Professional Documents
Culture Documents
Light Diesel Oil
Light Diesel Oil
Uploaded by
Trans AmOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Light Diesel Oil
Light Diesel Oil
Uploaded by
Trans AmCopyright:
Available Formats
HW1: Due May 24th 2023
17
Name: _____________ ID:__________
1- Follow the example on the lecture slides (isooctane) and assume that each
fuel is burnt completely then balance the Combustion Equation. The general
equations are listed at the end
a- Propane burns with air. (1%)
b- Light diesel oil burns with air. (1%)
c- Ethanol burns with air. (1%)
2- Assume Light diesel oil is burnt with 15% access air:
a- Write the new combustion equation. (3%)
b- Find 𝐹𝐴𝑅, (𝐹𝐴𝑅)𝑠𝑡𝑜𝑖𝑐ℎ , and equivalence ration. (3%)
Internal Combustion Engines March – 18 June 202312 محركات االحتراق الداخلي:1_2_3-8044305_2_443
Dr. Moaz Allehaibi molehaibi@uqu.edu.sa:البريد اإللكتروني الدكتورمعاذ اللهيبي
Mechanical Engineering https://uqu.edu.sa/en/Profile/molehaibi الهندسة امليكانيكية
3-A car has a mass of 1000 [kg] and we like to accelerate it from 0 to 100
[km/h] in 10 [seconds].
a- What engine power is needed? (2%)
b- What torque is needed on the drive wheels to get the same acceleration
at the same time ( 10 s)?. Assume 𝑟 = 0.3075 (2%)
c- what force and power needed to overcome the rolling resistance? The car
is driven on fine Cobblestone. (2%)
d- what force and power needed to overcome the Air resistance of a car?
Assume frontal area of 𝐴 = 2.2 [𝑚2 ] and the car is reversed wedge
shape. (2%)
Internal Combustion Engines March – 18 June 202312 محركات االحتراق الداخلي:1_2_3-8044305_2_443
Dr. Moaz Allehaibi molehaibi@uqu.edu.sa:البريد اإللكتروني الدكتورمعاذ اللهيبي
Mechanical Engineering https://uqu.edu.sa/en/Profile/molehaibi الهندسة امليكانيكية
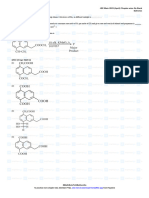
Supporting Equations:
𝑦 𝑦 𝑦
1- 𝐶 𝐻
𝑥 𝑦 + (𝑥 + ) (𝑂 + 3.76 𝑁 ) → 𝑥𝐶𝑂2 + 𝐻 𝑂 + 3.76 (𝑥 + )𝑁
4 2 2
2 2 4 2
𝑦 𝑧 𝑦 y z
2- . 𝐶 𝐻
𝑥 𝑦 + (𝑥 + − ) (𝑂2 + 3.76 𝑁2 ) → 𝑥𝐶𝑂2 + 𝐻 𝑂 +3.76 (x + − )N
4 2 2 2 4 2 2
𝑦 𝑦 𝑦 𝑦
3- 𝐶𝑥 𝐻𝑦 +𝐹 (𝑥 + ) (𝑂2 + 3.76 𝑁2 ) → 𝑥𝐶𝑂2 + 𝐻2 𝑂 + 𝐹 (𝑥 + ) (3.76 𝑁2 ) +(𝐹 − 1) (𝑥 + ) 𝑂2
4 2 4 4
4- 𝑚 = 𝑁 𝑀𝑊 , Where N is the number of moles and MW is the molecular
𝑔
weight[ ].
