Professional Documents
Culture Documents
Electrochemistry Formulas
Electrochemistry Formulas
Uploaded by
divyanshjoshidpsjkpCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Electrochemistry Formulas
Electrochemistry Formulas
Uploaded by
divyanshjoshidpsjkpCopyright:
Available Formats
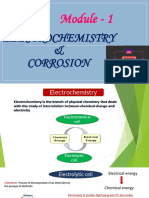
ELECTROCHEMISTRY
ELECTRODE POTENTIAL
For any electrode ’ oxidiation potential =-Reduction potential
E= R.P of cathode - R.P of anode
EFcell =R.P. of cathode + O.P of anode
E cell is always a +ve quantity &Anode will be electrode of low R.P
EoCell = SRP of cathode - SRP of anode.
Greater the SRP value greater will be oxidising power.
GIBBS FREE ENERGY CHANGE:
AG =- nFE"cell
AG° =- nFEOcell
NERNST EQUATION:(Effect of concentration andtemp on emf of cell)
AG = AG° + RT CnQ (where Qis raection quotient)
AG° =-RT n Kea
RT
E = Ecell ln Q
nF
2.303 RT
cell
= EOcell -log Q
nF
0.0591
Ecell E call log Q [At 298 K]
At chemical equilibrium
AG = 0 Eoell =0.
log Keq nEcell
0.0591
0.0591
EO cel log Keg
For an electrode M(s)/Mn*.
2.303RT 1
M /M log
nF M]
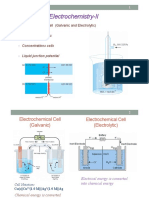
CONCENTRATION CELL:
A cell in which both the electrods are made up of same material.
For all concentration cell E=0.
(a) Electrolyte Concentration Cell :
eg. Zn(s) /Zn2* (c,) || Zn2*(c,) /Zn(s)
E=
0.0591
C2
I0g C
2
(b) Electrode ConcentrationCell:
eg. Pt, H,(P, atm) /H" (1M) 7H, (P, atm) /Pt
0.0591
E=
2
DIFFERENT TYPESOF ELECTRODES:
1. Metal-Metal ion Electrode M(s)/M*. Mnt + ne ’ M(s)
0.0591
E = E° t log[M]
n
2. Gas-ion Electrode PtH,(Patm) /H* (XM)
as a reduction electrode
1
H(aq) e’,H,
+ (Patm)
1
E= E°-0.0591 log PH,2
[H]
3. Oxidation-reduction Electrode Pt/ Fe2+, Fe+
as a reduction electrode Fe + e ’ Fe2+
E= E°-0.0591 log
(Fe²]
[Fe]
4. Metal-Metal insoluble salt Electrode eg. Ag/AgCl, C
as a reduction electrode AgCI(s) + e ’ Ag(s) + C
FcrIAgCiIAg = cr /AgC/ Ag -0.0591 log [C].
ELECTROLYSIS:
(a) K*, Ca2, Na, Mg*2, Al*3, Zn2, Fe2, H*, Cu*2, Ag", Au+s,
Increasingorder of deposition.
(b) Similarly the anion which is strogner reducing agent(low value of SRP)
is liberated first at the anode.
so, NO,. OH", CI, Br, I
Increa singorder of diposition
FARADAY'S LAW OFELECTROLYSIS:
First Law :
W= Zq W=Z it Z= Electrochemical equivalent of substance
Second Law :
WaE
W
= Constant
W, W2
E E E
W ixtx current efficiency factor
E 96500
actual mass deposited/produced x100
Current efficiency= Theoritical mass deposited/produced
CONDITION FOR SIMULTANEOUS DEPOSITION OF Cu & Fe AT CATHODE
0.0591 0.0591
Cu?t /Cu log Cu2+ = EFe (Fe log Fe2t
2 2
Condition for the simultaneous deposition of Cu & Fe on cathode.
