yet
- 1 s2.0 S0277538721003818 MainDocument11 pages1 s2.0 S0277538721003818 MainMoromi NathNo ratings yet
- Standard Molar Enthalpies of Formation of Hydroxy-, Chlor-, and BromapatiteDocument10 pagesStandard Molar Enthalpies of Formation of Hydroxy-, Chlor-, and BromapatiteDaiana IuliaNo ratings yet
- Chichkov ApplPhysA 1996Document7 pagesChichkov ApplPhysA 1996norbNo ratings yet
- Jahn-Teller VibronicDocument10 pagesJahn-Teller VibronicAlexanderNo ratings yet
- N ST J Braithwaite 2000 Plasma Sources Sci. Technol. 9 517Document12 pagesN ST J Braithwaite 2000 Plasma Sources Sci. Technol. 9 517yerson fabian barragan jimenezNo ratings yet
- EIS Tutorial Ver2bDocument10 pagesEIS Tutorial Ver2bodoalawayeNo ratings yet
- Rooney 1991 A Comprehensive Approach Analysis Interpretation Resonances Spins 3 2 Living SystemsDocument18 pagesRooney 1991 A Comprehensive Approach Analysis Interpretation Resonances Spins 3 2 Living SystemsAlfonso LemaNo ratings yet
- Younker 2013Document10 pagesYounker 2013Anonymous ZY43E2DTNo ratings yet
- Photoinduced Electron Transfer in Porphyrin-Quinone Cyclophanes 8Document11 pagesPhotoinduced Electron Transfer in Porphyrin-Quinone Cyclophanes 8Milton van PuttenNo ratings yet
- Cimoli No 1981Document5 pagesCimoli No 1981Omar José Cotazo MosqueraNo ratings yet
- Journal of Electroanalytical Chemistry: Tim Tichter, Dirk Andrae, Christina RothDocument11 pagesJournal of Electroanalytical Chemistry: Tim Tichter, Dirk Andrae, Christina RothALEJANDRA FONTALVO STUDENTNo ratings yet
- 1984 - MORSE - MICHALOPOULOS - Chem - Rev - Spectroscopic Studies of The Jet-Cooled Nickel DimerDocument7 pages1984 - MORSE - MICHALOPOULOS - Chem - Rev - Spectroscopic Studies of The Jet-Cooled Nickel DimerAlejandra AwimbaweNo ratings yet
- Capacitance of Two-Dimensional Titanium Carbide (MXene) and MXenecarbon Nanotube Composites in Organic ElectrolytesDocument6 pagesCapacitance of Two-Dimensional Titanium Carbide (MXene) and MXenecarbon Nanotube Composites in Organic Electrolytessj hNo ratings yet
- NMR Cross-Relaxation Study of Ultraslow Motion of The Domain Wall in Channel Inclusion CompoundDocument4 pagesNMR Cross-Relaxation Study of Ultraslow Motion of The Domain Wall in Channel Inclusion CompoundnokiaistNo ratings yet
- Paper 2Document8 pagesPaper 2Alexander AndradeNo ratings yet
- Analysis of Porous Electrodes With Sparingly Soluble Reactants - III - Short Time TransientsDocument7 pagesAnalysis of Porous Electrodes With Sparingly Soluble Reactants - III - Short Time Transientssumit singhNo ratings yet
- Chouaib 2016Document9 pagesChouaib 2016nabilNo ratings yet
- JonesC EndoEndo24Diphosphabicyclo110Butane Orbital Isomers CC 2001 663-4Document2 pagesJonesC EndoEndo24Diphosphabicyclo110Butane Orbital Isomers CC 2001 663-4jazmurdochNo ratings yet
- EPJD - 2018 - Impact of Single ParticleDocument8 pagesEPJD - 2018 - Impact of Single ParticleramazanNo ratings yet
- Electronic Control of The Co-Ordination Mode of An Alkyne To Trimetallic Cluster X-Ray Crystal Structure of 0S3 (C0) 7 (P3-Q2 - PHCDPH) (Ph2Pch2Pph2)Document3 pagesElectronic Control of The Co-Ordination Mode of An Alkyne To Trimetallic Cluster X-Ray Crystal Structure of 0S3 (C0) 7 (P3-Q2 - PHCDPH) (Ph2Pch2Pph2)Nikhil BhoumikNo ratings yet
- Rieger1994 Capìtulo 7 ElectrolisisDocument56 pagesRieger1994 Capìtulo 7 Electrolisisyazmin zapata garciaNo ratings yet
- Band-Selective Holstein Polaron in Luttinger Liquid Material A Moo (A K, RB)Document7 pagesBand-Selective Holstein Polaron in Luttinger Liquid Material A Moo (A K, RB)Scribd ScribdNo ratings yet
- Electron-Phonon C CDocument11 pagesElectron-Phonon C CArdani MunaqiNo ratings yet
- The Direct Electrochemical Synthesis of ( (C6H5) 3Ph) 2 (CoCl4)Document2 pagesThe Direct Electrochemical Synthesis of ( (C6H5) 3Ph) 2 (CoCl4)Pavle RadojkovićNo ratings yet
- Quasiparticle Dynamics in GrapheneDocument6 pagesQuasiparticle Dynamics in GrapheneThamyres F. Messa MoreiraNo ratings yet
- Martinezsaavedra 2014Document14 pagesMartinezsaavedra 2014snehasis banerjeeNo ratings yet
- Gaynor 2015Document5 pagesGaynor 2015Nasos IalexanthNo ratings yet
- Chimia Face Ce Vrea EaDocument5 pagesChimia Face Ce Vrea EaRomano AlbertNo ratings yet
- Separation of Thiocyanato Complexes of IronDocument2 pagesSeparation of Thiocyanato Complexes of Ironchm12No ratings yet
- A Mathematical Model For The Porous Lead Dioxide ElectrodeDocument10 pagesA Mathematical Model For The Porous Lead Dioxide Electrodesumit singhNo ratings yet
- Binary Phase Diagram of WaterDocument10 pagesBinary Phase Diagram of WaterkmkmNo ratings yet
