Professional Documents
Culture Documents
Gastrointestinal Physiology and Nutrition in Wild Birds
Gastrointestinal Physiology and Nutrition in Wild Birds
Uploaded by
Layna RiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Gastrointestinal Physiology and Nutrition in Wild Birds
Gastrointestinal Physiology and Nutrition in Wild Birds
Uploaded by
Layna RiCopyright:
Available Formats
Proceedings of the Nutrition Society (1997), 56, 1049-1056 1049
Gastrointestinal physiology and nutrition in wild birds
BY GARY E. DUKE
Department of Veterinary PathoBiology, University of Minnesota, St Paul, MN 55126, USA
ANATOMY OF GASTROINTESTINAL TRACT
The avian digestive system clearly demonstrates evolutionary adaptations which reduce
body mass to improve efficiency of flying. Teeth and heavy jaw muscles and bones seen in
mammals and reptiles have been replaced by much lighter beak, jaw bones and jaw
muscles. Because birds to not chew their food, the oesophagus is large in diameter to
accomodate large items. The heavy muscular stomach (gizzard) takes over the function of
teeth in mechanical digestion of ingesta. The main mass of birds, the breast muscles and
viscera are ‘suspended’ under the wings for flying efficiency.
The tongue and beak are used for food manipulation and exhibit many interesting
adaptations in birds (McLelland, 1979). There is no soft palate in most birds and the hard
palate connects with the nasal cavities via a median slit.
Salivary glands are poorly developed in species that ingest aquatic food and well
developed in those eating dry food. The glands of some species (e.g. house sparrow (Passer
domesticus)) contain appreciable amounts of amylase (EC 3.2.1. l), but those of domestic
chickens and turkeys do not. Feeding stimulates salivary secretion. Birds have taste buds
and their location and density varies between species (Duke, 1986).
Birds have relatively long necks because their beak must serve the function of hands or
paws in food gathering, preening, etc. Their oesophagus is, therefore, also relatively long.
It has numerous mucous glands to help lubricate the passage of food. The crop is an
enlargement of the oesophagus and it serves to store food for subsequent passage to the
stomach. (This is analogous to the cardia in the mammalian stomach.) The size and shape
of crops vary between species. Pigeons have two large lateral pouches, gallinaceous birds
have a single pouch, hawks have a spindle-shaped enlargement of the oesophagus and owls
have no crop (Fig. 1).
The glandular stomach (proventriculus) also varies in size between species, being
relatively small in graminivores, but often quite large and distensible in carnivores that
ingest large food items and in ostriches which use it for water storage (Degen et al. 1994).
In most species, however, food passes rapidly through the glandular stomach and is held in
the muscular stomach wherein the actions of the gastric secretions (pepsin (EC 3.4.23.1),
HCl, and mucus) occur. The muscular stomach is complex in most species (Fig. 1) and
consists of two pairs of opposing muscles called the ‘thin’ and ‘thick‘ muscle pairs (1-
3 mm and 6-9 mm thick respectively) which consist entirely of smooth muscle. A more
simple muscular stomach is found in birds of prey and heron-like species (Duke, 1986).
There are variations in this general anatomical scheme for the stomach, most notable
being a third chamber below the muscular stomach called the pyloric stomach found in
many aquatic species. It contains feathers and bones usually and is believed to act as a filter
to prevent bones from entering the intestines.
The avian small intestine is histologically divided into only the duodenum and ileum
(Fig. 1). It tends to be longer in herbivores and graminivores than in carnivores,
presumably because plant tissues are more difficult to digest than animal tissues. The small
https://doi.org/10.1079/PNS19970109 Published online by Cambridge University Press
1050 G . E. DUKE
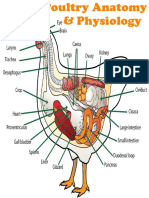
Fig. 1. Gastrointestinal tracts of: (a), domestic turkey; (b), great-homed owl (Bubo virginiunus); and (c), red-tailed
hawk (Buteo jumaicensis). Included are: (1) precrop oesophagus, (2) crop, (3) post-crop oesophagus, (4) glandular
stomach, (5) isthmus, (6) thin craniodorsal muscle, (6a) muscular stomach of raptors, (7) thick cranioventral muscle, (8)
thick caudodorsal muscle, (9) thin caudoventral muscle, (6-9) muscular stomach, (10) proximal duodenum, (1 1)
pancreas, (12) distal duodenum, (13) liver, (14) gall bladder, (15) ileum, (16) Meckel’s diverticulum, (17) ileo-caeco-
colic junction, (18) caecum, (19) rectum, (20) bursa of Fabricious, (21) cloaca, (22) vent, (Gc) greater curvature. (From
Duke, 1985.)
https://doi.org/10.1079/PNS19970109 Published online by Cambridge University Press
NUTRITION OF WILD AND CAPTIVE WILD ANIMALS 1051
intestine is the principal site of chemical digestion, using enzymes of both intestinal and
pancreatic origin. It is also the principal site of nutrient absorption (Duke, 1986).
