Professional Documents
Culture Documents
TG and Fragility Determination of Glass-Forming Sugars Using A Dynamic Mechanical Analyzer
TG and Fragility Determination of Glass-Forming Sugars Using A Dynamic Mechanical Analyzer
Uploaded by
lwnnrcCopyright:
Available Formats
You might also like
- Advanced Temperature Measurement and Control, Second EditionFrom EverandAdvanced Temperature Measurement and Control, Second EditionNo ratings yet
- Measuring Thermal Crystallinity in PET: Spe Antec Indianapolis 2016Document5 pagesMeasuring Thermal Crystallinity in PET: Spe Antec Indianapolis 2016Silviani SilvyNo ratings yet
- Chemical Names and Formulas: 9.1 Naming IonsDocument53 pagesChemical Names and Formulas: 9.1 Naming IonsLovieAlfonsoNo ratings yet
- Rheological Properties of Some Thermotropic Liquid Crystalline PolymersDocument7 pagesRheological Properties of Some Thermotropic Liquid Crystalline PolymersAdityaNo ratings yet
- Application of Diferential Scanning CalorimetryDocument4 pagesApplication of Diferential Scanning CalorimetryUsman GhaniNo ratings yet
- DSC Error & InterpretationDocument3 pagesDSC Error & InterpretationUsman GhaniNo ratings yet
- Differential Scanning CalorimetryDocument7 pagesDifferential Scanning CalorimetryKenesei GyörgyNo ratings yet
- Epoxy CureDocument7 pagesEpoxy CuregdritzNo ratings yet
- 1 s2.0 S0038109804000067 MainDocument4 pages1 s2.0 S0038109804000067 MainAbhishek ChaturvediNo ratings yet
- Aya Alla Mahmoud - 5632Document11 pagesAya Alla Mahmoud - 5632aya mahmoudNo ratings yet
- Thermal Conductivity Determination of Small Polymer Samples by Differential Scanning CalorimetryDocument5 pagesThermal Conductivity Determination of Small Polymer Samples by Differential Scanning Calorimetrycarlette11No ratings yet
- Dec 1984Document9 pagesDec 1984krishnaNo ratings yet
- Thermal Analysis of PolymersDocument86 pagesThermal Analysis of PolymersMarister OliveiraNo ratings yet
- Rahman2007 PDFDocument6 pagesRahman2007 PDFMD Mahmudul Hasan MasudNo ratings yet
- Composites: Part A: Oleksandr G. Kravchenko, Chunyu Li, Alejandro Strachan, Sergii G. Kravchenko, R. Byron PipesDocument9 pagesComposites: Part A: Oleksandr G. Kravchenko, Chunyu Li, Alejandro Strachan, Sergii G. Kravchenko, R. Byron Pipesmarco_ravelo_10No ratings yet
- Modeling The Thermal Response of Porcine Cartilage To Laser IrradiationDocument10 pagesModeling The Thermal Response of Porcine Cartilage To Laser IrradiationZina AliNo ratings yet
- Cálculo Del Calor Específico From DSCDocument7 pagesCálculo Del Calor Específico From DSCfabio1199No ratings yet
- SchaweDocument9 pagesSchawezhor El hallaouiNo ratings yet
- Determination of Polymer Crystallinity by DSC: AbstactDocument3 pagesDetermination of Polymer Crystallinity by DSC: AbstactJohana ValenciaNo ratings yet
- Rao Gunti, Asokan - 2012 - Thermodynamic, Raman and Electrical Switching Studies On Si15Te85-xAgx (4 X 20) Glasses (3) - AnnotatedDocument6 pagesRao Gunti, Asokan - 2012 - Thermodynamic, Raman and Electrical Switching Studies On Si15Te85-xAgx (4 X 20) Glasses (3) - AnnotatedJagan KbNo ratings yet
- Fouling Detection in A Cross - Ow Heat Exchanger Based On Physical ModelingDocument9 pagesFouling Detection in A Cross - Ow Heat Exchanger Based On Physical ModelingMounam MaitiNo ratings yet
- Determination of Arrhenius Kinetic Constants Differential Scanning CalorimetryDocument5 pagesDetermination of Arrhenius Kinetic Constants Differential Scanning CalorimetryNgocDiep PhamNo ratings yet
- Study of Mechanical AndcomparativeDocument9 pagesStudy of Mechanical Andcomparativenadir adelNo ratings yet