- Low-Temperature Tunneling of CH3 Quantum Rotor in Van Der Waals SolidsDocument15 pagesLow-Temperature Tunneling of CH3 Quantum Rotor in Van Der Waals SolidsNyiam HlubNo ratings yet
- 10.1515 - Zna 1983 0221Document6 pages10.1515 - Zna 1983 0221muthuNo ratings yet
- Ab Initio Simulation of Photoluminescence: Bi Iny O (S Site)Document10 pagesAb Initio Simulation of Photoluminescence: Bi Iny O (S Site)CamilaBurgosNo ratings yet
- Polar SolventDocument12 pagesPolar SolventReshma SaxenaNo ratings yet
- Effects of Lu and Ni Substitution On Thermoelectric Properties of Ca Co ODocument6 pagesEffects of Lu and Ni Substitution On Thermoelectric Properties of Ca Co OAhmed Khalid HussainNo ratings yet
- 1 StructuralDocument8 pages1 StructuralTJPRC PublicationsNo ratings yet
- Electroabsorption and Related Spectroscopic Studies of Bimetallic Tetraiminoethylenedimacrocyclic Complexes: Corroboration of Valence Electron DelocalizationDocument4 pagesElectroabsorption and Related Spectroscopic Studies of Bimetallic Tetraiminoethylenedimacrocyclic Complexes: Corroboration of Valence Electron DelocalizationAzizah MunitaNo ratings yet
- Thomas FermiDocument5 pagesThomas FermiMuhammad SaqibNo ratings yet
- Fundamentals of Atomic and Molecular Spectroscopy in Instrumental AnalysisDocument40 pagesFundamentals of Atomic and Molecular Spectroscopy in Instrumental Analysisnikusrivastva44043No ratings yet
- S0021889897001787 PDFDocument5 pagesS0021889897001787 PDFMario Misael Machado LòpezNo ratings yet
- Chalker FinalDocument44 pagesChalker FinalMuhammad Shifan JayadiNo ratings yet
- Plasmonic Physics of 2D Crystalline MaterialsDocument30 pagesPlasmonic Physics of 2D Crystalline Materials유재원No ratings yet
- Examination of Different Strengths of Octupole Correlations in Neutron-Rich PR and PM IsotopesDocument5 pagesExamination of Different Strengths of Octupole Correlations in Neutron-Rich PR and PM IsotopesabbeyNo ratings yet
- Chem Principles 7e ISM Major Techniques Even FINALDocument6 pagesChem Principles 7e ISM Major Techniques Even FINALSelma MeloNo ratings yet
- Electron-Phonon Heat Exchange in Quasi-Two-Dimensional NanolayersDocument17 pagesElectron-Phonon Heat Exchange in Quasi-Two-Dimensional NanolayersLiviu BadeaNo ratings yet
- Synthesis and Fluorescence Properties of Europium, Terbium DopedDocument5 pagesSynthesis and Fluorescence Properties of Europium, Terbium DopedRAQUEL GAMEZNo ratings yet
- Dynamic Effect NPDocument4 pagesDynamic Effect NPPurwojatmiko HandokoNo ratings yet
- Superconductivity and Charge Density Wave in ZrTe3-xSex (ZHU Et. Al, 2016)Document7 pagesSuperconductivity and Charge Density Wave in ZrTe3-xSex (ZHU Et. Al, 2016)Joao Pedro AlmeidaNo ratings yet
- 1 s2.0 001346869500077R MainDocument8 pages1 s2.0 001346869500077R MainAmit Kumar ChaurasiaNo ratings yet
- JP 1040234Document8 pagesJP 1040234Chem CU706No ratings yet
- PhysRev 87 835Document8 pagesPhysRev 87 835Miguel Angel CansecoNo ratings yet
- PhysRevC 65 014308Document10 pagesPhysRevC 65 014308Ravi Sankar Babu BalabhadrapatruniNo ratings yet
- Transcat 2014-EcatDocument678 pagesTranscat 2014-Ecatdilor19No ratings yet
- Process-Piping-Pipeline Engg PDP - Ray R10copiesDocument40 pagesProcess-Piping-Pipeline Engg PDP - Ray R10copiesVamsikrishna LakamsaniNo ratings yet
- Set Up Your Home Chemistry LabDocument16 pagesSet Up Your Home Chemistry LabDoohan Jemsy50% (2)
- Data Set Information WINE QUALITYDocument4 pagesData Set Information WINE QUALITYKustiara S100% (1)
- Climate Change PaperDocument3 pagesClimate Change Paperapi-320818400100% (1)
- Otec-Outline Full WordDocument27 pagesOtec-Outline Full WordAndrei SabaterNo ratings yet
- Answers For Practice Problems in Lesson 5: Practice Problem #1: Specific Gravity Calculation For Coarse AggregateDocument2 pagesAnswers For Practice Problems in Lesson 5: Practice Problem #1: Specific Gravity Calculation For Coarse AggregateDjonraeNarioGalvezNo ratings yet
- Catalogue of IRO WaterDocument90 pagesCatalogue of IRO WatershoyebNo ratings yet
- Waste Heat Recovery in Cement Industry at Mapl Leaf Cement FactoryDocument35 pagesWaste Heat Recovery in Cement Industry at Mapl Leaf Cement FactoryTafseer Abbas100% (2)
- Yhg/Yhf: Plastic Filters - On Line Filter Model 'H''Document2 pagesYhg/Yhf: Plastic Filters - On Line Filter Model 'H''Ted ThomsonNo ratings yet
- Material Safety Data Sheet: Section I - Chemical Product and Company IdentificationDocument2 pagesMaterial Safety Data Sheet: Section I - Chemical Product and Company IdentificationMu ClasNo ratings yet
- MASS TRANSFER Coefficient and Inter Phase Mass TransferDocument41 pagesMASS TRANSFER Coefficient and Inter Phase Mass TransferSannala Prudhvi100% (1)
