Professional Documents
Culture Documents
Monoethanolamine NIST
Monoethanolamine NIST
Uploaded by
Büşra BalcıCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Monoethanolamine NIST
Monoethanolamine NIST
Uploaded by
Büşra BalcıCopyright:
Available Formats
NIST Chemistry WebBook, SRD 69
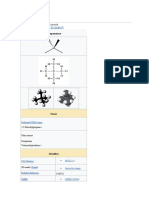
Monoethanolamine
Formula: C2H7NO
Molecular weight: 61.0831
IUPAC Standard InChI: InChI=1S/C2H7NO/c3-1-2-4/h4H,1-3H2
IUPAC Standard InChIKey: HZAXFHJVJLSVMW-UHFFFAOYSA-N
CAS Registry Number: 141-43-5
Chemical structure:
This structure is also available as a 2d Mol file or as a computed 3d SD file
The 3d structure may be viewed using Java or Javascript.
Other names: Ethanol, 2-amino-; β-Aminoethanol; β-Aminoethyl alcohol; β-
Hydroxyethylamine; Aminoethanol; Colamine; Ethanolamine; Ethylolamine; Glycinol; MEA;
Olamine; Thiofaco M-50; 2-Amino-1-Ethanol; 2-Aminoethan-1-ol; 2-Aminoethanol; 2-
Hydroxyethanamine; 2-Hydroxyethylamine; NH2CH2CH2OH; β-Ethanolamine; Aethanolamin;
2-Aminoaethanol; 2-Aminoetanolo; Etanolamina; Kolamin; Monoaethanolamin; UN 2491;
USAF EK-1597; 2-Ethanolamine; 1-Amino-2-hydroxyethane; 2-Aminoethyl alcohol
Permanent link for this species. Use this link for bookmarking this species for future
reference.
Information on this page:
Phase change data
References
Notes
Other data available:
Condensed phase thermochemistry data
Reaction thermochemistry data
Henry's Law data
Gas phase ion energetics data
Ion clustering data
IR Spectrum
Mass spectrum (electron ionization)
Gas Chromatography
Options:
Switch to calorie-based units
Data at NIST subscription sites:
NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical
data)
NIST subscription sites provide data under the NIST Standard Reference Data Program, but require
an annual fee to access. The purpose of the fee is to recover costs associated with the development
of data collections included in such sites. Your institution may already be a subscriber. Follow the
links above to find out more about the data in these sites and their terms of usage.
Phase change data
Go To: Top, References, Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Data compiled as indicated in comments:
BS - Robert L. Brown and Stephen E. Stein
TRC - Thermodynamics Research Center, NIST Boulder Laboratories, Chris Muzny director
AC - William E. Acree, Jr., James S. Chickos
Quantity Value Units Method Reference Comment
Weast and
Tboil 443.2 K N/A BS
Grasselli, 1989
Buckingham and
Tboil 444. K N/A BS
Donaghy, 1982
Anderson and
Tboil 444.15 K N/A Shimanskaya, Uncertainty assigned by TRC = 1.5 K; TRC
1969
Tboil 444.0 K N/A Lecat, 1947 Uncertainty assigned by TRC = 0.5 K; TRC
Quantity Value Units Method Reference Comment
McDonald,
Tfus 283.46 K N/A Shrader, et al., Uncertainty assigned by TRC = 0.05 K; TRC
1959
Quantity Value Units Method Reference Comment
Teja and
Tc 671.4 K N/A Uncertainty assigned by TRC = 1.5 K; TRC
Rosenthal, 1991
Uncertainty assigned by TRC = 50. K; Tc >
Anselme and Teja,
Tc 670. K N/A 670 K, which was observed with
1990
decomposition; TRC
Quantity Value Units Method Reference Comment
Teja and
Pc 80.30 bar N/A Uncertainty assigned by TRC = 0.40 bar; TRC
Rosenthal, 1991
Quantity Value Units Method Reference Comment
ΔvapH° 58. ± 3. kJ/mol AVG N/A Average of 9 values; Individual data points
Enthalpy of vaporization
ΔvapH Temperature
Method Reference Comment
(kJ/mol) (K)
Kim, Svendsen, et al.,
55.9 372. EB Based on data from 357. - 435. K.; AC
2008
Stephenson and
61.7 325. A Based on data from 310. - 444. K.; AC
Malanowski, 1987
McDonald, Shrader,
54.7 394. N/A Based on data from 379. - 443. K.; AC
et al., 1959, 2
Matthews, Sumner, et Based on data from 338. - 443. K. See
58.9 353. N/A
al., 1950 also Boublik, Fried, et al., 1984.; AC
Antoine Equation Parameters
log10(P) = A − (B / (T + C))
P = vapor pressure (bar)
T = temperature (K)
View plot Requires a JavaScript / HTML 5 canvas capable browser.