About half all avian species have caeca; they are absent, however, in hawks, parrots
and song birds. These are blind pouches originating at the juncture of the small and large
intestines. Several functions have been ascribed to the caeca, but the most important appear
to be participation in water balance, recycling of nitrogenous waste to create amino acids
and fermentation of fibre. In view of these functions, it is difficult to deduce what
evolutionary pressure led to their presence in owls but not in hawks. They do seem to be
important in water conservation in great-horned owls (Bubo virginianus; Duke et al. 1981).
The rectum is relatively short in most species and it is primarily involved in water
absorption from the indigesta and from urine which is passed orally from the ureters
(terminating in the cloaca) and in elimination of the indigesta.
GASTROINTESTINAL TRACT MOTILITY
Avian gastrointestinal contractile activity, i.e. motility, in those species with the thin and
thick muscles is unique relative to mammals. The thin and thick muscles contract
alternately; first the thin muscle pair contracts to push ingesta into the centre of the
muscular stomach, then the thick muscle pair contracts to grind the ingesta. Grit aids in this
grinding. This action is analogous to the cheeks and jaws of mammals. Gastric motility in
those avian species lacking the thin and thick muscles is similar to that of mammals with a
simple stomach, i.e. a contraction starts at the pylorus and proceeds around the greater
curvature to mix the gastric contents (Duke, 1989).
Small intestinal rejiux
Small intestinal motility is similar to that in mammals, except for the occurrence of small
intestinal refluxes. This is a contractile pattern that is totally unique to birds and involves a
periodic reverse peristalsis which moves virtually all the contents of the upper ileum and
duodenum back into the muscular stomach. The latter organ is simultaneously relaxed to
permit entry of the duodenal contents. These refluxes have been observed via image
intensification radiology in all species thus far examined, but they occur at different
frequencies between species. Leach’s storm petrel (Oceanodroma leuchorhoa) chicks
eating very-high-fat diets had approximately eight refluxes per h postprandially (Duke et
al. 1989). Raptors (i.e. birds of prey) had one to two per h (Duke et al. 1976) on a diet of
mice. Turkeys on standard poultry rations typically had two to three refluxes per h (Dziuk
& Duke, 1972; Duke et al. 197%). Only one small intestinal reflux was observed in cedar
waxwings (Bombycilla cedorum) during approximately 25 h of observation (Levey &
Duke, 1992). Similarly, only one was seen in ostriches (Struthio camelus) during 12 h of
observation (Degen et al. 1994). These observations led me to wonder what stimulated the
evolutionary development of small intestinal refluxes in birds.
Since birds have high metabolic rates, they must find, digest and metabolize more
nutrients per d to maintain normal activity, reproduction, etc. when compared with other
vertebrates. One way to achieve this increased digestive capacity would be to increase the
size of the gastrointestinal tract, a disadvantage to a flying animal because of added weight.
A second way would be to increase foraging time. Increased effort in foraging, however,
exposes birds to increased potential for predation. To reduce exposure to the latter risk and
also to reduce the volume of the gastrointestinal tract required to digest larger quantities of
food, the use of the same part of the gastrointestinal tract more than once in the digestion of
https://doi.org/10.1079/PNS19970109 Published online by Cambridge University Press
1052 G. E. DUKE
one meal would be an evolutionarily-wise response. A high nutrient density (such as in
prepared rations for poultry) or a high-fat diet (as eaten by petrels) would be more difficult
to digest than more simple diets and be more likely to lead to evolutionary development of
small intestinal reflux activity, i.e. the greater the small intestinal reflux frequency, the
greater would be the digestibility.
CARBOHYDRATE DIGESTION
The digestive strategies for handling the three classes of nutrients varies. More is known
about carbohydrate digestion and more carbohydrate sources (leaves, seeds, fruits, etc.)
have been tested than high-fat or protein foods.
Fibre
Many species eat mainly carbohydrate diets, but they employ different digestive strategies.
Even within similar species with similar diets, different strategies can be seen. If we
compare ratites, ostriches have relatively large caeca and a very long colon, both involved
in fermentation of plant fibre. They are also coprophagic. Through this combination of
strategies they achieve approximately 40-60 % digestibility of their diet of grasses, leaves
and occasional insects or lizards (Swart et al. 1993; Angel, 1994). Emus (Dromius
novaehollandiae) have relatively much shorter caeca and colon, but, they have a very long
small intestine. Their mainly carbohydrate diet of tender shoots, seeds, fruits and insects is
nutrient rich and readily digestible in the small intestine (Dawson & Herd, 1983).