- USP-NF OlanzapineDocument4 pagesUSP-NF OlanzapineDanielle De GuzmanNo ratings yet
- Application Note - Measuring KVP On An AMX 4 or 4plus-2013-10-30 PDFDocument2 pagesApplication Note - Measuring KVP On An AMX 4 or 4plus-2013-10-30 PDFLuis Fernando Garcia SanchezNo ratings yet
- CH 1Document19 pagesCH 1abdallah elsharnobyNo ratings yet
- E 1452 - 92 Standard Practice For Preparation of Calibration Solutions For Spectrophotometric and For Spectroscopic Atomic Analysis PDFDocument3 pagesE 1452 - 92 Standard Practice For Preparation of Calibration Solutions For Spectrophotometric and For Spectroscopic Atomic Analysis PDFBryanNo ratings yet
- Unit 5 Phase Equilibrium and CorrosionDocument29 pagesUnit 5 Phase Equilibrium and CorrosionDr. Ruma Arora SoniNo ratings yet
- 09 Science Notes Ch02 Is Matter Around Us PureDocument5 pages09 Science Notes Ch02 Is Matter Around Us PureApoorvaKashyapNo ratings yet
- Class 12 Chemistry Practical by Bharat PanchalDocument34 pagesClass 12 Chemistry Practical by Bharat PanchalSarita BhattNo ratings yet
- Current Concepts and Techniques For Caries Excavation and Adhesion To Residual Dentin, J Adhesi Dent 2011Document17 pagesCurrent Concepts and Techniques For Caries Excavation and Adhesion To Residual Dentin, J Adhesi Dent 2011Jesus NavarreteNo ratings yet
- Introduction To Cold Plasma.: Generation Techniques: 2-Radio Frequency Discharge. 3-Microwave Discharge PlasmaDocument21 pagesIntroduction To Cold Plasma.: Generation Techniques: 2-Radio Frequency Discharge. 3-Microwave Discharge PlasmaPakeeza DollNo ratings yet
- Effect of SO3 Content On Concrete StructureDocument2 pagesEffect of SO3 Content On Concrete StructureNam HuynhNo ratings yet
- Starch Characteristics of Black Bean, Chick Pea, Lentil, Navy Bean and Pinto Bean Cultivars Grown in CanadaDocument10 pagesStarch Characteristics of Black Bean, Chick Pea, Lentil, Navy Bean and Pinto Bean Cultivars Grown in CanadaJose perezNo ratings yet
- Forensics Crime Busters WebsitesDocument3 pagesForensics Crime Busters WebsitesMengyao Alice LiNo ratings yet
- Pengaruh Konsentrasi Gula Dan Lama Penyimpanan Terhadap Mutu Manisan Basah Batang Daun PepayaDocument8 pagesPengaruh Konsentrasi Gula Dan Lama Penyimpanan Terhadap Mutu Manisan Basah Batang Daun PepayaAlfi nur farchatin nufusNo ratings yet
- Bela Products IntoductionDocument12 pagesBela Products IntoductionShailesh ThakkarNo ratings yet
- JPC 119022Document2 pagesJPC 119022Juan Pablo EspinosaNo ratings yet
- PTC-40-1991 - Performance Test Codes Flue Gas Desulfurization Units PDFDocument70 pagesPTC-40-1991 - Performance Test Codes Flue Gas Desulfurization Units PDFSunil KumarNo ratings yet
- Soal LajuDocument3 pagesSoal Lajufhandayani8No ratings yet
Teixidor 1987
Teixidor 1987
Uploaded by
agcfilesloverOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Teixidor 1987
Teixidor 1987
Uploaded by
agcfilesloverCopyright:
Available Formats
Electronic Spectra of cis and trans Disubstituted
Octahedral Chromium(lll) Complexes
An Advanced Inorganic Chemistry Experiment
Francesc Teixidor and Jaume Casabol
Universitat Autonoma de Barcelona, Bellaterra, 08193 Barcelona, Spain
Andreu Solans
Productos UHPON S.A., Rubi, Barcelona. Spain
Electronic spectroscopy is a valuable tool used routinely in
coordination chemistry research laboratories. For a long
time it has been one of the most classical and powerful trans-[CrFz(en)2] CI (3)
techniques available to elucidate the fine details of coordina- A solution of 30 g (0.11 mol) of chromium(II1)chloride hexahy-
tion compound stereochemistry. It is well known that the drate in 100 mL of water was placed in a 400-mL polyethylene
understandine of the stereochemistrv of amine comnlexes of beaker and diluted with 24 g (0.6 mol) of a 48%solution of hydraflu-
chromium(llfi, cobalt(III), or ruthe&un(11) was almost en- oric acid. The beaker was packed in ice and 100 g (1.58 mol) of
tirelv based on their electronic soectra. aqueous 95% ethylenediamine was added dropwise over 1 h. After
A."B. P. Lever ( I ) says the addition of the ethylenediamine was completed, the dark red-
purple solution was heated for 2 h on a steam bath to complete the
Whilst many physical techniques play their part in the elueida- reaction. The solution, which had darkened in color during the
tion of the geometric and electronic structure of a metal complex, heating, was transferred to an evaporating dish, and the excess
a study of the electronic spectra can often provide the most water was removed by heating for an additional 2 h at 90-95 'C.