Temperature
A B C Reference Comment
(K)
Matthews, Sumner, et Coefficents calculated by NIST
338.6 - 444.1 4.29252 1408.873 -116.093
al., 1950, 2 from author's data.
In addition to the Thermodynamics Research Center (TRC) data
available from this site, much more physical and chemical
property data is available from the following TRC products:
SRD 103a – Thermo Data Engine (TDE) for pure
compounds.
SRD 103b – Thermo Data Engine (TDE) for pure
compounds, binary mixtures and chemical reactions
SRSD 2 – Web Thermo Tables (WTT), "lite" edition
SRSD 3 – Web Thermo Tables (WTT), professional edition
SRD 147 – Ionic Liquids Database
SRD 156 – Clathrate Hydrate Physical Property Database
References
Go To: Top, Phase change data, Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Weast and Grasselli, 1989
CRC Handbook of Data on Organic Compounds, 2nd Editon, Weast,R.C and Grasselli, J.G., ed(s).,
CRC Press, Inc., Boca Raton, FL, 1989, 1. [all data]
Buckingham and Donaghy, 1982
Buckingham, J.; Donaghy, S.M., Dictionary of Organic Compounds: Fifth Edition, Chapman and
Hall, New York, 1982, 1. [all data]
Anderson and Shimanskaya, 1969
Anderson, A.A.; Shimanskaya, M.V., Gas-Liquid Chromatography of some Aliphatic and Heterocyclic
Polyfunctional Amines: II. Solution Thermodyn. of Amines in Fix. Phas., Latv. PSR Zinat. Akad. Vestis,
Kim. Ser., 1969, No. 5, 527. [all data]
Lecat, 1947
Lecat, M., Orthobaric Azeotropes of Sulfides, Bull. Cl. Sci., Acad. R. Belg., 1947, 33, 160-82. [all data]
McDonald, Shrader, et al., 1959
McDonald, R.A.; Shrader, S.A.; Stull, D.R., Vapor Pressures and Freezing Points of 30 Organics, J. Chem.