The hoatzin (Opisthocomus hoazin) is folivorous but uses foregut fermentation (Grajal,
1995). The crop is enlarged and the oesophagus is elongated; together they account for
77 % of the total gut capacity. Both organs are quite muscular and both maceration and
fermentation occur therein. Approximately 60-80 % digestibility is achieved from a very
poor, coarse diet. Gallinaceous birds such as chickens, turkeys and ring-necked pheasants
(Phasianus colchicus) tend to eat more seeds and less foliage than ratites or the hoatzin,
and they achive 60-70% digestibility. Hindgut fermentation in these species can add an
extra 4-1 1 % in diet digestibility (Duke, 1986).
Poultry are generally not considered to be able to ‘digest’ fibre. However, if they are
pre-conditioned to a high-fibre diet before digestibility is tested, their digestibilities
improve. Domestic turkeys fed on either 28 or 161g oat hullskg in isoenergetic diets
labelled with 14C-labelledcellulose were only able to digest 2.3 % of it (as determined by
collecting 14C02in exhaled air) when fed on the diet containing 28g cellulosekg for 6
weeks, but digested 10.4 96 of the cellulose (on average) after eating the diet containing
161g cellulosekg (Duke et al. 1984).
Nectar
Another carbohydrate source used mainly by hummingbirds and passerines is nectar. In
feeding trials, hummingbirds were found to prefer sucrose over glucose, fructose or a
mixture of glucose and fructose. While all three sugars are highly assimilated by
hummingbirds (greater than 97 %), glucose was assimilated more slowly (birds rested
longer between feeding bouts), thus making a glucose diet less efficient (Martinez Del Rio,
1990~).Hummingbirds have very high rates of intestinal sucrose hydrolysis and glucose
transport (Martinez Del Rio, 1990~).A comparison of intestinal sucrase (EC 3.2.1.48) and
maltase (EC 3.2.1.20) activities between three species of hummingbirds and nine species of
https://doi.org/10.1079/PNS19970109 Published online by Cambridge University Press
NUTRITION OF WILD AND CAPTIVE WILD ANIMALS 1053
fruit-eating passerines that had significant intestinal sucrase activity indicated that
intestinal sucrase was between two times and 118 times higher in hummingbirds (Martinez
Del Rio, 1990b). Thus, these birds are highly adapted to specific carbohydrate diets.
Martinez Del Rio (1990~)found that the nectar secreted by flowers preferred by
hummingbirds is rich in sucrose, whereas, nectar secreted by flowers preferred by
passerines contains a mixture of glucose and fructose.
Fruits
A third diet high in carbohydrates and eaten by many species is fruits. Most fruit-eating
birds consume sizable quantities daily, absorb the sugars and defaecate the pulp, gaining
little nutrient value from it. Cedar waxwings (Bombycilla cedorum) eating artificial fruits
(sphere-shaped, red-dyed agar with 150g glucosekg and a plastic bead as a ‘seed’) were
observed radiographically (Levey & Duke, 1992). Typically two or three ‘fruits’ were
eaten at once and passed into the gizzard within 3 min (on dissection, this species does not
appear to have a crop, but the seeds were held in the upper oesophagus). The pulp and
seeds were separated within seconds by contractions of the muscular stomach and the pulp
was emptied into the intestine within about 8min. The seeds remained in the musclar
stomach for about 30min; however, once they were passed from the stomach they moved
through the small intestine in less than 10min. Pulp appeared to be ‘pushed’ through the
intestine by the seeds. Both pulp and seeds remained in the rectum for several minutes.
This presumably allowed absorption of nutrients not absorbed in the small intenstine before
defaecation of the pulp. Active absorption of D-glucose was found to be high in the rectum
(Levey & Duke, 1992). In previous studies it was found that glucose absorption averaged
92% on this diet (Karasov & Levey, 1990), thus processing and absorption are very
efficient. Absorption of a large proportion of ingested sugars is typical of most frugivores
(e.g. Karasov & Levey, 1990).
Digestion is complicated by seasonal changes in diet in some birds. American robins
(Turdus migratorius) switch from eating fruits in the autumn to insects in the spring (Levey
& Karasov, 1992). This switch is accompanied by changes in digestive enzyme secretions
from the intestinal epithelium and appears to be seasonally induced (probably by
photoperiod) rather than in response to diet. In some cases, seasonal dietary changes
involve changes in volume eaten due to the bulk of the new diet (Walsberg & Thompson,
1990) or due to eating more in response to a lower environmental temperature (Dykstra &
Karasov, 1992). So, gut structure, size and function may all change seasonally.