- Differential Scanning Calorimetry (DSC) : Mr. Sagar Kishor SavaleDocument27 pagesDifferential Scanning Calorimetry (DSC) : Mr. Sagar Kishor SavaleDivya Tripathy100% (1)
- Unit 1 NotesDocument16 pagesUnit 1 NotesSAJITH NFNo ratings yet
- 04 474Document18 pages04 474webhareggebru06No ratings yet
- Chemical Kinetics On Thermal Decompositions of CumeneDocument8 pagesChemical Kinetics On Thermal Decompositions of CumeneMario Alonso Velasquez FlorezNo ratings yet
- Differential Scanning CalorimetryDocument7 pagesDifferential Scanning CalorimetryAli HussnainNo ratings yet
- Differential Scanning Calorimeter (DSC)Document7 pagesDifferential Scanning Calorimeter (DSC)Fi FialaNo ratings yet
- Creep Parameters of Spruce Wood in High Temperature EnvironmentDocument4 pagesCreep Parameters of Spruce Wood in High Temperature EnvironmentBentea MarcelNo ratings yet
- Using Thermal Analysis Methods To Better Understand Asphalt RheologyDocument6 pagesUsing Thermal Analysis Methods To Better Understand Asphalt RheologyNatalia KovalovaNo ratings yet
- Study On The Influence of Ageing On Chemical and Mechanical Properties of N, N - Dimethyl-N, N - Diphenylcarbamide Stabilized PropellantsDocument8 pagesStudy On The Influence of Ageing On Chemical and Mechanical Properties of N, N - Dimethyl-N, N - Diphenylcarbamide Stabilized Propellantstotenkopf0424No ratings yet
- Sanchez Et Al., (2008)Document8 pagesSanchez Et Al., (2008)radzul abyanNo ratings yet
- Experimental Determination of the Thermal Di ffusivity of α‑Cryolite up to 810 K and Comparison with First Principles PredictionsDocument7 pagesExperimental Determination of the Thermal Di ffusivity of α‑Cryolite up to 810 K and Comparison with First Principles PredictionsIbraheem AlQadiNo ratings yet
- 10 1002@star 19970490704 PDFDocument5 pages10 1002@star 19970490704 PDFuwuNo ratings yet
- Isothermal Microcalorimetry: An Analytical Technique For Assessing The Dynamic Chemical Stability of UHMWPEDocument6 pagesIsothermal Microcalorimetry: An Analytical Technique For Assessing The Dynamic Chemical Stability of UHMWPEmurugandevaNo ratings yet
- 33Document7 pages33ninhhoanghai_lhdNo ratings yet
- 1 PaperDocument3 pages1 PaperMadhavanIceNo ratings yet
- Solidification of SteelDocument7 pagesSolidification of SteelDan Pascu100% (1)
- Fardapaper Method For Lifetime Estimation of Power Transformer Mineral OilDocument7 pagesFardapaper Method For Lifetime Estimation of Power Transformer Mineral OilbenlahnecheNo ratings yet
- Chen2019-Thermal Response Characteristics of A SPRIGT Primary Thermometry SystemDocument6 pagesChen2019-Thermal Response Characteristics of A SPRIGT Primary Thermometry SystemJacyNo ratings yet
- Error Analysis of The Thermal Cell For Soil Thermal Conductivity MeasurementDocument10 pagesError Analysis of The Thermal Cell For Soil Thermal Conductivity MeasurementMasha RamirezNo ratings yet
- Lifetime Prediction of Thermo-Mechanical Fatigue For Exhaust ManifoldDocument10 pagesLifetime Prediction of Thermo-Mechanical Fatigue For Exhaust Manifoldram shyamNo ratings yet
- An Examination of The Thermal ExpansionDocument2 pagesAn Examination of The Thermal Expansionmap vitcoNo ratings yet
- Engineering Failure Analysis: H.S. Da Costa Mattos, L.M. Paim, J.M.L. ReisDocument13 pagesEngineering Failure Analysis: H.S. Da Costa Mattos, L.M. Paim, J.M.L. ReisFelipe Perissé Duarte LopesNo ratings yet