Eng. Data, 1959, 4, 311. [all data]
Teja and Rosenthal, 1991
Teja, A.S.; Rosenthal, D.J., The critical pressures and temperatures of ten substances using a low
residence time flow apparatus in Experimental Results for Phase Equilibria and Pure Component
Properties, DIPPR DATA Series No. 1, 1991. [all data]
Anselme and Teja, 1990
Anselme, M.J.; Teja, A.S., The critical properties of rapidly reacting substances, AIChE Symp. Ser.,
1990, 86, 279, 128-32. [all data]
Kim, Svendsen, et al., 2008
Kim, Inna; Svendsen, Hallvard F.; Børresen, Eli, Ebulliometric Determination of Vapor-Liquid Equilibria
for Pure Water, Monoethanolamine, N -Methyldiethanolamine, 3-(Methylamino)-propylamine, and Their
Binary and Ternary Solutions, J. Chem. Eng. Data, 2008, 53, 11, 2521-2531,
https://doi.org/10.1021/je800290k . [all data]
You might also like
- Generalized Rackett-Type Correlations To Predict The Density of Saturated Liquids and Petroleum FractionsDocument14 pagesGeneralized Rackett-Type Correlations To Predict The Density of Saturated Liquids and Petroleum FractionsLizeth RamirezNo ratings yet
- Thelma G. Manning and Zafar Iqbal - Polymeric Nitrogen Stabilized On Carbon Nanotubes: A Highly Energetic, Green ExplosiveDocument13 pagesThelma G. Manning and Zafar Iqbal - Polymeric Nitrogen Stabilized On Carbon Nanotubes: A Highly Energetic, Green ExplosiveYsam2No ratings yet
- Tutorial Lle Ii-1920Document5 pagesTutorial Lle Ii-1920Muhammad HazwanNo ratings yet
- DESUPERHEATERDocument4 pagesDESUPERHEATERghkashyap1No ratings yet
- Standard ReferenceDocument8 pagesStandard Referenceapi-3828258No ratings yet
- 2 Butanone PropertiesDocument7 pages2 Butanone PropertiesRāmji ParvathinaNo ratings yet
- Experimental Study of The Binary Nb-Te System (LIMA Et. Al., 2019)Document9 pagesExperimental Study of The Binary Nb-Te System (LIMA Et. Al., 2019)Joao Pedro AlmeidaNo ratings yet
- Differential Thermal Analysis (DTA) and Differential Scanning CalorimetryDocument27 pagesDifferential Thermal Analysis (DTA) and Differential Scanning CalorimetryAgustynho MagimbaNo ratings yet
- Psychrometric Data PDFDocument9 pagesPsychrometric Data PDFWolveringClaydermanNo ratings yet
- 1973 - Das - Reed - Eubank - PVT Surface and Thermodynamic Properties of N-ButaneDocument10 pages1973 - Das - Reed - Eubank - PVT Surface and Thermodynamic Properties of N-ButaneAlexanderNo ratings yet
- 1973 - Das - Reed - Eubank - PVT Surface and Thermodynamic Properties of Isobutane PDFDocument10 pages1973 - Das - Reed - Eubank - PVT Surface and Thermodynamic Properties of Isobutane PDFAlexanderNo ratings yet
- CCCBDB Listing of Experimental Data Page 2Document1 pageCCCBDB Listing of Experimental Data Page 2Amir ChNo ratings yet
- Melting, Boiling, Triple, and Critical Points of The ElementsDocument3 pagesMelting, Boiling, Triple, and Critical Points of The ElementsantonioNo ratings yet
- Propiedades Físicas, Hidrocarburos (GPSA)Document8 pagesPropiedades Físicas, Hidrocarburos (GPSA)David EscobarNo ratings yet
- V005t15a026 98 GT 347Document11 pagesV005t15a026 98 GT 347Zaidi IsmailNo ratings yet
- Reactor Design With Matlab in A Manufacturing EnvironmentDocument11 pagesReactor Design With Matlab in A Manufacturing Environmentமுத்துக்குமார் சிவகாமி0% (1)