detailed information. In the spectrum we see a map of the energy During this time a crustwas formed on the surface of the liquid, and
bands within the molecule-the trick is to learn how to read this this was broken up frequentlyduring the late stages of the evapora-
map. tion. At the end of the 2 hours the reaction mixture was cooled to 10
OC in an ice bath and filtered.
Since visible and UV spectrophotometers are now very com- Caution: All work should he done in a hood. The manipulation of
mon in teaching lnboratorieii] in our opinion ir is veriinter- 95%ethylenediamineor 48% HF solutions is potentially dangerous;
esting to let thestudents learn ah0111the largr analytical and the latter can seriously damage the skin.
structural elucidation powers of this technique. The crystals that had precipitated were collected, washed with
Among the most dramatic examples that show the power 100 mL of EtOH followed by 100 mL of acetone, and air-dried.The
of electronic soectra to elucidate stereochemistm are the above procedure yielded 15.5 g (56%)of reddish orange crystals.
spectra of ris and trans diauhitituted octahedral romplexes
ol Crtllli or Cu(II1).whichdis~laswell-known oattrrns. I'se
T o the mother liquor from thes!nthe+ of Iran? isomer (150ml.l
of these also shows'the powe;ofthe group theory in quan- were a d d ~ d i.n i,rder, dry \leOH t3OO ml.,, Mr?CO (300 mL,, nnd
tum mechanics. At present,, elementary group theory is part Et.4<200ml.) u i t h viaorousrirrinr, whereupon a pinksolid slowlv
of the inorganic chemistry curricula of most universities. began to precipitate. Caution: All procedures involving ether are
In our department the students spend part of their labora- hazardous. Oecasionallv. the .orecinitation
. had to he started bv
tory time in synthesizing of cis- and trans-[CrFz(en)z]Cl, scratching the henkrr's walls. In n r d ~ ro
r assure complete prrripita-
recording their spectra, and interpreting them. By doing this rion, t he mixture uns kept at h w temperature (freezer)for 2 h. 'l'hr
they get first-hand knowledge about cis/trans isomeric solid wuifilterednnd naihed withdry hlr,C'OandFt?Oanddried in
chemistry, learn about electronic spectra, and study the in- a dry air stream to avoid the formation of resins or gums.
tricate differences between cis and trans electronic spectra, Electronic Spectra
which are currently neglected in almost all textbooks. Usual-
ly, only trans isomers are explained, apparently because they It is convenient to record the electronic apeetra in aqueous solu-
tion and to record the spectra of both isomers superimposed at the
fit elementary group predictions and the cis isomer spectra same molar concentration to show their similarities and differences
do not. in hand positions and molar ahsorptivities. Alsoit would be instruc-
Furthermore, we think the preparation of trans- and cis- tional to record the spectrum of the initialsolution since it displays a
[CrF2(en)2]CIcomplexes is also interesting from the synthe- cis pattern, although cis and trans species are present in the solu-
sis point of view since they are both formed during the tion.
reaction and their separation is easily achieved because of
their different solubilities. It is not clear, a t present, whether Theory
both isomers really are formed simultaneously or one of the Octahedral chromium(1II) complexes exhibit three spin-
isomers is being isomerized to the other during the reaction. allowed transitions (5) from the ground state 'AA to the
The easiness of trans-cis isomerization in this kind of com- excited states4TQ(F),4T1p(F),4Tlg(P).It is well known that
plex is well known. On the other hand their electronic spec- onlv the first two transitions are aenerallv dis~lavedin the
tra are very typical, showing all the details expected for spectrum because the highest in energy is in fait overlapped
-
them. The cation trans-CrFz(en)2+ is of particular interest
because of the large splitting of the (AQ (Tzg and 'Azg
'TI, bands (2).
- bv charae-transfer bands. The cis and trans octahedral di-
sibstituied complexes belong to CPUandD4hsymmetry point
croups,
- . respectively, but, from the view of the electronic
environmedt around the metal ion, both symmetry groups
are in practice the tetragonal one, Ddh. This fact is not gener-
ally recognized and is neglected in almost all textbooks, but
it is well explained in Ballhausen's classic Introduction to
' Author to whom correspondence should be addressed. Ligand Field Theory (6).