CONDUCTANCE:
1
Conductance =
Resistance
Specific conductance or conductivity :
1
(Reciprocal of specific resistance) K=
K= specific conductance
Equivalent conductance:
Kx1000
Normality unit: -ohm- cm² eq-1
Molar conductance:
Kx1000
unit: -ohm- cm² mole-1
Molarity
specific conductance = conductance x a
KOHLRAUSCH'S LAW:
Variation of o /a, of a solution with concentration :
Strong electrolyte
A=h-b/c
(ii) Weak electrolytes : 2,=n, +n_.
where is the molar conductivity
n, = No of cations obtained after dissociation per formula unit
n_= No of anions obtained after dissociation per formula unit
APPLICATION OF KOHLRAUSCH LAW:
1. Calculation of 29, of weak electrolytes :
M
(CH3CoOH) =20 +20M(HCI) -20M(NaC)
M(CH3COONa)
2. To calculate degree of diossociation of a week electrolyte
ca?
=
m Keg (1-a)
3. Solubility (S) of sparingly soluble salt &their K
1000
=M = K X
solubility
Kyp = S2.
Transport Number :
Ha
t,= Hc +Ha. Ha tHc
Where t,= Transport Number of cation &t, = Transport Number of
anion
You might also like
- Comparison Between Api Standard 5L - 45TH & 46TH - Rev. 1Document17 pagesComparison Between Api Standard 5L - 45TH & 46TH - Rev. 1Rushabh Kapadia100% (2)
- Prayer Service For The DeadDocument16 pagesPrayer Service For The DeadRodel Ramos DaquioagNo ratings yet
- Massage Sequence Full Body PDFDocument19 pagesMassage Sequence Full Body PDFNorbs Victorio100% (2)
- ElectrochemistryDocument4 pagesElectrochemistrySiya ThakkarNo ratings yet
- 5 ElectrochemistryDocument21 pages5 Electrochemistry1k subscribers with flimNo ratings yet
- 5 Electrochemistry PDFDocument21 pages5 Electrochemistry PDFP. E. I. AcademicsNo ratings yet
- Electrochemistry FinalDocument58 pagesElectrochemistry Finalrp7workNo ratings yet
- ElectrochemistryDocument40 pagesElectrochemistryՏɑʍ ՏɑղԵհօՏհNo ratings yet
- Cheatsheets For Important Chapters - Chemistry: Chapter - ElectrochemistryDocument11 pagesCheatsheets For Important Chapters - Chemistry: Chapter - ElectrochemistryPrasad YarraNo ratings yet
- ElectrochemistryDocument40 pagesElectrochemistryakshaysinghofficial007No ratings yet
- ElectrolysisDocument24 pagesElectrolysisSMELLY CATNo ratings yet
- Electrochemical SystemDocument19 pagesElectrochemical SystemJim tanNo ratings yet
- Electrochemistry: Electrochemical CellsDocument10 pagesElectrochemistry: Electrochemical CellsTr Mazhar PunjabiNo ratings yet
- Electrochemistry 1Document38 pagesElectrochemistry 1Google CloudNo ratings yet
- Electrochemistry FinalDocument76 pagesElectrochemistry Finalsmudgegaming4989No ratings yet
- Electrode Potential - Nernst EquationDocument13 pagesElectrode Potential - Nernst EquationTrung HảiNo ratings yet
- Electrochemical CellDocument30 pagesElectrochemical CellSubhu100% (1)
- Electrochemistry Anil HssliveDocument10 pagesElectrochemistry Anil HssliveRanit MukherjeeNo ratings yet
- Chapter 3 Electrochemistry (Notes)Document14 pagesChapter 3 Electrochemistry (Notes)DivyanshuNo ratings yet
- Physical Analysis 2010 - Electroanalytical Methods PDFDocument40 pagesPhysical Analysis 2010 - Electroanalytical Methods PDFCh AswadNo ratings yet