- Meyer p566-577 01Document12 pagesMeyer p566-577 01mauricio rojas alvarezNo ratings yet
- Nuclear Engineering and Design: A B C B DDocument9 pagesNuclear Engineering and Design: A B C B DDevia GahanaNo ratings yet
- B.E. Poling, J.M. Prausnitz, J.P. O'Connell, 'The Properties of Gases and Liquids' 5ht Ed. Property Data Bank. Appendix ADocument61 pagesB.E. Poling, J.M. Prausnitz, J.P. O'Connell, 'The Properties of Gases and Liquids' 5ht Ed. Property Data Bank. Appendix AIsaac A Vazquez MedranoNo ratings yet
- Viscosidad Inorganicos PDFDocument8 pagesViscosidad Inorganicos PDFJuan Carlos VazquezNo ratings yet
- Koilgas Standings SPE7905Document3 pagesKoilgas Standings SPE7905JeffGreenNo ratings yet
- 1977 - Das - Reed - Eubank - PVT Surface and Thermodynamic Properties of IsopentaneDocument7 pages1977 - Das - Reed - Eubank - PVT Surface and Thermodynamic Properties of IsopentaneAlexanderNo ratings yet
- A Generalized Set of Correlations For Plus Fraction Characterization, JAMIALAHMADI Mohamad, 2012Document9 pagesA Generalized Set of Correlations For Plus Fraction Characterization, JAMIALAHMADI Mohamad, 2012joreliNo ratings yet
- Govpub C13Document130 pagesGovpub C13st.shenppNo ratings yet
- Thermophysical Properties of Propane, National Bureau of Standars.Document256 pagesThermophysical Properties of Propane, National Bureau of Standars.Ezra E. Rangel AcedoNo ratings yet
- Hydroquinone:: C: 110.1106 Iupac Standard InchiDocument6 pagesHydroquinone:: C: 110.1106 Iupac Standard InchinurdiyaNo ratings yet
- Preparation, Crystal Structure and Enhanced Bipolar Response of 0.90BLNT-0.10BCT Lead-Free PiezoceramicsDocument5 pagesPreparation, Crystal Structure and Enhanced Bipolar Response of 0.90BLNT-0.10BCT Lead-Free Piezoceramicshéma tologieNo ratings yet
- CCMT Crystal Structure - Vm2096Document4 pagesCCMT Crystal Structure - Vm2096Uttam PawarNo ratings yet
- Isobaric Vapor Liquid Equilibria of The Water 2-Propanol System at 30, 60, and 100 KpaDocument4 pagesIsobaric Vapor Liquid Equilibria of The Water 2-Propanol System at 30, 60, and 100 KpaRafael HenriqueNo ratings yet
- Thermal Analysis of Fuel + Core Temperature Distributions: - Fall 2010 Problem Set 4Document4 pagesThermal Analysis of Fuel + Core Temperature Distributions: - Fall 2010 Problem Set 4nayeemNo ratings yet
- Thermal Dissociation Kinetics of SolventsDocument7 pagesThermal Dissociation Kinetics of SolventsaccofaceNo ratings yet
- 1992 BenderDocument12 pages1992 BenderJohn PACHON MORALESNo ratings yet
- Chemrj 2017 02 02 113 115Document3 pagesChemrj 2017 02 02 113 115editor chemrjNo ratings yet
- November 1987: ASTM International 100 Barr Harbor Drive West Conshohocken, PA 19428-2959Document214 pagesNovember 1987: ASTM International 100 Barr Harbor Drive West Conshohocken, PA 19428-2959eduardo.ald1213No ratings yet
- Je000301o PDFDocument20 pagesJe000301o PDFRLNo ratings yet
- Fe-Nb-Ni (Iron-Niobium-Nickel) : Binary SystemsDocument4 pagesFe-Nb-Ni (Iron-Niobium-Nickel) : Binary Systemsabdul basitNo ratings yet
- Physical Properties of Petroleum Fractions: Appendix LDocument9 pagesPhysical Properties of Petroleum Fractions: Appendix LMeetu KaurNo ratings yet
- Compare Nagata & KameiDocument5 pagesCompare Nagata & KameiPhượng NguyễnNo ratings yet
- Thermal AnalysisDocument28 pagesThermal AnalysisYuppie RajNo ratings yet