Chitin
One last type of carbohydrate eaten by many birds is chitin, one of the most abundant
biopolymers on earth. In feeding trials with six species of seabirds, sooty albatross
(Phoebetria fusca), white-chinned petrels (Procellaria aequinotialis), Leach’s storm
petrels, rockhopper penguins (Eudyptes chlysocome), gentoo penguins (Pygoscelis papua)
and king penguins (Aptenodytes patagonicus) digestibilities of 35-84% were observed
(average 50.6 %; Jackson et al. 1992).
PROTEIN DIGESTION
Since chitin provides the exoskeleton of all arthropods, birds eating chitin are also very
likely to be eating protein from the muscles, internal organs, etc. of the arthropods. Protein
https://doi.org/10.1079/PNS19970109 Published online by Cambridge University Press
1054 G. E. DUKE!
is essential for growth and development in all young birds and for maintenance and
replacement in adult birds. Thus, all birds are capable of digesting some protein; however,
protein makes up most of the diet in carnivorous birds.
Birds eating high-protein diets generally have less complicated digestive systems than
those eating complex carbohydrates (Fig. 1). The muscular stomach of raptors and fish-
eating birds (e.g. herons and penguins; McLelland, 1979; Duke, 1985) is simple, without
the thin and thick pairs of muscles (described previously). Although hawks have a poorly
developed crop, it does function in food storage postprandially. Owls have no crop so
swallowed food passes immediately into the glandular stomach.
Gastric digestion
Four phases of gastric digestion can be observed in great-horned owls: (1) filling of the
stomach characterized by high amplitude and moderately-high-frequency contractions (1-
1.5 per min); (2) chemical digestion which is a long (4-8 h) period of very-low-amplitude,
low-frequency contractions (0-1 per min); (3) fluid evacuation which occurs over a 1-2 h
period during which contractile activity slowly increases to about 1.5 contractions per min;
(4) pellet egestion which involves very-high-amplitude and high-frequency contractions (2
per min) for 4-10 min followed by pellet egestion during which vigorous oesophageal anti-
peristalsis moves the pellet from the stomach to the mouth in about 8-12s (Kostuch &
Duke, 1975; Duke et al. 1976).
During gastric digestion, the pH of gastric contents averaged 1.6 (range 1-3-1.8) in
samples taken orally from five species of falconiforms and 2.35 (range 2.2-2.5) in samples
from two species of owls (Duke et al. 197%). The pellets of falconiforms contain virtually
no bones from their prey, while those from owls contain nearly all the bones of their prey.
Using mouse bones cleaned from mouse carcasses by dermested beetles, it was found that a
solution of HC1 at pH 1.6 at 40" entirely dissolved the bones overnight. Gastric proteolytic
activity was found to be similar between the two orders of raptors. Food metabolizability
averaged approximately 60 % in great-homed owls eating a mouse diet.
Pellet egestion
The single most unique gastrointestinal event occurring in raptors and many other avian
carnivores is pellet egestion. Pellets are formed in the stomach from the undigested remains
of hair (falconiforms) or hair and bones (owls). This oral egestion avoids having to digest
bones and hair and pass them through the entire gastrointestinal tract. The physiological
mechanism of pellet egestion differs from vomition in mammals with simple stomachs and
from regurgitation of the cud in ruminants. Implanted strain-gauge transducers or bipolar
electrodes on the oesophagus, muscular stomach and on the abdominal muscles indicated
that at about 12min before egestion, vigorous gastric contractions formed the pellet and
pushed it into the lower oesophagus. Only one gastric contraction precedes vomition and
regurgitation of the cud. The pellet was moved orally by oesophageal anti-peristalsis,
which occurs during cud regurgitation, but not in vomiting. This orally-directed movement
of the pellet required 8-10s to move the pellet to the mouth. No abdominal muscle
contractions occurred in owls as they do during vomiting (Duke et al. 1976).
Small intestinal refluxes and colonic anti-peristalsis (Dzuik, 1971; Duke, 1985) have
been observed in raptors, the former presumably increases digestion and the latter enhances
water balance.
https://doi.org/10.1079/PNS19970109 Published online by Cambridge University Press
NUTRITION OF WILD AND CAPTIVE WILD ANIMALS 1055
In owls, meal-to-pellet intervals vary between species and with meal size. Meal-to-
pellet interval is shorter for smaller owls and for relatively smaller meals in all species. In
contrast, the falconiforms tend to egest pellets at dawn unless meals are eaten late on the
previous day. Great-homed owls and most other owls may hunt during both daylight and
darkness, whereas falconiforms hunt strictly during daylight. Thus, egesting at dawn would
be advantageous because by emptying the stomach early in the day, a new meal may be
ingested as soon as it can be captured. If the first meal is small, one or more additional
meals may be eaten before darkness.