Volume 64 Number 5 May 1967 461
The electrostatic potential, V , a t the point (x,y,z) due to Spectral Data and Assignments
two point charges, ql and 92, placed on the x axis a t a dis-
tance f a from the center of the coordinate system, is given complex Ion h (nm)' "2 (nmIb ~nm)~
by CrFBs- 671 441 291
Cr(en)2+* 458 351 -
~IsCrF~(en)~+ 517 378 235
transCrFden)f 530' 3979 248"
In this expression Pz(x) and Pa(x) are the first two even 485' 3511 229"
Legendre functions and r the radius vector of the point
(xY,z).Notice that only the sum of ql and qa isof importance
for the crystalline field potential. In other words the crystal-
line potential for an octahedron can be written as the sum of
three contributions, each of them containing the sum of the
axial electronic charges.
If the sum of the charges on the x, y, and z axes are pz, py,
"B,, -
"B1*-'E0
'EgIOmdcomponem of u,.
(Olnl component of u,.
and p, and if p, = II, = pz, the complex is said to have a cubic "B,, -
"'B,, -'A,,(D,d mmponem of vs; calculated Com reference 2.
component of u,.
crvstalline field. If u, = u, # u,. the comnlex is said to have a
tetragonal crystall& fikfd. 0 r i f p, # py.# p,, the complex is
said to have a rhombic crvstalline field. I t is nossible to
-
J's,.-'A,, lD,d component of u,.
"'B,, 'E0(D4d component of nS: calculated from reference 2.
define a tetragonal contribution, VT,to the octaKedra1 crys-
talline field, f(x,y,z), as:
nal contribution with respect to trans isomers and generally
the splitting is not observed in the spectrum (eq 1). The
minus sign means that the order of splitting in the energy
levels would be inverted in both isomers.
and Thus, in theory our three-band spectrum of an octahedral
d3 complex, becomes a six-hand spectrum, but all six bands
are rarely seen. I t is generally true that the lowest energy
therefore spin-allowed transition is most susceptible to the effects of
lower symmetry and the highest energy one is generally
overlapped by charge-transfer hands.
consequently, for trans-MA4B2 Results and Dlscusslon
PZ = %
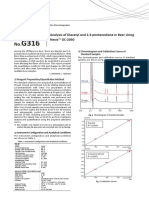
A' Reported in the table are the electronic spectra of both
P7 = %A cis- and trans-CrFde&+ isomers (3.4) and the band assien-
P, = 248 ments as they are givenby ~ u b i c kkt ; al. based on a sinGe-
crystal, polarized electronic spectral analysis (2).
and for cis-MA4B2 The rule of "average environment" (5)relates the pseudo-
octahedral mixed complex CrF2(enIzf with CrF$ and
C r ( e n ) ~ ~By
+ . applying this rule we may obtain an approxi-
mate value of 10 Dq for ci~-CrFz(en)2~+ and predict its spec-
trum in this fashion:
where, q~ and q~ are the point charges due to the ligands A
and B. As can be seen, both isomers have equal values for p,
and py and one different value for pz so they belong to the
same D4kelectronic symmetry point group. Furthermore, for and
the trans complex
This is in good agreement with the experimentalvalue of 517
and for the cis complex nm for the c i ~ - C r F ~ ( e ncomplex.
)~+
Using the graphic methods outlined in several textbooks
or in reference 5, one can obtain the B value, which is 674
And -
cm-I. Using these B and Dq values for cis-CrFz(en)2+, the
highest energy transition, 4A2g 4T2g(P), is calculated at
that overlap and obscure this d d transition.
Literature Cited
-
235 nm. In this region there are strong charge-transfer bands
From these expressions i t may be seen that 1. LFYFI,A.B.P.Cmrd. Ckem.Reu. 1968,3.119.
2. Dubieki.L.;Hitchman.M.A.:Day.P.Inorg.Ckem. 1970.9. 188.
3. Vsughn.J. W.:Stvsn,O.J.: Magnusson. V. E.lnorg. Chem 1968,7,736.
4. Cssabb,J.;Solans.A.;Resl,J. J.Synlk.Reoct.Inorg. Me1.018. Chorn. 1983.13.835.
Both cis and trans isomers should show the tetraeonal 6. Figgi~,B. N. Intmducfian l o Llmnd Fields; Intorscience: New York, 1966.
6. Ballhausen, C. J.lnlroducfion to LigondPidd Theo~y; MeGiaw-Hill: New York, 1962.
splitting of the octahedral transitions, but only the trans 7. J o r g e n m , C. K. A b a o r p t i i Spadm and Ckrmieol Bonding in Complrier:Pergamon:
isomers exhibit it. The cis isomers have only a half tetrago- London. 1962.