- Thermal Decomposition of Tetrazene at 90Document22 pagesThermal Decomposition of Tetrazene at 90Omar valdesNo ratings yet
- Comparison of Power Number For Paddle Type Impellers by Three Methods-UnlockedDocument4 pagesComparison of Power Number For Paddle Type Impellers by Three Methods-UnlockedBánh Cuốn Tôm ThịtNo ratings yet
- Tutorial Data AnalysisDocument4 pagesTutorial Data Analysisshuhui383838No ratings yet
- Kinetics and Fixed-Bed Reactor Modeling of Butane Oxidation Maleic AnhydrideDocument9 pagesKinetics and Fixed-Bed Reactor Modeling of Butane Oxidation Maleic AnhydrideMario Garcia MarquezNo ratings yet
- Thermal Expansion of Anatase and Rutile Between 300 and 575 K Using Synchrotron Powder X-Ray DiffractionDocument7 pagesThermal Expansion of Anatase and Rutile Between 300 and 575 K Using Synchrotron Powder X-Ray Diffractionmalika mudaliarNo ratings yet
- The Neutron Structure of and Thermal Motion in 7-Aminobutyrlc Acid (GABA) at 122 KDocument7 pagesThe Neutron Structure of and Thermal Motion in 7-Aminobutyrlc Acid (GABA) at 122 KGabrielNo ratings yet
- Robie and Hemingway PDFDocument464 pagesRobie and Hemingway PDFtermmasterNo ratings yet
- An Examination of The Thermal ExpansionDocument2 pagesAn Examination of The Thermal Expansionmap vitcoNo ratings yet
- Dehydrogenation of Butan-8-01 On Zinc Oxide Catalyst: Continuous Stirred Tank Reactor StudyDocument6 pagesDehydrogenation of Butan-8-01 On Zinc Oxide Catalyst: Continuous Stirred Tank Reactor StudyRuben GonzalezNo ratings yet
- POLAND For ACE InsercjaDocument7 pagesPOLAND For ACE Insercjamaracaibo35100% (2)
- Vapor-Liquid Equilibrium Measurements For The BinaDocument9 pagesVapor-Liquid Equilibrium Measurements For The BinaMariaFernandaHernandezZuñigaNo ratings yet
- D 3588 - 98 R03 Rdm1odgDocument9 pagesD 3588 - 98 R03 Rdm1odginoveita24No ratings yet
- Conductividad Termica Inorganicos PDFDocument7 pagesConductividad Termica Inorganicos PDFGerardoMgNo ratings yet
- Vapor-Liquid Equilibria in The Nitrogen + Carbon Dioxide + Propane System From 240 To 330 K at Pressures To 15 MpaDocument6 pagesVapor-Liquid Equilibria in The Nitrogen + Carbon Dioxide + Propane System From 240 To 330 K at Pressures To 15 MpadynamischmystischerbussardNo ratings yet
- 1992 AbhariDocument10 pages1992 AbhariMuhammad SaqlainNo ratings yet
- Miyashita1977 PiridiniumDocument3 pagesMiyashita1977 PiridiniumROCIO ISABEL RAMIREZ PANTINo ratings yet
- Chapter 4Document4 pagesChapter 4hendik suwotoNo ratings yet
- Heats of Combustion ReportDocument8 pagesHeats of Combustion ReportNikoNo ratings yet
- The Equation State of AmmoniaDocument34 pagesThe Equation State of AmmoniaempanadaNo ratings yet
- Viscosity Liq InorgDocument3 pagesViscosity Liq InorgCristian GallegoNo ratings yet
- Neopentane: Jump To Navigation Jump To SearchDocument5 pagesNeopentane: Jump To Navigation Jump To SearchGood GuyNo ratings yet
- Decay Heat CalculationDocument12 pagesDecay Heat CalculationUdit OjhaNo ratings yet
- Photochemistry – 6: Plenary Lectures Presented at the Sixth International Symposium on Photochemistry, Aix-En-Provence, France, 19-23 July, 1976From EverandPhotochemistry – 6: Plenary Lectures Presented at the Sixth International Symposium on Photochemistry, Aix-En-Provence, France, 19-23 July, 1976A. GilbertNo ratings yet