𝑚𝑜𝑙𝑒
𝑔 𝑔
5- 𝑀𝑊𝑎𝑖𝑟 = 0.21 𝑂2 + 0.79 𝑁2 = 0.21 (2 ∗ 16 [ ]) ∗ 0.79 (2 ∗ 14[ ]) =
𝑚𝑜𝑙𝑒 𝑚𝑜𝑙𝑒
𝑔
28.84 [ ]
𝑚𝑜𝑙𝑒
Formula Fuel QHHV [MJ/kg] QLHV [MJ/kg]
Assume isooctane
Gasoline 47.3 44.0
C10 H18 Light diesel oil 46.1 43.2
Gas chromatographic
Heavy fuel oil 45.5 42.8
Methane + ethane+ propane
Natural Gas (US) 50 45
CH4 Methane 55.5 50
C3 H8 Propane 50.4 46.4
C8 H18 Isooctane 47.8 44.3
C16 H34 Cetane 47.3 44.0
C6 H6 Benzene 41.9 40.2
C6 H5 CH3 Toluene 42.5 40.6
CH3OH Methanol 22.7 20.0
C2 H6 O Ethanol 29.7 26.9
Internal Combustion Engines March – 18 June 202312 محركات االحتراق الداخلي:1_2_3-8044305_2_443
Dr. Moaz Allehaibi molehaibi@uqu.edu.sa:البريد اإللكتروني الدكتورمعاذ اللهيبي
Mechanical Engineering https://uqu.edu.sa/en/Profile/molehaibi الهندسة امليكانيكية
Internal Combustion Engines March – 18 June 202312 محركات االحتراق الداخلي:1_2_3-8044305_2_443
Dr. Moaz Allehaibi molehaibi@uqu.edu.sa:البريد اإللكتروني الدكتورمعاذ اللهيبي
Mechanical Engineering https://uqu.edu.sa/en/Profile/molehaibi الهندسة امليكانيكية
You might also like
- 3410 3411 H179Service TrainingDocument668 pages3410 3411 H179Service TrainingWilliam Giovanni Madariaga Malebrán97% (35)
- Bell B25D & B30D PartsDocument752 pagesBell B25D & B30D PartsJoaoVr82% (11)
- JCB 3.0D 4×4, 3.5D 4×4 TELETRUK Service Repair Manual SN 78001 Onwards PDFDocument50 pagesJCB 3.0D 4×4, 3.5D 4×4 TELETRUK Service Repair Manual SN 78001 Onwards PDFfjjskemdme29% (7)
- Shop Manual PC200 8 (Ing)Document1,082 pagesShop Manual PC200 8 (Ing)John MkCito KI88% (160)
- Full Download Sustainable Energy Edition 1st Edition Dunlap Solutions ManualDocument35 pagesFull Download Sustainable Energy Edition 1st Edition Dunlap Solutions Manualgiedreeluyzay100% (29)
- VT Supercharged V6 (L67) Tech SpecsDocument3 pagesVT Supercharged V6 (L67) Tech SpecsVYSUPER667% (3)
- 01 - CHEM 102 Sample Midterm 2 QuestionsDocument10 pages01 - CHEM 102 Sample Midterm 2 QuestionsPallavi RawatNo ratings yet
- Components and Installation Handbook Landirenzo Omega GiDocument49 pagesComponents and Installation Handbook Landirenzo Omega GiJose GuerrNo ratings yet
- Steam EngineDocument10 pagesSteam EngineKristian Taruc100% (2)
- Escavadora 135C RTSDocument543 pagesEscavadora 135C RTSGilberto Torres50% (2)
- Cet300s Fisa 2014 MemoDocument17 pagesCet300s Fisa 2014 Memocarleston thurgoodNo ratings yet
- LECTUREDocument5 pagesLECTUREkarim shahNo ratings yet
- Sample Calculations Bio-OilDocument8 pagesSample Calculations Bio-OilJames Matthew LimpinNo ratings yet
- FINAL EXAM CombustionDocument5 pagesFINAL EXAM CombustionJepoy torresNo ratings yet
- S6 Chemistry: Exam 2Document12 pagesS6 Chemistry: Exam 2Aine VisionNo ratings yet
- IJRAH2022020309Document7 pagesIJRAH2022020309Johanes olo tua ManullangNo ratings yet
- PP Cep FinalDocument7 pagesPP Cep Finalchzain0912No ratings yet
- New Correlation For Calculating Acentric Factor of Petroleum 2 FRDocument7 pagesNew Correlation For Calculating Acentric Factor of Petroleum 2 FRتامر دندش100% (1)
- Fuel Consumption For Boiler SPDocument20 pagesFuel Consumption For Boiler SPaqilah liyanaNo ratings yet
- As1143a1 - Lab 1 - CHM213Document14 pagesAs1143a1 - Lab 1 - CHM213Emeer EllyasNo ratings yet
- HW7 2023Document4 pagesHW7 2023writersleedNo ratings yet
- New Sch4u PT AnswersDocument10 pagesNew Sch4u PT Answersleafyfun100No ratings yet
- Licta March 2024 Paper 2 A Level Chemistry Marking GuideDocument14 pagesLicta March 2024 Paper 2 A Level Chemistry Marking Guidenkafor7No ratings yet
- New Correlation For Calculating Critical Pressure of Petroleum FractionsDocument6 pagesNew Correlation For Calculating Critical Pressure of Petroleum FractionsaliNo ratings yet
- An Optimal Fourth Order Method For Solving Nonlinear Equations-1602246038Document13 pagesAn Optimal Fourth Order Method For Solving Nonlinear Equations-1602246038Oktarina 1903113284No ratings yet