FAT DIGESTION
Most animals which eat meat are incidently eating fat; similarly frugivores consume many
fruits which contain lipid. Many avian species periodically seek high-fat diets in order to
increase their levels of body fat in preparation for migration or in anticipation of food
scarcity. However, some birds eat very-high-fat diets routinely; the best examples are
seabirds. Seabirds are uniquely able to assimilate wax esters at efficiencies greater than
90 % (Jackson & Place, 1990); efficiencies in mammals are less than 50 %. Marine foods
available to seabirds have a high lipid content as an energy store, for buoyancy or for
insulation in marine mammals (eaten as carrion). This high efficiency is accomplished by:
(1) a comparatively very high intestinal bile salt concentration to aid lipid digestion; and
(2) regular small intestinal refluxes which repeatedly expose ingesta to pancreatic and
duodenal lipases and bile (Duke et al. 1989). Radiographic studies which disclosed the
high frequency of refluxes (about eight per h) also allowed observation of how the high-fat
meal was processed in the gastrointestinal tract of Leach's storm petrel chicks. Ingesta
accumulated in the glandular stomach and eventually separated into two phases, a lipid
phase floating on an aqueous phase. The latter phase, presumably containing amino acids
(from digested protein) and small amounts of lipid, regularly emptied from the stomach
into the duodenum.
Three terrestrial species are efficient also at digesting lipids and they consume high-
lipid diets. The lesser honey guide (Indicator minor) which consumes wax esters in
honeycomb, and yellow-rumped warblers (Dendroicu coronata) and tree swallows
(Tuchycinetu bicolor) which eat fruits with a waxy coating (Place & Stiles, 1992).
Virtually all organic matter on earth (and some inorganic ions) serves as food for some
species of animal. By fitting into a myriad of niches, birds have taken advantage of this
organic matter to meet their energy and protein needs. The feeding and digestive
mechanisms they use are just part of what makes them interesting to man.
REFERENCES
Angel, C. R. (1994). Age changes in digestibility of nutrients in ostriches. American Ostrich June issue, 2 6 3 1 .
Dawson, T. J. & Herd, R. M. (1983). Digestion in the Emu: Low energy and nitrogen requirements of this large
ratite bird. Comparative Biochemistry and Physiology 75A, 4 1 4 3 .
Degen, A. A., Duke, G . E. & Reynhout, J. K. (1994). Gastroduodenal motility and glandular stomach function in
young ostriches. Auk 111, 750-755.
Duke, G . E. (1985). Raptor physiology. In Zoo and Wildlife Medicine, 2nd ed., pp. 370-376 [M. E. Fowler,
editor]. Philadelphia: W. B. Saunders Co.
Duke, G . E. (1986). Alimentary canal: anatomy, regulation of feeding and motility. In Avian Physiology, 4th ed.,
pp. 269-288 [P. D. Sturkie, editor]. New York Springer-Verlag.
Duke, G. E. (1989). Avian gastrointestinal motor function. In Handbook of Physiology - The Gastrointestinal
System, Part 2, pp. 1283-1300 [J. D. Wood, editor]. Bethesda, MD: American Physiological Society.
Duke, G . E., Bird, J. E., Daniels, K. A. & Bertoy, R. W. (1981). Food metabolizability and water balance in
intact and cecectomized Great-Horned Owls. Comparative Biochemistry and Physiology 68A, 237-240.
https://doi.org/10.1079/PNS19970109 Published online by Cambridge University Press
1056 G. E. DUKE
Duke, G. E., Eccelstein, E., Kirkwood, S., Louis, C. F. & Bedbury, H. P. (1984). Cellulose digestion by domestic
turkeys fed low or high fiber diets. Journal of Nutrition 114, 95-102.
Duke, G. E., Evanson, 0. A., Redig, P. T. & Rhoades, P. T. (1976). Mechanism of pellet egestion in Great-
Homed Owls (Bubo virginianus). American Journal of Physiology 231, 1824-1829.
Duke, G. E., Jegers, A. A., Loff, G . & Evanson, 0. A. (1975~).Gastric digestion in some raptors. Comparative
Biochemistry and Physiology 15A, 649-656.
Duke, G. E., Kostuch, T. E. & Evanson, 0. A. (19756). Electrical activity and intraluminal pressure changes in
the lower small intestine of turkeys. American Journal of Digestive Diseases 20, 1040-1046.
Duke, G. E., Place, A. R. & Jones, B. (1989). Gastric emptying and gastrointestinal motility in Leach’s Storm-
Petrel chicks (Oceanodromu leuchorhoa). Auk 106, 80-85.
