Professional Documents
Culture Documents
Pure Substances
Pure Substances
Uploaded by
Soul Gaming0 ratings0% found this document useful (0 votes)
2 views1 pageCopyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
2 views1 pagePure Substances
Pure Substances
Uploaded by
Soul GamingCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 1
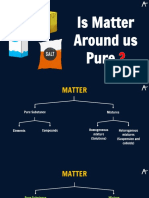
Pure substances
Pure substances are elements or compounds.
They are made up of only one kind of substance.
They cannot be broken down into simpler substance by chemical or physical methods.
They have a fixed composition.
Example: Diamond, carbon dioxide.
Types of Pure substance –
The pure substance is divided in 2 types on the basis of their chemical
composition-
1-Elements
2-Compounds
Elements-
Robert Boyle was the first scientist to use the term element
Element is a basic form of matter that cannot be broken down into
simpler substance by chemical reactions
It is divided in 3 types which are metal, non-metals, metalloids
Ex- Gold, hydrogen
Properties of metal-
They are lustrous
They are malleable
They are ductile
They are sonorous
Properties of non metal-
They are not lustrous.
They are not malleable.
They are not sonorous.
They are not ductile.
Metalloids- Elements having intermediate properties between those of
metals and non metals are called metalloids. EX-Boron, Silicon
You might also like
- Chapter 2 - Is Matter Around Us Pure - Summary Note: Sub-TopicsDocument14 pagesChapter 2 - Is Matter Around Us Pure - Summary Note: Sub-TopicsRanjeet SinghNo ratings yet
- Metals and Non MetalDocument4 pagesMetals and Non MetalPaul NelsonNo ratings yet
- Year 8 Chmistry NoteDocument2 pagesYear 8 Chmistry NoteFranca OkechukwuNo ratings yet
- SaveDocument2 pagesSaveSteven TiczonNo ratings yet
- Classification of MatterDocument17 pagesClassification of MatterAshmyra ManaloNo ratings yet
- Study Unit 1Document8 pagesStudy Unit 1Mphoka SalomeNo ratings yet
- 2nd 7 Unit 3Document45 pages2nd 7 Unit 3Tijani Basit AbiodunNo ratings yet
- Classification of ElementsDocument1 pageClassification of Elementsdan964No ratings yet
- Is Matter Around Us Pure Class 9 Notes CBSE Science Chapter 2 (PDF)Document16 pagesIs Matter Around Us Pure Class 9 Notes CBSE Science Chapter 2 (PDF)DKNo ratings yet
- BLEPT ReviewerDocument5 pagesBLEPT ReviewerJuliet Ileto Villaruel - AlmonacidNo ratings yet
- Properties of MetalsDocument4 pagesProperties of MetalsKevin Joe CuraNo ratings yet
- Metal, Metalloids, and NonmetalsDocument7 pagesMetal, Metalloids, and Nonmetalsmicahhobbs50No ratings yet
- Semi DLPDocument2 pagesSemi DLPAlleen Joy SolivioNo ratings yet
- Foundation and FoundamentalDocument37 pagesFoundation and Foundamentalkrishal khadka 9ENo ratings yet
- Science ReviewerDocument2 pagesScience ReviewerSamantha CabarlesNo ratings yet
- Properties of MetalsDocument4 pagesProperties of MetalsYanika BarasNo ratings yet
- Properties of MaterialsDocument55 pagesProperties of MaterialstereveNo ratings yet
- Properties of Elements1Document23 pagesProperties of Elements1diamondtressNo ratings yet
- VOCABULARY Minerals and OresDocument2 pagesVOCABULARY Minerals and OresRichard CLNo ratings yet
- L1. What Makes Up Matter - 2023-2024Document41 pagesL1. What Makes Up Matter - 2023-2024mohammed-tellaNo ratings yet
- Is Matter Around Us Pure PDFDocument81 pagesIs Matter Around Us Pure PDFAwdesh SinghNo ratings yet
- St. John'S School Greater Noida WestDocument5 pagesSt. John'S School Greater Noida WestIndia Tech with AstitvaNo ratings yet
- Periodic TableDocument42 pagesPeriodic TableInform7105No ratings yet