462 Journal of Chemical Education
You might also like
- Method Statement For I&C Calibration and LaboratoryDocument20 pagesMethod Statement For I&C Calibration and LaboratoryUtku Can Kılıç100% (1)
- ACETANILIDEDocument10 pagesACETANILIDEDiego AlonsoNo ratings yet
- Low Cost, High Efficiency Solar Cell Based On Dye-Sensitized Colloidal TiO2 FilmDocument4 pagesLow Cost, High Efficiency Solar Cell Based On Dye-Sensitized Colloidal TiO2 FilmWildan MocholladNo ratings yet
- Ed 079 P 1092Document2 pagesEd 079 P 1092Júlia Comas BarcelóNo ratings yet
- Closed Paramagnetic Electron-Precise Cluster'Document2 pagesClosed Paramagnetic Electron-Precise Cluster'Nikhil BhoumikNo ratings yet
- Double Layer I. Qualitative Detection of Adsorption of Ions and Neutral Molecules On The Mercury Electrode and Excess Charge On The Electrode PDFDocument3 pagesDouble Layer I. Qualitative Detection of Adsorption of Ions and Neutral Molecules On The Mercury Electrode and Excess Charge On The Electrode PDFAndresQuesadaNo ratings yet
- Thermal Conductivity Superlattices: Revie%Document6 pagesThermal Conductivity Superlattices: Revie%venkatsrNo ratings yet
- 1 s2.0 S0277538721003818 MainDocument11 pages1 s2.0 S0277538721003818 MainMoromi NathNo ratings yet
- Standard Molar Enthalpies of Formation of Hydroxy-, Chlor-, and BromapatiteDocument10 pagesStandard Molar Enthalpies of Formation of Hydroxy-, Chlor-, and BromapatiteDaiana IuliaNo ratings yet
- Chichkov ApplPhysA 1996Document7 pagesChichkov ApplPhysA 1996norbNo ratings yet
- Jahn-Teller VibronicDocument10 pagesJahn-Teller VibronicAlexanderNo ratings yet
- N ST J Braithwaite 2000 Plasma Sources Sci. Technol. 9 517Document12 pagesN ST J Braithwaite 2000 Plasma Sources Sci. Technol. 9 517yerson fabian barragan jimenezNo ratings yet
- EIS Tutorial Ver2bDocument10 pagesEIS Tutorial Ver2bodoalawayeNo ratings yet
- Rooney 1991 A Comprehensive Approach Analysis Interpretation Resonances Spins 3 2 Living SystemsDocument18 pagesRooney 1991 A Comprehensive Approach Analysis Interpretation Resonances Spins 3 2 Living SystemsAlfonso LemaNo ratings yet
- Younker 2013Document10 pagesYounker 2013Anonymous ZY43E2DTNo ratings yet
- Photoinduced Electron Transfer in Porphyrin-Quinone Cyclophanes 8Document11 pagesPhotoinduced Electron Transfer in Porphyrin-Quinone Cyclophanes 8Milton van PuttenNo ratings yet
- Cimoli No 1981Document5 pagesCimoli No 1981Omar José Cotazo MosqueraNo ratings yet
- Journal of Electroanalytical Chemistry: Tim Tichter, Dirk Andrae, Christina RothDocument11 pagesJournal of Electroanalytical Chemistry: Tim Tichter, Dirk Andrae, Christina RothALEJANDRA FONTALVO STUDENTNo ratings yet
- 1984 - MORSE - MICHALOPOULOS - Chem - Rev - Spectroscopic Studies of The Jet-Cooled Nickel DimerDocument7 pages1984 - MORSE - MICHALOPOULOS - Chem - Rev - Spectroscopic Studies of The Jet-Cooled Nickel DimerAlejandra AwimbaweNo ratings yet
- Capacitance of Two-Dimensional Titanium Carbide (MXene) and MXenecarbon Nanotube Composites in Organic ElectrolytesDocument6 pagesCapacitance of Two-Dimensional Titanium Carbide (MXene) and MXenecarbon Nanotube Composites in Organic Electrolytessj hNo ratings yet
- NMR Cross-Relaxation Study of Ultraslow Motion of The Domain Wall in Channel Inclusion CompoundDocument4 pagesNMR Cross-Relaxation Study of Ultraslow Motion of The Domain Wall in Channel Inclusion CompoundnokiaistNo ratings yet
- Paper 2Document8 pagesPaper 2Alexander AndradeNo ratings yet
- Analysis of Porous Electrodes With Sparingly Soluble Reactants - III - Short Time TransientsDocument7 pagesAnalysis of Porous Electrodes With Sparingly Soluble Reactants - III - Short Time Transientssumit singhNo ratings yet
- Chouaib 2016Document9 pagesChouaib 2016nabilNo ratings yet
- JonesC EndoEndo24Diphosphabicyclo110Butane Orbital Isomers CC 2001 663-4Document2 pagesJonesC EndoEndo24Diphosphabicyclo110Butane Orbital Isomers CC 2001 663-4jazmurdochNo ratings yet
- EPJD - 2018 - Impact of Single ParticleDocument8 pagesEPJD - 2018 - Impact of Single ParticleramazanNo ratings yet
- Electronic Control of The Co-Ordination Mode of An Alkyne To Trimetallic Cluster X-Ray Crystal Structure of 0S3 (C0) 7 (P3-Q2 - PHCDPH) (Ph2Pch2Pph2)Document3 pagesElectronic Control of The Co-Ordination Mode of An Alkyne To Trimetallic Cluster X-Ray Crystal Structure of 0S3 (C0) 7 (P3-Q2 - PHCDPH) (Ph2Pch2Pph2)Nikhil BhoumikNo ratings yet