Dyksua, C. R. & Karasov, W. H. (1992). Changes in gut structure and function of House wrens (Troglodytes
aedon) in response to increased energy demands. Physiological Zoology 65, 4 2 2 4 2 .
Dziuk, H. E. (1971). Reverse flow of gastrointestinal contents in turkeys. Federation Proceedings 30, 610.
Dziuk, H. E. & Duke, G. E. (1972). Cineradiographic studies of gastric motility in turkeys. American Journal of
Physiology 222, 159-166.
Grajal, A. (1995). Structure and function of the digestive tract of the Hoatzin (Opisthocomus hoazin): a folivorus
bird with foregut fermentation. Auk 112, 20-28.
Jackson, S. & Place, A. R. (1990). Gastrointestinal transit and lipid assimilation efficiencies in three species of
sub-antarctic seabird. Journal of Experimental Zoology 255, 141-154.
Jackson, S., Place, A. R. & Seiderer, L. J. (1992). Chitin digestion and assimilation by seabirds. Auk 109, 758-
770.
Karasov, W. H. & Levey, D. J. (1990). Digestive system trade-offs and adaptations of frugivorous Passerine
birds. Physiological Zoology 63, 1248-1270.
Kostuch, T. E. & Duke, G. E. (1975). Gastric motility in Great-Homed owls. Comparative Biochemistry and
Physiology 51, 201-205.
Levey, D. J. & Duke, G. E. (1992). How do frugivores process fruit? Gastrointestinal transit and glucose
absorption in Cedar waxwings (Bombycilla cedorum). Auk 109,722-730.
Levey, D. J. & Karasov, W. H. (1992). Digestive modulation in a seasonal frugivore, the American robin
(Turdus migmtorius). American Journal of Physiology 262, G7114718.
McLelland, J. (1979). Digestive system. In Form and Function in Birds, pp. 69-181 [A. S. King and J.
McLelland, editors]. London: Academic Press.
Martinez Del Rio, C . (1990~).Sugar preferences in hummingbirds: The influence of subtle chemical differences
on food choice. Condor 92, 1022-1030.
Martinez Del Rio, C . (1990b). Dietary, phylogenetic, and ecological correlates of intestinal sucrase and maltase
activity in birds. Physiological Zoology 63, 987-101 1.
Place, A. R. & Stiles, E. W. (1992). Living off the wax of the land: Bayberries and Yellow-rumped warblers.
Auk 109, 334-345.
Swart, D., Mackie, R. I. & Hayes, J. P. (1993). Influence of live mass, rate of passage and site of digestion on
energy metabolism and fibre digestion in the Ostrich (Struthio camelus). South African Journal of Animal
Science 23, 119-124.
Walsberg, G. E. & Thompson, C. W. (1990). Annual changes in gizzard size and function in the frugivorous
bird. Condor 92, 794-795.
0Nutrition Society 1997
https://doi.org/10.1079/PNS19970109 Published online by Cambridge University Press
You might also like
- Global HACCP PlanDocument65 pagesGlobal HACCP Plansyed zia ul hassan100% (3)
- SITHCCC013 Assessment 2 - Practical ObservationDocument15 pagesSITHCCC013 Assessment 2 - Practical ObservationajayNo ratings yet
- 1 Digestion in Teleost Fishes - 2Document23 pages1 Digestion in Teleost Fishes - 2Evi Nurul IhsanNo ratings yet
- SCD Food ListDocument4 pagesSCD Food ListAnuj MittalNo ratings yet
- ZaguDocument5 pagesZaguNatalie Jane Martinet100% (2)
- Sandhurst Situational AnalysisDocument35 pagesSandhurst Situational AnalysisIshanShikarkhaneNo ratings yet
- CBR Animal StructureDocument7 pagesCBR Animal StructureArifahNo ratings yet
- Disorders of The Psittacine Gastrointestinal TractDocument21 pagesDisorders of The Psittacine Gastrointestinal TractRebeca GalvãoNo ratings yet
- Rees DaviesDocument15 pagesRees DaviesAaron WoodardNo ratings yet
- The Digestive System of Fowl: VAN Practical AssignmentDocument6 pagesThe Digestive System of Fowl: VAN Practical AssignmentSampreet SaikiaNo ratings yet
- Gastropoda: Gastropoda Temporal Range: Late Cambrian-PresentDocument6 pagesGastropoda: Gastropoda Temporal Range: Late Cambrian-Presentchiluka87No ratings yet