- 3 Electro Chemistry 1Document40 pages3 Electro Chemistry 1Kalpana BidhanNo ratings yet
- CHAP 6 SEM 1 2020-2021 TERKINI Part 2 NewDocument46 pagesCHAP 6 SEM 1 2020-2021 TERKINI Part 2 NewNUR KHALISAH AZMANNo ratings yet
- Short-Cut Revision Notes: Chapter: ElectrochemistryDocument6 pagesShort-Cut Revision Notes: Chapter: ElectrochemistryANIKET BATTINWARNo ratings yet
- ElectrochemistryDocument16 pagesElectrochemistryMidhunRameshThuvassery100% (1)
- Hsslive Xii CH 2 Electro Chemistry AnilDocument10 pagesHsslive Xii CH 2 Electro Chemistry AnilFathima NithinshaNo ratings yet
- Concentration Cell Batteries CorrosionDocument76 pagesConcentration Cell Batteries Corrosionannida latifahNo ratings yet
- CHEMISTRY (XI, XII & Medical) by VIJAY KUMAR (M.Sc. B.Ed.)Document10 pagesCHEMISTRY (XI, XII & Medical) by VIJAY KUMAR (M.Sc. B.Ed.)Vijay KumarNo ratings yet
- 3 ElectrochemDocument4 pages3 ElectrochemFelven Leo AbayaNo ratings yet
- CLS JEEAD-19-20 XII Che Target-1 Level-1 Chapter-3Document26 pagesCLS JEEAD-19-20 XII Che Target-1 Level-1 Chapter-3Archita Ray0% (1)
- Module - I: I/Ii Sem Be, Engineering ChemistryDocument35 pagesModule - I: I/Ii Sem Be, Engineering ChemistryPradeep SiddhamNo ratings yet
- ElectroChemistry Slides PDFDocument44 pagesElectroChemistry Slides PDFHenry OkoyeNo ratings yet
- Electrochemical CellDocument5 pagesElectrochemical Cellrekhadohrey450No ratings yet
- Module 1 - Electrode Potential & CellsDocument13 pagesModule 1 - Electrode Potential & CellsrashmiNo ratings yet
- Redox Reactions and Electrochemistry: I. Redox Reactions II. Galavanic or Voltaic CellsDocument48 pagesRedox Reactions and Electrochemistry: I. Redox Reactions II. Galavanic or Voltaic CellsAdzimahNo ratings yet
- ElectrochemistryDocument70 pagesElectrochemistryMark Cidric RoqueroNo ratings yet
- Chapter 18 - Rev PDFDocument17 pagesChapter 18 - Rev PDFalaa al sahmaraniNo ratings yet
- Electrode System Notes - FinalDocument13 pagesElectrode System Notes - Finalrockymounesh177No ratings yet
- Engineering Chemistry Notes For Vtu Stud PDFDocument70 pagesEngineering Chemistry Notes For Vtu Stud PDFPraveenkrishnanNo ratings yet
- Potentiometry: Dr. Kathlia A. de Castro-Cruz Analytical Chemistry For CheDocument59 pagesPotentiometry: Dr. Kathlia A. de Castro-Cruz Analytical Chemistry For CheCyrus VizonNo ratings yet
- ELectrochemistryDocument24 pagesELectrochemistryAuliaNo ratings yet
- PoteniometryDocument85 pagesPoteniometrymalyaaNo ratings yet
- Electrochemical ThermodynamicsDocument38 pagesElectrochemical ThermodynamicsikamelyaastutiNo ratings yet
- Physical Chemistry 2 - Kinetics of Electrochemical ProcessesDocument44 pagesPhysical Chemistry 2 - Kinetics of Electrochemical ProcessesNguyễn Thu HàNo ratings yet
- Topic 2: Potentiometric Methods of AnalysisDocument19 pagesTopic 2: Potentiometric Methods of AnalysisYting TanNo ratings yet
- Chap.1 ElectrochemistryDocument93 pagesChap.1 ElectrochemistryAnushkaSinhaNo ratings yet
- Unit 3 ElectrochemistryDocument8 pagesUnit 3 ElectrochemistryYashvee GuptaNo ratings yet