- Rieger1994 Capìtulo 7 ElectrolisisDocument56 pagesRieger1994 Capìtulo 7 Electrolisisyazmin zapata garciaNo ratings yet
- Band-Selective Holstein Polaron in Luttinger Liquid Material A Moo (A K, RB)Document7 pagesBand-Selective Holstein Polaron in Luttinger Liquid Material A Moo (A K, RB)Scribd ScribdNo ratings yet
- Electron-Phonon C CDocument11 pagesElectron-Phonon C CArdani MunaqiNo ratings yet
- The Direct Electrochemical Synthesis of ( (C6H5) 3Ph) 2 (CoCl4)Document2 pagesThe Direct Electrochemical Synthesis of ( (C6H5) 3Ph) 2 (CoCl4)Pavle RadojkovićNo ratings yet
- Quasiparticle Dynamics in GrapheneDocument6 pagesQuasiparticle Dynamics in GrapheneThamyres F. Messa MoreiraNo ratings yet
- Martinezsaavedra 2014Document14 pagesMartinezsaavedra 2014snehasis banerjeeNo ratings yet
- Gaynor 2015Document5 pagesGaynor 2015Nasos IalexanthNo ratings yet
- Chimia Face Ce Vrea EaDocument5 pagesChimia Face Ce Vrea EaRomano AlbertNo ratings yet
- Separation of Thiocyanato Complexes of IronDocument2 pagesSeparation of Thiocyanato Complexes of Ironchm12No ratings yet
- A Mathematical Model For The Porous Lead Dioxide ElectrodeDocument10 pagesA Mathematical Model For The Porous Lead Dioxide Electrodesumit singhNo ratings yet
- Binary Phase Diagram of WaterDocument10 pagesBinary Phase Diagram of WaterkmkmNo ratings yet
- Low-Temperature Tunneling of CH3 Quantum Rotor in Van Der Waals SolidsDocument15 pagesLow-Temperature Tunneling of CH3 Quantum Rotor in Van Der Waals SolidsNyiam HlubNo ratings yet
- 10.1515 - Zna 1983 0221Document6 pages10.1515 - Zna 1983 0221muthuNo ratings yet
- Ab Initio Simulation of Photoluminescence: Bi Iny O (S Site)Document10 pagesAb Initio Simulation of Photoluminescence: Bi Iny O (S Site)CamilaBurgosNo ratings yet
- Polar SolventDocument12 pagesPolar SolventReshma SaxenaNo ratings yet
- Effects of Lu and Ni Substitution On Thermoelectric Properties of Ca Co ODocument6 pagesEffects of Lu and Ni Substitution On Thermoelectric Properties of Ca Co OAhmed Khalid HussainNo ratings yet
- 1 StructuralDocument8 pages1 StructuralTJPRC PublicationsNo ratings yet
- Electroabsorption and Related Spectroscopic Studies of Bimetallic Tetraiminoethylenedimacrocyclic Complexes: Corroboration of Valence Electron DelocalizationDocument4 pagesElectroabsorption and Related Spectroscopic Studies of Bimetallic Tetraiminoethylenedimacrocyclic Complexes: Corroboration of Valence Electron DelocalizationAzizah MunitaNo ratings yet
- Thomas FermiDocument5 pagesThomas FermiMuhammad SaqibNo ratings yet
- Fundamentals of Atomic and Molecular Spectroscopy in Instrumental AnalysisDocument40 pagesFundamentals of Atomic and Molecular Spectroscopy in Instrumental Analysisnikusrivastva44043No ratings yet
- S0021889897001787 PDFDocument5 pagesS0021889897001787 PDFMario Misael Machado LòpezNo ratings yet
- Chalker FinalDocument44 pagesChalker FinalMuhammad Shifan JayadiNo ratings yet
- Plasmonic Physics of 2D Crystalline MaterialsDocument30 pagesPlasmonic Physics of 2D Crystalline Materials유재원No ratings yet
- Examination of Different Strengths of Octupole Correlations in Neutron-Rich PR and PM IsotopesDocument5 pagesExamination of Different Strengths of Octupole Correlations in Neutron-Rich PR and PM IsotopesabbeyNo ratings yet
- Chem Principles 7e ISM Major Techniques Even FINALDocument6 pagesChem Principles 7e ISM Major Techniques Even FINALSelma MeloNo ratings yet
- Electron-Phonon Heat Exchange in Quasi-Two-Dimensional NanolayersDocument17 pagesElectron-Phonon Heat Exchange in Quasi-Two-Dimensional NanolayersLiviu BadeaNo ratings yet
- Synthesis and Fluorescence Properties of Europium, Terbium DopedDocument5 pagesSynthesis and Fluorescence Properties of Europium, Terbium DopedRAQUEL GAMEZNo ratings yet
- Dynamic Effect NPDocument4 pagesDynamic Effect NPPurwojatmiko HandokoNo ratings yet
- Superconductivity and Charge Density Wave in ZrTe3-xSex (ZHU Et. Al, 2016)Document7 pagesSuperconductivity and Charge Density Wave in ZrTe3-xSex (ZHU Et. Al, 2016)Joao Pedro AlmeidaNo ratings yet
- 1 s2.0 001346869500077R MainDocument8 pages1 s2.0 001346869500077R MainAmit Kumar ChaurasiaNo ratings yet
- JP 1040234Document8 pagesJP 1040234Chem CU706No ratings yet
- PhysRev 87 835Document8 pagesPhysRev 87 835Miguel Angel CansecoNo ratings yet
- PhysRevC 65 014308Document10 pagesPhysRevC 65 014308Ravi Sankar Babu BalabhadrapatruniNo ratings yet