- Da DieuDocument16 pagesDa DieuVu NguyenNo ratings yet
- The Human Vermiform AppendixDocument33 pagesThe Human Vermiform AppendixpawchanNo ratings yet
- Vertebrate DiversityDocument14 pagesVertebrate DiversityKacang PeasNo ratings yet
- Facts About LizardDocument9 pagesFacts About LizardJatin ChaudharyNo ratings yet
- Ross 2000Document40 pagesRoss 2000nicole delgadoNo ratings yet
- Cap. 02 - General Anatomy and Physiology of ReptilesDocument23 pagesCap. 02 - General Anatomy and Physiology of ReptilesNailson JúniorNo ratings yet
- Morphological Comparison of Proventricular and Gizzard in Starling Birds Sturnus Vulgaris and PigeonDocument4 pagesMorphological Comparison of Proventricular and Gizzard in Starling Birds Sturnus Vulgaris and PigeonDr. Suhaib A.H. Al-TaaiNo ratings yet
- Beltzer Et Al - Algunas Ardeidas Del Valle de Inundación Del Río ParanáDocument28 pagesBeltzer Et Al - Algunas Ardeidas Del Valle de Inundación Del Río ParanáAlejandra ArreolaNo ratings yet
- Foraging Strategies of Deep-Sea Fish: Mauchline D. GordonDocument12 pagesForaging Strategies of Deep-Sea Fish: Mauchline D. Gordonfaiz ismaNo ratings yet
- Anatomy DigestiveDocument8 pagesAnatomy DigestiveGalih Kurniawan100% (2)
- Gut MorphologyDocument27 pagesGut MorphologyrezqNo ratings yet
- Roberts T. 2017. Anatony and Physiology of The Digestive Systema of The Oarfish Regalecus Rusellii. Ichtyol Res 64. 475-477Document3 pagesRoberts T. 2017. Anatony and Physiology of The Digestive Systema of The Oarfish Regalecus Rusellii. Ichtyol Res 64. 475-477José Vélez TacuriNo ratings yet
- #2 Chapter IIDocument17 pages#2 Chapter IIAngelyn Bantilo SerronaNo ratings yet
- Oleracea Contain 13.2% Dry Matter, 15.7% Crude Protein, 5.4% Ether ExtractionDocument47 pagesOleracea Contain 13.2% Dry Matter, 15.7% Crude Protein, 5.4% Ether ExtractionJakin Aia TapanganNo ratings yet
- Digestive System of The PoultryDocument9 pagesDigestive System of The Poultrydr.mostafa.alyahyaNo ratings yet
- JejunumDocument3 pagesJejunumspiraldaoNo ratings yet
- Stomach in VertebratesDocument6 pagesStomach in VertebratesTuhinNo ratings yet
- Assignment of Diversity of AnimalDocument6 pagesAssignment of Diversity of AnimalA TNo ratings yet
- Assignment#3-Vertebral ColumnDocument5 pagesAssignment#3-Vertebral ColumnvinlavisoNo ratings yet
- Ratory Rowth, L S Ygmy ,: Labo G Reproduction and Ife Pan of The Pacific P OctopusDocument18 pagesRatory Rowth, L S Ygmy ,: Labo G Reproduction and Ife Pan of The Pacific P OctopusGadisNo ratings yet
- Chicken Anatomy & PhysiologyDocument26 pagesChicken Anatomy & PhysiologySanjeev ChaudharyNo ratings yet
- Finite Element Analysis of Ungulate Jaws Can Mode PDFDocument16 pagesFinite Element Analysis of Ungulate Jaws Can Mode PDFNonoNo ratings yet
- Sustained Mass Culture Amphiascoides Atopus A Marine Harpacticoid Copepod in A Recirculating SystemDocument9 pagesSustained Mass Culture Amphiascoides Atopus A Marine Harpacticoid Copepod in A Recirculating SystemJordan IsmaelNo ratings yet
- Segers 2003 BDocument16 pagesSegers 2003 BEstefania SarangoNo ratings yet
- XXV No.3 112 215 1966 SaleDocument10 pagesXXV No.3 112 215 1966 SaleYoussefNo ratings yet
- Module 7 - Digestive SystemDocument19 pagesModule 7 - Digestive SystemkeziahlibberfNo ratings yet
- Development of The Digestive Tract of MiDocument17 pagesDevelopment of The Digestive Tract of MiLaura IbañezNo ratings yet
- 1 Lect THR 1 Intro MuscaDocument22 pages1 Lect THR 1 Intro MuscaSaadNo ratings yet
- Bird Anatomy: From Wikipedia, The Free EncyclopediaDocument12 pagesBird Anatomy: From Wikipedia, The Free Encyclopediahd_catwolfieNo ratings yet
- GastropodsDocument12 pagesGastropodsLeoregine RodriguezNo ratings yet
- Lily Issa - Bio Fun Fact! - 2Document4 pagesLily Issa - Bio Fun Fact! - 2lilyissa70No ratings yet