- Science-Grade-7-Handout-2-Element Vs CompoundDocument5 pagesScience-Grade-7-Handout-2-Element Vs CompoundClinton YmbongNo ratings yet
- Final Report MathDocument10 pagesFinal Report MathFe BalizaNo ratings yet
- Classifying MatterDocument53 pagesClassifying MatterTanya MaheshwariNo ratings yet
- ElementsDocument39 pagesElementsJona Geron ApolonioNo ratings yet
- Metals and NonmetalsDocument2 pagesMetals and NonmetalsAnnisa Ekaputri FebrianiNo ratings yet
- Groups of The Periodic TableDocument5 pagesGroups of The Periodic TableEmikah TaylorNo ratings yet
- Me Non MeDocument25 pagesMe Non MeTulga EddieNo ratings yet
- Metals, Nonmetals, MetalloidsDocument17 pagesMetals, Nonmetals, Metalloidszendy llima malicdemNo ratings yet
- Chem Chap-2 NotesDocument2 pagesChem Chap-2 NotesgrandmastersakshamNo ratings yet
- Atomic Structure and PeriodicityDocument9 pagesAtomic Structure and PeriodicityYash BhattNo ratings yet
- Section 2Document11 pagesSection 2Jimmy gogoNo ratings yet
- Metaullargy NotesDocument14 pagesMetaullargy Noteswama ojhaNo ratings yet
- Chemistry Reviewer 1 2Document10 pagesChemistry Reviewer 1 2Ian PadillaNo ratings yet
- Element Compound Mixtures NotesDocument18 pagesElement Compound Mixtures NotesSumit ParicharakNo ratings yet
- Unit IiiDocument12 pagesUnit IiiIvy Marie ToyonganNo ratings yet
- Articlechem 1Document4 pagesArticlechem 1api-233267698No ratings yet
- Week 7 Trends of Periodic TableDocument3 pagesWeek 7 Trends of Periodic TableDaniel DowdingNo ratings yet
- Metal & Non-MetalDocument5 pagesMetal & Non-MetalAakarshNo ratings yet
- PWSUNP223620230816131206848PPT - Metals, Nonmetals, MetalloidsDocument18 pagesPWSUNP223620230816131206848PPT - Metals, Nonmetals, Metalloidsrishit.yadav1102No ratings yet
- Chemical Properties:: M M M MDocument13 pagesChemical Properties:: M M M MRana BarakatNo ratings yet
- Periodic Table Quiz: Can You Guess The Element From Its Chemical Symbol?Document43 pagesPeriodic Table Quiz: Can You Guess The Element From Its Chemical Symbol?Kyla Renz de LeonNo ratings yet
- By:-Utsah Sharma - at Crazy ScienceDocument21 pagesBy:-Utsah Sharma - at Crazy ScienceUtsah SharmaNo ratings yet
- Secondary One Chemistry 2015: ElementsDocument18 pagesSecondary One Chemistry 2015: ElementsPing HuiNo ratings yet
- Is Matter Around Us PureDocument46 pagesIs Matter Around Us Pureparamjeet164No ratings yet
- Chapter 1Document10 pagesChapter 1KENT CARLO PAYENo ratings yet
- Revision Notes For Checkpoint ChemistryDocument13 pagesRevision Notes For Checkpoint ChemistryKostas TampakisNo ratings yet
- Science 7 WEEK 3Document58 pagesScience 7 WEEK 3Rochel MarasiganNo ratings yet
- Lecture 2 Periodic TableDocument30 pagesLecture 2 Periodic TableInaya ImranNo ratings yet
- Metal and Non MetalDocument14 pagesMetal and Non MetalZia UllahNo ratings yet
- Metals and NonmetalsDocument2 pagesMetals and NonmetalsSenthil Kumar PNo ratings yet
- Deformation of Solids AsDocument14 pagesDeformation of Solids AsWaseem AminNo ratings yet
- CBSE Class 9 Science Chapter 2 Is Matter Around Us Pure Revision NotesDocument42 pagesCBSE Class 9 Science Chapter 2 Is Matter Around Us Pure Revision NotesPiyush YadavNo ratings yet
- Activity 2 Nakakapanibago Pero Bongga Sa PagkatutoDocument6 pagesActivity 2 Nakakapanibago Pero Bongga Sa PagkatutoJay Rostata TuraNo ratings yet
- Properties of MetalsDocument18 pagesProperties of MetalsMohan RaiNo ratings yet
- Chemistry Repaso Test 1 Periodo 3 10 GradoDocument7 pagesChemistry Repaso Test 1 Periodo 3 10 GradoRebeca BenavidesNo ratings yet
- Chemistry Test 1 Periodo 3 10 GradeDocument7 pagesChemistry Test 1 Periodo 3 10 GradeRebeca BenavidesNo ratings yet
- The Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookNo ratings yet