- Transcat 2014-EcatDocument678 pagesTranscat 2014-Ecatdilor19No ratings yet
- Process-Piping-Pipeline Engg PDP - Ray R10copiesDocument40 pagesProcess-Piping-Pipeline Engg PDP - Ray R10copiesVamsikrishna LakamsaniNo ratings yet
- Set Up Your Home Chemistry LabDocument16 pagesSet Up Your Home Chemistry LabDoohan Jemsy50% (2)
- Data Set Information WINE QUALITYDocument4 pagesData Set Information WINE QUALITYKustiara S100% (1)
- Climate Change PaperDocument3 pagesClimate Change Paperapi-320818400100% (1)
- Otec-Outline Full WordDocument27 pagesOtec-Outline Full WordAndrei SabaterNo ratings yet
- Answers For Practice Problems in Lesson 5: Practice Problem #1: Specific Gravity Calculation For Coarse AggregateDocument2 pagesAnswers For Practice Problems in Lesson 5: Practice Problem #1: Specific Gravity Calculation For Coarse AggregateDjonraeNarioGalvezNo ratings yet
- Catalogue of IRO WaterDocument90 pagesCatalogue of IRO WatershoyebNo ratings yet
- Waste Heat Recovery in Cement Industry at Mapl Leaf Cement FactoryDocument35 pagesWaste Heat Recovery in Cement Industry at Mapl Leaf Cement FactoryTafseer Abbas100% (2)
- Yhg/Yhf: Plastic Filters - On Line Filter Model 'H''Document2 pagesYhg/Yhf: Plastic Filters - On Line Filter Model 'H''Ted ThomsonNo ratings yet
- Material Safety Data Sheet: Section I - Chemical Product and Company IdentificationDocument2 pagesMaterial Safety Data Sheet: Section I - Chemical Product and Company IdentificationMu ClasNo ratings yet
- MASS TRANSFER Coefficient and Inter Phase Mass TransferDocument41 pagesMASS TRANSFER Coefficient and Inter Phase Mass TransferSannala Prudhvi100% (1)
- USP-NF OlanzapineDocument4 pagesUSP-NF OlanzapineDanielle De GuzmanNo ratings yet
- Application Note - Measuring KVP On An AMX 4 or 4plus-2013-10-30 PDFDocument2 pagesApplication Note - Measuring KVP On An AMX 4 or 4plus-2013-10-30 PDFLuis Fernando Garcia SanchezNo ratings yet
- CH 1Document19 pagesCH 1abdallah elsharnobyNo ratings yet
- E 1452 - 92 Standard Practice For Preparation of Calibration Solutions For Spectrophotometric and For Spectroscopic Atomic Analysis PDFDocument3 pagesE 1452 - 92 Standard Practice For Preparation of Calibration Solutions For Spectrophotometric and For Spectroscopic Atomic Analysis PDFBryanNo ratings yet
- Unit 5 Phase Equilibrium and CorrosionDocument29 pagesUnit 5 Phase Equilibrium and CorrosionDr. Ruma Arora SoniNo ratings yet
- 09 Science Notes Ch02 Is Matter Around Us PureDocument5 pages09 Science Notes Ch02 Is Matter Around Us PureApoorvaKashyapNo ratings yet
- Class 12 Chemistry Practical by Bharat PanchalDocument34 pagesClass 12 Chemistry Practical by Bharat PanchalSarita BhattNo ratings yet
- Current Concepts and Techniques For Caries Excavation and Adhesion To Residual Dentin, J Adhesi Dent 2011Document17 pagesCurrent Concepts and Techniques For Caries Excavation and Adhesion To Residual Dentin, J Adhesi Dent 2011Jesus NavarreteNo ratings yet
- Introduction To Cold Plasma.: Generation Techniques: 2-Radio Frequency Discharge. 3-Microwave Discharge PlasmaDocument21 pagesIntroduction To Cold Plasma.: Generation Techniques: 2-Radio Frequency Discharge. 3-Microwave Discharge PlasmaPakeeza DollNo ratings yet
- Effect of SO3 Content On Concrete StructureDocument2 pagesEffect of SO3 Content On Concrete StructureNam HuynhNo ratings yet
- Starch Characteristics of Black Bean, Chick Pea, Lentil, Navy Bean and Pinto Bean Cultivars Grown in CanadaDocument10 pagesStarch Characteristics of Black Bean, Chick Pea, Lentil, Navy Bean and Pinto Bean Cultivars Grown in CanadaJose perezNo ratings yet
- Forensics Crime Busters WebsitesDocument3 pagesForensics Crime Busters WebsitesMengyao Alice LiNo ratings yet
- Pengaruh Konsentrasi Gula Dan Lama Penyimpanan Terhadap Mutu Manisan Basah Batang Daun PepayaDocument8 pagesPengaruh Konsentrasi Gula Dan Lama Penyimpanan Terhadap Mutu Manisan Basah Batang Daun PepayaAlfi nur farchatin nufusNo ratings yet
- Bela Products IntoductionDocument12 pagesBela Products IntoductionShailesh ThakkarNo ratings yet
- JPC 119022Document2 pagesJPC 119022Juan Pablo EspinosaNo ratings yet
- PTC-40-1991 - Performance Test Codes Flue Gas Desulfurization Units PDFDocument70 pagesPTC-40-1991 - Performance Test Codes Flue Gas Desulfurization Units PDFSunil KumarNo ratings yet
- Soal LajuDocument3 pagesSoal Lajufhandayani8No ratings yet