- Dynamic Prediction of Wastewater Aeration Basin Temperature: Journal of Environmental Engineering September 1995Document11 pagesDynamic Prediction of Wastewater Aeration Basin Temperature: Journal of Environmental Engineering September 1995g.ahmedNo ratings yet
- Carson 2014Document3 pagesCarson 2014Alex Samuel SilvaNo ratings yet
- Predict The Glass Transition Temperature of Glycerol-Water Binary Cryoprotectant by Molecular Dynamic SimulationDocument7 pagesPredict The Glass Transition Temperature of Glycerol-Water Binary Cryoprotectant by Molecular Dynamic Simulationfadhillah ivanNo ratings yet
- 2 Finalizado 1145-11Document5 pages2 Finalizado 1145-11Luis AliagaNo ratings yet
- Etd Chapt 6Document69 pagesEtd Chapt 6Miruna PetriaNo ratings yet
- Tom 5th UnitDocument9 pagesTom 5th UnitmysterypopzaswinNo ratings yet
- Tshwane University of Technology Department of Chemical and Mettalugical EngineeringDocument14 pagesTshwane University of Technology Department of Chemical and Mettalugical EngineeringSamuel GaétanNo ratings yet
- Pele de CaçãoDocument8 pagesPele de CaçãoJuliano SouzaNo ratings yet
- Thermal Characterization of Polymers (MSE LAb 7)Document2 pagesThermal Characterization of Polymers (MSE LAb 7)GhostInTheFlameNo ratings yet
- Polyester-Based Coil CoatingsDocument7 pagesPolyester-Based Coil CoatingsRodolvano EmilianoNo ratings yet
- Gathers 1986Document57 pagesGathers 1986Михаил ПарамоновNo ratings yet
- Enthalpyof FusionanddegreeofcrystallinityDocument7 pagesEnthalpyof FusionanddegreeofcrystallinityCamila BascuNo ratings yet
- JNN 2008 1178Document7 pagesJNN 2008 1178Triều Huỳnh NhậtNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Paracetamol ImpuritiesDocument2 pagesParacetamol ImpuritiesVeeprho LaboratoriesNo ratings yet
- Fierascu-2023-Metal and Metal Oxide NanoparticDocument31 pagesFierascu-2023-Metal and Metal Oxide NanoparticEduard-Marius LungulescuNo ratings yet
- Boyles LawDocument3 pagesBoyles Lawaiza larrozaNo ratings yet
- SUPPOSITORIESDocument38 pagesSUPPOSITORIESRahul Lakhani100% (1)
- Gem Webinar - 4Document36 pagesGem Webinar - 4venu r sNo ratings yet
- Haloalkanes HaloareneDocument23 pagesHaloalkanes HaloarenegtfhrfvhwcvfrwdpitNo ratings yet
- MSDSTransocean Longlife Antifouling (ID) - En53Document11 pagesMSDSTransocean Longlife Antifouling (ID) - En53akasNo ratings yet
- Bardeen Regular Black Hole With An Electric Source: PACS Numbers: 04.50.Kd, 04.70.BwDocument9 pagesBardeen Regular Black Hole With An Electric Source: PACS Numbers: 04.50.Kd, 04.70.BwAshima SoodNo ratings yet
- 9701 s09 Ms 31Document7 pages9701 s09 Ms 31Hubbak KhanNo ratings yet
- DK Did You Know Earth PDFDocument144 pagesDK Did You Know Earth PDFThuy Pham85% (13)
- CIE IGCSE Forces Hookes Law OnlyDocument22 pagesCIE IGCSE Forces Hookes Law Onlyh aNo ratings yet
- Ergonomics & Industrial Safety Ii-Tie 1204: Non-Destructive Testing (NDT)Document35 pagesErgonomics & Industrial Safety Ii-Tie 1204: Non-Destructive Testing (NDT)phillip chirongweNo ratings yet
- Extrusion Concept - 1Document129 pagesExtrusion Concept - 1subhoNo ratings yet
- 1CL QMDocument6 pages1CL QMshankasurNo ratings yet
- Dowlath Form 4 Physics Past Papers DynamicsDocument4 pagesDowlath Form 4 Physics Past Papers DynamicsRuqayya ImranNo ratings yet
- Soal EnzymeDocument3 pagesSoal EnzymeluliNo ratings yet
- Physics: Mechanical Waves (3 Hours)Document11 pagesPhysics: Mechanical Waves (3 Hours)Nur Farhana SuhaimiNo ratings yet
- Brochure-Innovic Best PLC SCADA Industrial Automation Training InstituteDocument6 pagesBrochure-Innovic Best PLC SCADA Industrial Automation Training InstituteRaaz NayakNo ratings yet