- Thermochemistry - DPP-11 - GATE Crash Course 2023 MechanicalDocument4 pagesThermochemistry - DPP-11 - GATE Crash Course 2023 MechanicalhibominNo ratings yet
- Baschem Q3Document6 pagesBaschem Q3Harvey LimNo ratings yet
- INChO2024 Solution 20240206Document6 pagesINChO2024 Solution 20240206Keka MandalNo ratings yet
- Final Exam - Sem 2 AY1718Document6 pagesFinal Exam - Sem 2 AY1718Dhadkan D KCNo ratings yet
- Water Vaporization Calculation 250420 R2Document3 pagesWater Vaporization Calculation 250420 R2Kanthan DevanNo ratings yet
- Lecture#1Document6 pagesLecture#1Anmar A. Al-joboryNo ratings yet
- GARCIA, Krizzi Eve D. 3CHEM1Document6 pagesGARCIA, Krizzi Eve D. 3CHEM1Krizzi Dizon GarciaNo ratings yet
- CMY 285 Experiment 2 - U20518073Document11 pagesCMY 285 Experiment 2 - U20518073Suné MartinsNo ratings yet
- STOICHIOMETRYDocument17 pagesSTOICHIOMETRYboluwatifeajiboye371No ratings yet
- Lampiran Mass BalanceDocument18 pagesLampiran Mass BalanceIrma SariNo ratings yet
- 2 ReportDocument9 pages2 Reportsvzdv asdqNo ratings yet
- Theory 2 - Moment Distribution Method Sample ProblemsDocument20 pagesTheory 2 - Moment Distribution Method Sample ProblemsJohn Paul Liwaliw100% (2)
- LULU, SHIELNEY G. - ACTIVITY No.2Document5 pagesLULU, SHIELNEY G. - ACTIVITY No.2Shielney LuluNo ratings yet
- CHEN309 Test (2019 2020)Document1 pageCHEN309 Test (2019 2020)Saidu WaziriNo ratings yet
- PAPER SIDIQ ICOS FixDocument7 pagesPAPER SIDIQ ICOS FixAtika AssalafiyahNo ratings yet
- CAGAMPANG, Julius D. CHE 15 A1 Assignment 5B: Required: AnswerDocument2 pagesCAGAMPANG, Julius D. CHE 15 A1 Assignment 5B: Required: AnswerJulius CagampangNo ratings yet
- CE 264 - PS4 - NCJajurieDocument28 pagesCE 264 - PS4 - NCJajurieRanji JajurieNo ratings yet
- Performance Emission and Efficiency AnalysisDocument11 pagesPerformance Emission and Efficiency AnalysisGlobal Research and Development ServicesNo ratings yet
- V6 90 Engine Vibration Analysis and Discussion 1706352546Document13 pagesV6 90 Engine Vibration Analysis and Discussion 1706352546Francesco MasillaNo ratings yet
- Exam 2021 - MBS3111 - Solution - AT - 1Document6 pagesExam 2021 - MBS3111 - Solution - AT - 1kennethdevera01No ratings yet
- 02 Ee044 3 3 THT Fe MSDocument5 pages02 Ee044 3 3 THT Fe MSMohamed AltijaniNo ratings yet
- Exercise 2 Nuclear Reaction - Lucas Damien F. ManceraDocument6 pagesExercise 2 Nuclear Reaction - Lucas Damien F. ManceraDamien ManceraNo ratings yet
- SRG SPS-04 08 Nov SoDocument3 pagesSRG SPS-04 08 Nov Sotechnicalfacts31No ratings yet
- Espartero Activity 2 EnergyDocument3 pagesEspartero Activity 2 EnergyJahrel DaneNo ratings yet
- Mechanical Engineering Design of IPA DistillationDocument5 pagesMechanical Engineering Design of IPA DistillationSanthosh RockNo ratings yet
- Merged 20240208 0716Document27 pagesMerged 20240208 0716sophiaccharlotte876No ratings yet
- TL102 0 2024 Che3701 0Document12 pagesTL102 0 2024 Che3701 0sollomontlou06No ratings yet
- Application of System Linear Diophantine Equations in Balancing Chemical EquationsDocument6 pagesApplication of System Linear Diophantine Equations in Balancing Chemical EquationsIJRASETPublicationsNo ratings yet
- Coal EbuDocument19 pagesCoal Ebupippo pappiNo ratings yet
- New Correlation For Calculating Critical Pressure of Petroleum Fractions, Sayed Gomaa, 2016, 7 PGDocument7 pagesNew Correlation For Calculating Critical Pressure of Petroleum Fractions, Sayed Gomaa, 2016, 7 PGjoselosse desantosNo ratings yet
- Stoichiometry 1Document17 pagesStoichiometry 1Abraham JosephNo ratings yet
- Work Sheet # 6: College of Engineering and ArchitectureDocument3 pagesWork Sheet # 6: College of Engineering and Architecturekhadijah shabanNo ratings yet
- MTT II - Engineering Mathematics - Set BDocument2 pagesMTT II - Engineering Mathematics - Set BVibhorNo ratings yet