- CH 2 Fundamental Relationships May 2010 RevDocument43 pagesCH 2 Fundamental Relationships May 2010 RevJayden WangNo ratings yet
- (2091) Lecture Notes Electrochemistry E.pdf - TMPDocument43 pages(2091) Lecture Notes Electrochemistry E.pdf - TMPRamJiPandeyNo ratings yet
- 202004021930364692ranvijay ElectrochemistryDocument11 pages202004021930364692ranvijay ElectrochemistryThantzinNo ratings yet
- B. Electrochemistry 2 1Document37 pagesB. Electrochemistry 2 1Dank CoderNo ratings yet
- Engineering Chemistry Notes: For Vtu StudentsDocument73 pagesEngineering Chemistry Notes: For Vtu StudentsShravanNo ratings yet
- Module 1 Electrochemistry PPT Slides Part 2Document30 pagesModule 1 Electrochemistry PPT Slides Part 2May TampusNo ratings yet
- ElectrochemistryDocument80 pagesElectrochemistryNitin NishantNo ratings yet
- Module 2 24 01 2022Document6 pagesModule 2 24 01 2022Ananya JayasimhaNo ratings yet
- Electrochemistry and Storage CellsDocument14 pagesElectrochemistry and Storage CellsDivithNo ratings yet
- M1 ElectrochemistryDocument11 pagesM1 ElectrochemistryMalvika RkNo ratings yet
- 22CHE22-notesModule 1Document24 pages22CHE22-notesModule 1Vinay AdariNo ratings yet
- Engineering Chemistry NotesDocument83 pagesEngineering Chemistry Notess. EswarNo ratings yet
- Electrochemistry & CorrosionDocument28 pagesElectrochemistry & CorrosionAdharshNo ratings yet
- CH 14Document27 pagesCH 14NAMENo ratings yet
- Chem101 Ho4Document4 pagesChem101 Ho4cyrusryan21No ratings yet
- Feynman Lectures Simplified 2C: Electromagnetism: in Relativity & in Dense MatterFrom EverandFeynman Lectures Simplified 2C: Electromagnetism: in Relativity & in Dense MatterNo ratings yet
- Quiz - WEPDocument5 pagesQuiz - WEPdivyanshjoshidpsjkpNo ratings yet
- CPP - 4 ElectrochemistryDocument3 pagesCPP - 4 ElectrochemistrydivyanshjoshidpsjkpNo ratings yet
- CPP - Alcohol Ether PhenolDocument12 pagesCPP - Alcohol Ether PhenoldivyanshjoshidpsjkpNo ratings yet
- Ipmat Indore 2022 PaperDocument26 pagesIpmat Indore 2022 PaperdivyanshjoshidpsjkpNo ratings yet
- Ipmat Indore 2022 SolutionDocument12 pagesIpmat Indore 2022 SolutiondivyanshjoshidpsjkpNo ratings yet
- Ipmat Indore 2021 PaperDocument8 pagesIpmat Indore 2021 PaperdivyanshjoshidpsjkpNo ratings yet
- Fipt-02-Pcm-Jee MainDocument18 pagesFipt-02-Pcm-Jee MaindivyanshjoshidpsjkpNo ratings yet
- Quadratic Equations PYQ'sDocument6 pagesQuadratic Equations PYQ'sdivyanshjoshidpsjkpNo ratings yet
- Trigonometry PYQ'sDocument9 pagesTrigonometry PYQ'sdivyanshjoshidpsjkpNo ratings yet
- Physical Education (Batch 1)Document1 pagePhysical Education (Batch 1)divyanshjoshidpsjkpNo ratings yet
- Trignometric Equations With ExeDocument8 pagesTrignometric Equations With ExedivyanshjoshidpsjkpNo ratings yet
- Christian Leadership Who Is A Christian?Document10 pagesChristian Leadership Who Is A Christian?Kunle AkingbadeNo ratings yet
- A Novel Ultrafast Transient Constant On-Time Buck Converter For Multiphase OperationDocument11 pagesA Novel Ultrafast Transient Constant On-Time Buck Converter For Multiphase OperationzzhbpainNo ratings yet