- AA'BB' SpectraDocument11 pagesAA'BB' SpectraSaurav PaulNo ratings yet
- 1 - Piping CBT API 571 QuestionDocument5 pages1 - Piping CBT API 571 QuestionAMALENDU PAULNo ratings yet
- Compressor DesignDocument34 pagesCompressor DesignFarrell AsdyatamaNo ratings yet
- Aits 1819 Ot Jeea Paper 1 SolDocument10 pagesAits 1819 Ot Jeea Paper 1 SolwhybangadNo ratings yet
- Atoms V2Document15 pagesAtoms V2Doaa NassarNo ratings yet
- Productflyer - 978 3 540 45469 4Document1 pageProductflyer - 978 3 540 45469 4SairaightNo ratings yet
- Centrifuge Modeling On The Effect of Mechanical Connection On The Dynamic Performance of Narrow Geosynthetic Reinforced Soil WallDocument17 pagesCentrifuge Modeling On The Effect of Mechanical Connection On The Dynamic Performance of Narrow Geosynthetic Reinforced Soil WallpiyushparikNo ratings yet
- 09 JJ H2 Prelim P2Document15 pages09 JJ H2 Prelim P2Gopi KupuchittyNo ratings yet
- A Model For The Graphite Formation in Ductile Cast Iron Part I Inoculation MechanismsDocument25 pagesA Model For The Graphite Formation in Ductile Cast Iron Part I Inoculation MechanismsAdams GodoyNo ratings yet
- Luo Et Al. Analytical Modeling and Thermal Analysis of Deep Coaxial Borehole Heat PDFDocument17 pagesLuo Et Al. Analytical Modeling and Thermal Analysis of Deep Coaxial Borehole Heat PDFPekas GuareñaNo ratings yet
- Mag3 - Magnetic Separation Efficiency in Food Industriy, 2014Document4 pagesMag3 - Magnetic Separation Efficiency in Food Industriy, 2014Alifah MauludinahNo ratings yet
TG and Fragility Determination of Glass-Forming Sugars Using A Dynamic Mechanical Analyzer
TG and Fragility Determination of Glass-Forming Sugars Using A Dynamic Mechanical Analyzer
Uploaded by
lwnnrcOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
TG and Fragility Determination of Glass-Forming Sugars Using A Dynamic Mechanical Analyzer
TG and Fragility Determination of Glass-Forming Sugars Using A Dynamic Mechanical Analyzer
Uploaded by
lwnnrcCopyright:
Available Formats
and Fragility Determination of Glass-Forming Sugars using a Dynamic Mechanical Analyzer
Lindong Weng, Gloria D. Elliott.
Department of Mechanical Engineering and Engineering Sciences, University of North Carolina at Charlotte,
Charlotte, NC 28223
Statement of Purpose: Glass-forming sugars are used in wherein is a universal constant of 16. The values of
a number of fields, including the preservation of and are obtained by best-fitting with the
biologics. Sugars are widely used as protectants primarily limitation of > to Eq. (1). When the relaxation time
due to their high glass transition temperature ( )1 which, ( ) at is set to 100s,4 the kinetic fragility index ( )
by convention, is the temperature at which the viscosity of
can be obtained by = | .
the liquid reaches 1012 Pa·s (i.e. becomes physically ⁄
“solid”). The glass transition is a second-order, reversible
dynamic phenomenon which exhibits a discontinuity of
the second-order properties such as a step change of
thermal expansion or heat capacity ( ).2 Due to the
nature of the glass transition, one of the most widely-used
techniques for measuring is differential scanning
calorimetry (DSC). Another important property of glass-
forming materials used for preservation purposes is
fragility, which is generally recognized as a way of
classifying the strength of glass-forming liquids.3 It is
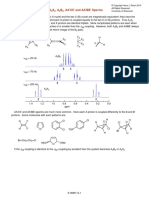
defined as the deviation of the temperature dependence of Figure 1. Generation of master curve for loss modulus ".
the α-relaxation time ( ) from simple Arrhenius behavior.