- New Correlation For Calculating Critical Pressure of Petroleum FractionsDocument7 pagesNew Correlation For Calculating Critical Pressure of Petroleum FractionsBitan chowdhuryNo ratings yet
- Hydrocarbons - JEE Main 2023 April Chapterwise PYQ - MathonGoDocument5 pagesHydrocarbons - JEE Main 2023 April Chapterwise PYQ - MathonGoAnjani Kumar SinghNo ratings yet
- MTA Grade 10 March Test 1 2022Document3 pagesMTA Grade 10 March Test 1 2022skhosana807No ratings yet
- Science Talks: Mekala Manikanta, D.R. SrinivasanDocument7 pagesScience Talks: Mekala Manikanta, D.R. SrinivasanNatán Pérez SánchezNo ratings yet
- Dwnload Full Sustainable Energy Edition 1st Edition Dunlap Solutions Manual PDFDocument35 pagesDwnload Full Sustainable Energy Edition 1st Edition Dunlap Solutions Manual PDFjaneaneriez100% (14)
- Test 1 - 2020 - SolutionDocument5 pagesTest 1 - 2020 - SolutionKHÁNH VÂN DIỆPNo ratings yet
- The Partial Regularity Theory of Caffarelli, Kohn, and Nirenberg and its SharpnessFrom EverandThe Partial Regularity Theory of Caffarelli, Kohn, and Nirenberg and its SharpnessNo ratings yet
- ATP IndexDocument2,352 pagesATP IndexRicardo zafra100% (1)
- As 4427-1996 Automotive Repairs - Code of Practice For Reconditioning Reciprocating Compression Ignition EngiDocument6 pagesAs 4427-1996 Automotive Repairs - Code of Practice For Reconditioning Reciprocating Compression Ignition EngiSAI Global - APACNo ratings yet
- Ge Lms100 Brochure March2015Document7 pagesGe Lms100 Brochure March2015smartleo_waloNo ratings yet
- VI1603 Spring R2 FinalDocument28 pagesVI1603 Spring R2 Finalrodruren01100% (2)
- QSG12-G2: Specification SheetDocument3 pagesQSG12-G2: Specification SheetAlejandro Dominado100% (1)
- SSI Artificial Lift Integrated SolutionsDocument24 pagesSSI Artificial Lift Integrated SolutionsmghareebNo ratings yet
- Engine Class-3 BD Question BankDocument11 pagesEngine Class-3 BD Question BankRakibul JubayerNo ratings yet
- Solvingweek 9Document22 pagesSolvingweek 9Christopher Lennon Dela Cruz0% (1)
- The New BMW 2.0 L Four Cylinder Gasoline Engine With TurbochargerDocument9 pagesThe New BMW 2.0 L Four Cylinder Gasoline Engine With TurbochargerMarculescu Nicolae CatalinNo ratings yet
- Session 1.2 - Willson - 0 - Methane SlipDocument10 pagesSession 1.2 - Willson - 0 - Methane SlipFauzan PhoneNo ratings yet
- In-Cylinder FlowDocument105 pagesIn-Cylinder FlowdebelaNo ratings yet
- Crystallizers: Chapter 16 Cost Accounting and Capital Cost EstimationDocument1 pageCrystallizers: Chapter 16 Cost Accounting and Capital Cost EstimationDeiver Enrique SampayoNo ratings yet
- Arecanut Harvesting MachineDocument14 pagesArecanut Harvesting MachineAnchith JoshiNo ratings yet
- 2011 AC & DC Hydraulic Power Packs CompactDocument97 pages2011 AC & DC Hydraulic Power Packs CompactNicolae MogosNo ratings yet
- Part 2: Mixture Formation, Combustion Method and TurbochargingDocument7 pagesPart 2: Mixture Formation, Combustion Method and TurbochargingÁdám DomokosNo ratings yet
- Ficha Tecnica Del ManliftDocument7 pagesFicha Tecnica Del ManliftJAIRO ANDRES SOLANO LOBONo ratings yet
- Chassis Design ImportantDocument27 pagesChassis Design ImportantNandan PoojaryNo ratings yet
- Reciprocating PumpDocument7 pagesReciprocating PumpMd. Tariqul Islam MunnaNo ratings yet
- Operator'S Manual: 21" Rotary Mower - Model Series 410 Thru 420Document16 pagesOperator'S Manual: 21" Rotary Mower - Model Series 410 Thru 420GorneauNo ratings yet
- Specification Sheet Sany SCC500EDocument17 pagesSpecification Sheet Sany SCC500Ejessica.chandrinovaNo ratings yet
- 741-742-743-743ds 6566109 SM 4-88 PDFDocument6 pages741-742-743-743ds 6566109 SM 4-88 PDFJavier AnezNo ratings yet
- Sistem Pelumasan: - ReviewDocument71 pagesSistem Pelumasan: - ReviewM Lazuardi Imani S100% (1)