- Assignment 6 On Python: Simulations: March 6, 2018Document5 pagesAssignment 6 On Python: Simulations: March 6, 2018Vinayak Nishant Gudipaty ee19b129No ratings yet
- Photovoltaic Cable (Solar) : Solar Energy. Ful Lling The Energy Needs of TomorrowDocument4 pagesPhotovoltaic Cable (Solar) : Solar Energy. Ful Lling The Energy Needs of TomorrowNitinNo ratings yet
- Lived Experiences of Muslim High SchoolDocument12 pagesLived Experiences of Muslim High SchoolJustine ReveloNo ratings yet
- List of SOC Related DocumentsDocument1 pageList of SOC Related DocumentsRavi Yadav0% (1)
- Case Nancy: ExampleDocument38 pagesCase Nancy: Exampleshweta GNo ratings yet
- The Conclusion of The Book Revelation 22Document5 pagesThe Conclusion of The Book Revelation 22Ruth AramburoNo ratings yet
- UntitledDocument321 pagesUntitled谷光生No ratings yet
- 03 - Information PackagesDocument13 pages03 - Information Packagesyusi cantikNo ratings yet
- Chevron Australia Project Overview - PPTDocument15 pagesChevron Australia Project Overview - PPTzawamaNo ratings yet
- Steam Air Ejector Performance and Its Dimensional ParametersDocument296 pagesSteam Air Ejector Performance and Its Dimensional ParametersGuru Raja Ragavendran NagarajanNo ratings yet
- Matrix CitateDocument1 pageMatrix CitateluizetteNo ratings yet
- Sample Test 1Document3 pagesSample Test 1Bình Phạm ThịNo ratings yet
- 2300 SIGE 2016 FinalDocument6 pages2300 SIGE 2016 FinalCarlos JuniorNo ratings yet
- SS Ind A21 BVX002 - Approval of Permanent Joining Procedure and PersonnelDocument1 pageSS Ind A21 BVX002 - Approval of Permanent Joining Procedure and PersonnelTuTuy AnNo ratings yet
- Benefits For Blue Collar Employees - Oishik-1.docx (1) 3Document73 pagesBenefits For Blue Collar Employees - Oishik-1.docx (1) 3Anonymous Fsv7B0No ratings yet
- Padre Island National Seashore Superintendent's StatementDocument7 pagesPadre Island National Seashore Superintendent's StatementcallertimesNo ratings yet
- Formulation Development and Compatibility Study of Dexketoprofen Injection Used in The Management of Post-Operative PainDocument7 pagesFormulation Development and Compatibility Study of Dexketoprofen Injection Used in The Management of Post-Operative PainAdeeva MaulidaNo ratings yet
- Working Memory Model of Memory QuestionsDocument26 pagesWorking Memory Model of Memory QuestionssophiaNo ratings yet
- Analysis of ToothGrowth Data SetDocument4 pagesAnalysis of ToothGrowth Data SetJavo SantibáñezNo ratings yet
- A Presentation On: "Gswan"Document19 pagesA Presentation On: "Gswan"Sunil PillaiNo ratings yet
- Snail AssignmentDocument7 pagesSnail AssignmentTekla FabriczyNo ratings yet
- Ics Lab Manual PDFDocument46 pagesIcs Lab Manual PDFEyes FlikerNo ratings yet
- CPAR LessonDocument2 pagesCPAR LessonAnabelle MoyamoyNo ratings yet
- English Chapter 5Document20 pagesEnglish Chapter 5Kumar sankar SNo ratings yet
- Factoring Flow Sheet PDFDocument7 pagesFactoring Flow Sheet PDFReginald LopezNo ratings yet