This deviation determines the steepness of the Arrhenius Results: The and fragility index of trehalose was
plot near and thus the “sharpness” of the glass determined to be 388.8±0.6 K and 115.2±6.3,
transition.3 The fragility is usually measured by using respectively, both of which are in good agreement with
dielectric spectroscopy, dynamic mechanical analyzer the literature values of 388.15 K5 (DSC measurement)
(DMA) or nuclear magnetic resonance. Because of the and 107±34 (dielectric measurement). In addition, the
importance of these parameters, a comprehensive method of sucrose was determined to be 343.0±0.4 K and the
to determine both and fragility of the same samples fragility index 98.1±0.9, consistent with the literature
would be highly desirable. In this study, we propose a values of 350 K6 (DSC measurement) and 98-1007
method based on DMA to estimate both the and (dielectric measurement).
fragility index. Results were validated by comparing with
literature values of commonly used sugars. Conclusions: Given that the glass transition temperature
and fragility are both important properties of glass-
DMA Method: A TA Q800 DMA was used to conduct forming liquids, a comprehensive method is proposed to
temperature step/frequency sweep experiments on simultaneously determine these properties by applying the
amorphous samples of trehalose or sucrose enveloped in a time-temperature superposition principle to relaxation
steel material pocket. The temperature range was 298-423 data obtained by DMA. The method was used to
K with an increment of 5 K and the oscillation frequency determine the and fragility index of trehalose and
was swept from 20 to 0.01 Hz at each temperature step. sucrose, yielding good agreement with literature values.
For a specific temperature series, the loss modulus ( ") This DMA-based and fragility determination method
data as a function of frequency can be shifted by (x- represents a new approach for identifying optimal
shift factor) to fit a master curve using a time-temperature compositions for preservation of biologics.
superposition (TTS) model. The α-relaxation time can be
calculated by where is the relaxation time at References:
the reference temperature ( ) and equal to 1/ .The 1. Crowe, JH. Annu. Rev. Physiol. 1998;60:73-103.
corresponds to the maximum loss modulus in the 2 Weng, L. Phys. Chem. Chem. Phys. 2014;16:11555-
TTS master curve (See Figure 1 (b)). The profile of 11565.
supercooled liquids above follows the Williams- 3. Angell, CA. Chem. Rev. 2002;102:2627-2650.
Landel-Ferry (WLF) equation as given by Eq. (1). 4. De Gusseme, A. J. Phys. Chem. B. 2003;107:10879-
10886.
5. Weng, L. Cryobiology 2014;68:155-158.
log ⁄ (1)
6. Hancock, BC. Pharm. Res. 1995;12:799-806.
7. Wang, LM. J. Chem. Phys. 2006;125:74505.
You might also like
- Advanced Temperature Measurement and Control, Second EditionFrom EverandAdvanced Temperature Measurement and Control, Second EditionNo ratings yet
- Measuring Thermal Crystallinity in PET: Spe Antec Indianapolis 2016Document5 pagesMeasuring Thermal Crystallinity in PET: Spe Antec Indianapolis 2016Silviani SilvyNo ratings yet
- Chemical Names and Formulas: 9.1 Naming IonsDocument53 pagesChemical Names and Formulas: 9.1 Naming IonsLovieAlfonsoNo ratings yet
- Rheological Properties of Some Thermotropic Liquid Crystalline PolymersDocument7 pagesRheological Properties of Some Thermotropic Liquid Crystalline PolymersAdityaNo ratings yet
- Application of Diferential Scanning CalorimetryDocument4 pagesApplication of Diferential Scanning CalorimetryUsman GhaniNo ratings yet
- DSC Error & InterpretationDocument3 pagesDSC Error & InterpretationUsman GhaniNo ratings yet