- Floating Roof Tank - Design PDFDocument2 pagesFloating Roof Tank - Design PDFlinustec100% (5)
- Test Bank For Microbiology An Introduction 11Th Edition Tortora Funke Case 0321733606 9780321733603 Full Chapter PDFDocument33 pagesTest Bank For Microbiology An Introduction 11Th Edition Tortora Funke Case 0321733606 9780321733603 Full Chapter PDFlisa.seeholzer270100% (10)
- GalvanizingDocument4 pagesGalvanizingAlsonChinNo ratings yet
- E-D-07-01 - Fire Detection - 0-0m PDFDocument1 pageE-D-07-01 - Fire Detection - 0-0m PDFspeaker_john-1No ratings yet
- Section-4 Concrete WorksDocument35 pagesSection-4 Concrete Worksery achjariNo ratings yet
- Canning EquipmentDocument1 pageCanning EquipmentDhias Cahya HakikaNo ratings yet
- Dissolved Oxygen MeasurementDocument5 pagesDissolved Oxygen Measurementraju1559405No ratings yet
- Final-Module 5-The AlkynesDocument5 pagesFinal-Module 5-The Alkynesjohncarlodc99No ratings yet
- Ethyl Benzene Plant DesignDocument45 pagesEthyl Benzene Plant DesignfaridzawiNo ratings yet
- Use of Rapid Prototyping For Rapid Tooling - PPTDocument17 pagesUse of Rapid Prototyping For Rapid Tooling - PPTSudhanwa KulkarniNo ratings yet
- ATEX User Guide: Addendum To The System's User Guide For Installation in Hazardous AreasDocument28 pagesATEX User Guide: Addendum To The System's User Guide For Installation in Hazardous AreasrehanNo ratings yet
- Electrolysis SummaryDocument1 pageElectrolysis SummaryrgblackmanNo ratings yet
- Appendix C12 The Teas Graph 2014 Cleaning With SolventsDocument4 pagesAppendix C12 The Teas Graph 2014 Cleaning With SolventsMenee Love U TooNo ratings yet
- Sample Psychrometrics and Basic Hvac System Calculations Study Problems For Hvacr ExamDocument23 pagesSample Psychrometrics and Basic Hvac System Calculations Study Problems For Hvacr ExamAhmed Ebrahim100% (1)
- Student Notes: Hema 1: Davao Doctors College Medical Laboratory Science DepartmentDocument8 pagesStudent Notes: Hema 1: Davao Doctors College Medical Laboratory Science DepartmentJym TampusNo ratings yet
- Oil Management PDFDocument5 pagesOil Management PDFBhushan VermaNo ratings yet
- Sewage Fungus - A Field and Microscopic Guide (Version 3)Document33 pagesSewage Fungus - A Field and Microscopic Guide (Version 3)Chris WestNo ratings yet
- AeroShell 41Document3 pagesAeroShell 41chandan sahooNo ratings yet
- NCSE 2010 Integrated ScienceDocument21 pagesNCSE 2010 Integrated Sciencevinita_9574233% (3)
- 6 2017 01 21!08 16 33 PMDocument4 pages6 2017 01 21!08 16 33 PMValeria HornaNo ratings yet
- Tig200ac DCDocument41 pagesTig200ac DCTallerSoldaduraAluminioInoxidableNo ratings yet
- Tyson 1999Document5 pagesTyson 1999Darwin BurgosNo ratings yet
- Elasticity BoardworksDocument27 pagesElasticity BoardworksMurugan.SubramaniNo ratings yet
- Salt Analysis ProcedureDocument8 pagesSalt Analysis ProcedureIzuku MidoriaNo ratings yet
- Jsci Ppt0202 BilDocument68 pagesJsci Ppt0202 Bilherculescast0852No ratings yet
- Abstract (Replacement of Cement With Glass Powder in Concrete)Document9 pagesAbstract (Replacement of Cement With Glass Powder in Concrete)pawan kumar100% (5)
- Ejhg 201324Document7 pagesEjhg 201324sssssNo ratings yet
- V RV Ambient Air Vaporizer Data SheetDocument3 pagesV RV Ambient Air Vaporizer Data SheetGilberto Yoshida100% (1)