- Differential Scanning CalorimetryDocument7 pagesDifferential Scanning CalorimetryKenesei GyörgyNo ratings yet
- Epoxy CureDocument7 pagesEpoxy CuregdritzNo ratings yet
- 1 s2.0 S0038109804000067 MainDocument4 pages1 s2.0 S0038109804000067 MainAbhishek ChaturvediNo ratings yet
- Aya Alla Mahmoud - 5632Document11 pagesAya Alla Mahmoud - 5632aya mahmoudNo ratings yet
- Thermal Conductivity Determination of Small Polymer Samples by Differential Scanning CalorimetryDocument5 pagesThermal Conductivity Determination of Small Polymer Samples by Differential Scanning Calorimetrycarlette11No ratings yet
- Dec 1984Document9 pagesDec 1984krishnaNo ratings yet
- Thermal Analysis of PolymersDocument86 pagesThermal Analysis of PolymersMarister OliveiraNo ratings yet
- Rahman2007 PDFDocument6 pagesRahman2007 PDFMD Mahmudul Hasan MasudNo ratings yet
- Composites: Part A: Oleksandr G. Kravchenko, Chunyu Li, Alejandro Strachan, Sergii G. Kravchenko, R. Byron PipesDocument9 pagesComposites: Part A: Oleksandr G. Kravchenko, Chunyu Li, Alejandro Strachan, Sergii G. Kravchenko, R. Byron Pipesmarco_ravelo_10No ratings yet
- Modeling The Thermal Response of Porcine Cartilage To Laser IrradiationDocument10 pagesModeling The Thermal Response of Porcine Cartilage To Laser IrradiationZina AliNo ratings yet
- Cálculo Del Calor Específico From DSCDocument7 pagesCálculo Del Calor Específico From DSCfabio1199No ratings yet
- SchaweDocument9 pagesSchawezhor El hallaouiNo ratings yet
- Determination of Polymer Crystallinity by DSC: AbstactDocument3 pagesDetermination of Polymer Crystallinity by DSC: AbstactJohana ValenciaNo ratings yet
- Rao Gunti, Asokan - 2012 - Thermodynamic, Raman and Electrical Switching Studies On Si15Te85-xAgx (4 X 20) Glasses (3) - AnnotatedDocument6 pagesRao Gunti, Asokan - 2012 - Thermodynamic, Raman and Electrical Switching Studies On Si15Te85-xAgx (4 X 20) Glasses (3) - AnnotatedJagan KbNo ratings yet
- Fouling Detection in A Cross - Ow Heat Exchanger Based On Physical ModelingDocument9 pagesFouling Detection in A Cross - Ow Heat Exchanger Based On Physical ModelingMounam MaitiNo ratings yet
- Determination of Arrhenius Kinetic Constants Differential Scanning CalorimetryDocument5 pagesDetermination of Arrhenius Kinetic Constants Differential Scanning CalorimetryNgocDiep PhamNo ratings yet
- Study of Mechanical AndcomparativeDocument9 pagesStudy of Mechanical Andcomparativenadir adelNo ratings yet
- Differential Scanning Calorimetry (DSC) : Mr. Sagar Kishor SavaleDocument27 pagesDifferential Scanning Calorimetry (DSC) : Mr. Sagar Kishor SavaleDivya Tripathy100% (1)
- Unit 1 NotesDocument16 pagesUnit 1 NotesSAJITH NFNo ratings yet
- 04 474Document18 pages04 474webhareggebru06No ratings yet
- Chemical Kinetics On Thermal Decompositions of CumeneDocument8 pagesChemical Kinetics On Thermal Decompositions of CumeneMario Alonso Velasquez FlorezNo ratings yet
- Differential Scanning CalorimetryDocument7 pagesDifferential Scanning CalorimetryAli HussnainNo ratings yet
- Differential Scanning Calorimeter (DSC)Document7 pagesDifferential Scanning Calorimeter (DSC)Fi FialaNo ratings yet
- Creep Parameters of Spruce Wood in High Temperature EnvironmentDocument4 pagesCreep Parameters of Spruce Wood in High Temperature EnvironmentBentea MarcelNo ratings yet
- Using Thermal Analysis Methods To Better Understand Asphalt RheologyDocument6 pagesUsing Thermal Analysis Methods To Better Understand Asphalt RheologyNatalia KovalovaNo ratings yet
- Study On The Influence of Ageing On Chemical and Mechanical Properties of N, N - Dimethyl-N, N - Diphenylcarbamide Stabilized PropellantsDocument8 pagesStudy On The Influence of Ageing On Chemical and Mechanical Properties of N, N - Dimethyl-N, N - Diphenylcarbamide Stabilized Propellantstotenkopf0424No ratings yet
- Sanchez Et Al., (2008)Document8 pagesSanchez Et Al., (2008)radzul abyanNo ratings yet
- Experimental Determination of the Thermal Di ffusivity of α‑Cryolite up to 810 K and Comparison with First Principles PredictionsDocument7 pagesExperimental Determination of the Thermal Di ffusivity of α‑Cryolite up to 810 K and Comparison with First Principles PredictionsIbraheem AlQadiNo ratings yet
- 10 1002@star 19970490704 PDFDocument5 pages10 1002@star 19970490704 PDFuwuNo ratings yet
- Isothermal Microcalorimetry: An Analytical Technique For Assessing The Dynamic Chemical Stability of UHMWPEDocument6 pagesIsothermal Microcalorimetry: An Analytical Technique For Assessing The Dynamic Chemical Stability of UHMWPEmurugandevaNo ratings yet
- 33Document7 pages33ninhhoanghai_lhdNo ratings yet
- 1 PaperDocument3 pages1 PaperMadhavanIceNo ratings yet
- Solidification of SteelDocument7 pagesSolidification of SteelDan Pascu100% (1)
- Fardapaper Method For Lifetime Estimation of Power Transformer Mineral OilDocument7 pagesFardapaper Method For Lifetime Estimation of Power Transformer Mineral OilbenlahnecheNo ratings yet
- Chen2019-Thermal Response Characteristics of A SPRIGT Primary Thermometry SystemDocument6 pagesChen2019-Thermal Response Characteristics of A SPRIGT Primary Thermometry SystemJacyNo ratings yet
- Error Analysis of The Thermal Cell For Soil Thermal Conductivity MeasurementDocument10 pagesError Analysis of The Thermal Cell For Soil Thermal Conductivity MeasurementMasha RamirezNo ratings yet
- Lifetime Prediction of Thermo-Mechanical Fatigue For Exhaust ManifoldDocument10 pagesLifetime Prediction of Thermo-Mechanical Fatigue For Exhaust Manifoldram shyamNo ratings yet
- An Examination of The Thermal ExpansionDocument2 pagesAn Examination of The Thermal Expansionmap vitcoNo ratings yet
- Engineering Failure Analysis: H.S. Da Costa Mattos, L.M. Paim, J.M.L. ReisDocument13 pagesEngineering Failure Analysis: H.S. Da Costa Mattos, L.M. Paim, J.M.L. ReisFelipe Perissé Duarte LopesNo ratings yet
- Dynamic Prediction of Wastewater Aeration Basin Temperature: Journal of Environmental Engineering September 1995Document11 pagesDynamic Prediction of Wastewater Aeration Basin Temperature: Journal of Environmental Engineering September 1995g.ahmedNo ratings yet
- Carson 2014Document3 pagesCarson 2014Alex Samuel SilvaNo ratings yet
- Predict The Glass Transition Temperature of Glycerol-Water Binary Cryoprotectant by Molecular Dynamic SimulationDocument7 pagesPredict The Glass Transition Temperature of Glycerol-Water Binary Cryoprotectant by Molecular Dynamic Simulationfadhillah ivanNo ratings yet
- 2 Finalizado 1145-11Document5 pages2 Finalizado 1145-11Luis AliagaNo ratings yet
- Etd Chapt 6Document69 pagesEtd Chapt 6Miruna PetriaNo ratings yet
- Tom 5th UnitDocument9 pagesTom 5th UnitmysterypopzaswinNo ratings yet
- Tshwane University of Technology Department of Chemical and Mettalugical EngineeringDocument14 pagesTshwane University of Technology Department of Chemical and Mettalugical EngineeringSamuel GaétanNo ratings yet
- Pele de CaçãoDocument8 pagesPele de CaçãoJuliano SouzaNo ratings yet
- Thermal Characterization of Polymers (MSE LAb 7)Document2 pagesThermal Characterization of Polymers (MSE LAb 7)GhostInTheFlameNo ratings yet
- Polyester-Based Coil CoatingsDocument7 pagesPolyester-Based Coil CoatingsRodolvano EmilianoNo ratings yet
- Gathers 1986Document57 pagesGathers 1986Михаил ПарамоновNo ratings yet
- Enthalpyof FusionanddegreeofcrystallinityDocument7 pagesEnthalpyof FusionanddegreeofcrystallinityCamila BascuNo ratings yet
- JNN 2008 1178Document7 pagesJNN 2008 1178Triều Huỳnh NhậtNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Paracetamol ImpuritiesDocument2 pagesParacetamol ImpuritiesVeeprho LaboratoriesNo ratings yet
- Fierascu-2023-Metal and Metal Oxide NanoparticDocument31 pagesFierascu-2023-Metal and Metal Oxide NanoparticEduard-Marius LungulescuNo ratings yet
- Boyles LawDocument3 pagesBoyles Lawaiza larrozaNo ratings yet
- SUPPOSITORIESDocument38 pagesSUPPOSITORIESRahul Lakhani100% (1)
- Gem Webinar - 4Document36 pagesGem Webinar - 4venu r sNo ratings yet
- Haloalkanes HaloareneDocument23 pagesHaloalkanes HaloarenegtfhrfvhwcvfrwdpitNo ratings yet
- MSDSTransocean Longlife Antifouling (ID) - En53Document11 pagesMSDSTransocean Longlife Antifouling (ID) - En53akasNo ratings yet
- Bardeen Regular Black Hole With An Electric Source: PACS Numbers: 04.50.Kd, 04.70.BwDocument9 pagesBardeen Regular Black Hole With An Electric Source: PACS Numbers: 04.50.Kd, 04.70.BwAshima SoodNo ratings yet
- 9701 s09 Ms 31Document7 pages9701 s09 Ms 31Hubbak KhanNo ratings yet
- DK Did You Know Earth PDFDocument144 pagesDK Did You Know Earth PDFThuy Pham85% (13)
- CIE IGCSE Forces Hookes Law OnlyDocument22 pagesCIE IGCSE Forces Hookes Law Onlyh aNo ratings yet
- Ergonomics & Industrial Safety Ii-Tie 1204: Non-Destructive Testing (NDT)Document35 pagesErgonomics & Industrial Safety Ii-Tie 1204: Non-Destructive Testing (NDT)phillip chirongweNo ratings yet
- Extrusion Concept - 1Document129 pagesExtrusion Concept - 1subhoNo ratings yet
- 1CL QMDocument6 pages1CL QMshankasurNo ratings yet
- Dowlath Form 4 Physics Past Papers DynamicsDocument4 pagesDowlath Form 4 Physics Past Papers DynamicsRuqayya ImranNo ratings yet
- Soal EnzymeDocument3 pagesSoal EnzymeluliNo ratings yet
- Physics: Mechanical Waves (3 Hours)Document11 pagesPhysics: Mechanical Waves (3 Hours)Nur Farhana SuhaimiNo ratings yet
- Brochure-Innovic Best PLC SCADA Industrial Automation Training InstituteDocument6 pagesBrochure-Innovic Best PLC SCADA Industrial Automation Training InstituteRaaz NayakNo ratings yet
- AA'BB' SpectraDocument11 pagesAA'BB' SpectraSaurav PaulNo ratings yet
- 1 - Piping CBT API 571 QuestionDocument5 pages1 - Piping CBT API 571 QuestionAMALENDU PAULNo ratings yet
- Compressor DesignDocument34 pagesCompressor DesignFarrell AsdyatamaNo ratings yet
- Aits 1819 Ot Jeea Paper 1 SolDocument10 pagesAits 1819 Ot Jeea Paper 1 SolwhybangadNo ratings yet
- Atoms V2Document15 pagesAtoms V2Doaa NassarNo ratings yet
- Productflyer - 978 3 540 45469 4Document1 pageProductflyer - 978 3 540 45469 4SairaightNo ratings yet
- Centrifuge Modeling On The Effect of Mechanical Connection On The Dynamic Performance of Narrow Geosynthetic Reinforced Soil WallDocument17 pagesCentrifuge Modeling On The Effect of Mechanical Connection On The Dynamic Performance of Narrow Geosynthetic Reinforced Soil WallpiyushparikNo ratings yet
- 09 JJ H2 Prelim P2Document15 pages09 JJ H2 Prelim P2Gopi KupuchittyNo ratings yet
- A Model For The Graphite Formation in Ductile Cast Iron Part I Inoculation MechanismsDocument25 pagesA Model For The Graphite Formation in Ductile Cast Iron Part I Inoculation MechanismsAdams GodoyNo ratings yet
- Luo Et Al. Analytical Modeling and Thermal Analysis of Deep Coaxial Borehole Heat PDFDocument17 pagesLuo Et Al. Analytical Modeling and Thermal Analysis of Deep Coaxial Borehole Heat PDFPekas GuareñaNo ratings yet
- Mag3 - Magnetic Separation Efficiency in Food Industriy, 2014Document4 pagesMag3 - Magnetic Separation Efficiency in Food Industriy, 2014Alifah MauludinahNo ratings yet