- MOLLUSCA 3 GastropodaDocument14 pagesMOLLUSCA 3 GastropodaCARLOSNo ratings yet
- A Novel Amniote Model of Epimorphic Regeneration The Leopard Gecko, Eublepharis MaculariusDocument25 pagesA Novel Amniote Model of Epimorphic Regeneration The Leopard Gecko, Eublepharis MaculariusSandra Clavero ClopèsNo ratings yet
- Aenigmamus (Rodentia: Diatomyidae) С Эволюционной Точки ЗренияDocument16 pagesAenigmamus (Rodentia: Diatomyidae) С Эволюционной Точки ЗренияFildzah AliyahNo ratings yet
- Comparative Vertebrate Anatomy of The Digestive System Source: Http://people - Eku.edu/ritchisong/342notes7.htmlDocument14 pagesComparative Vertebrate Anatomy of The Digestive System Source: Http://people - Eku.edu/ritchisong/342notes7.htmlYeyen M. EvoraNo ratings yet
- Anatomical Adaptations of Aquatic MammalsDocument7 pagesAnatomical Adaptations of Aquatic MammalsArturo CastroNo ratings yet
- Biology I Lecture Outline 6 Digestion & Nutrition: Pages 633 Lab 2Document17 pagesBiology I Lecture Outline 6 Digestion & Nutrition: Pages 633 Lab 2Chessking Siew HeeNo ratings yet
- Topic 8 Digestive SystemDocument10 pagesTopic 8 Digestive SystemMary Ann Morillo TenederoNo ratings yet
- Chickenanatomyphysiology 140119134735 Phpapp02Document30 pagesChickenanatomyphysiology 140119134735 Phpapp02Justine Mae Balodong BumalayNo ratings yet
- Jurnal MamaliaDocument10 pagesJurnal MamaliaClarissa B100% (1)
- 53 Publication Snakes Survive StarvationDocument10 pages53 Publication Snakes Survive StarvationÑiýāž MāłïķNo ratings yet
- Digestive System in ChordatesDocument45 pagesDigestive System in ChordatesDr. Sudesh RathodNo ratings yet
- Comparative Vertebrate Anatomy Lecture Notes - Digestive SystemDocument14 pagesComparative Vertebrate Anatomy Lecture Notes - Digestive SystemMielah Ruth100% (1)
- The Effect of Insoluble Fiber and Intermittent Feeding On Gizzard Development, Gut Motility, and Performance of Broiler ChickensDocument8 pagesThe Effect of Insoluble Fiber and Intermittent Feeding On Gizzard Development, Gut Motility, and Performance of Broiler ChickensAndre FahmiNo ratings yet
- Arthropoda Characteristics: Circulatory SystemDocument15 pagesArthropoda Characteristics: Circulatory SystemBello AjetayoNo ratings yet
- JurnalDocument4 pagesJurnalBunga Giftha NurhamsryNo ratings yet
- EntomologyDocument7 pagesEntomologymoniqueNo ratings yet
- Breeding Biology of Guppy Fish, Poecilia Reticulata: (Peters, 1859) in The LaboratoryDocument9 pagesBreeding Biology of Guppy Fish, Poecilia Reticulata: (Peters, 1859) in The LaboratoryKatamoNo ratings yet
- Breeding Biology of Guppy Fish, Poecilia Reticulata: (Peters, 1859) in The LaboratoryDocument9 pagesBreeding Biology of Guppy Fish, Poecilia Reticulata: (Peters, 1859) in The LaboratorypacifistlightNo ratings yet
- Chapter 11 - Digestive System - (Ramos, Vanny L.)Document14 pagesChapter 11 - Digestive System - (Ramos, Vanny L.)Vanny RamosNo ratings yet
- Gross and Histological Studies of Digestive Tract of Broilers During Postnatal Growth and DevelopmentDocument9 pagesGross and Histological Studies of Digestive Tract of Broilers During Postnatal Growth and DevelopmentyessiesektiNo ratings yet
- Lecture 16 Notes 2013Document4 pagesLecture 16 Notes 2013Richard HampsonNo ratings yet
- Review Article Comparison Anatomy, Physiology, A N D Biochemistry Commonly Used Laboratory AnimalsDocument30 pagesReview Article Comparison Anatomy, Physiology, A N D Biochemistry Commonly Used Laboratory AnimalsJony KechapNo ratings yet
- Horizons in Nutritional Science Carbohydrate BioavailabilityDocument11 pagesHorizons in Nutritional Science Carbohydrate BioavailabilityNurmaNo ratings yet
- Англи Хэл Тест ХариуDocument15 pagesАнгли Хэл Тест Хариуdeegijdeegij34No ratings yet
- Federal Register / Vol. 73, No. 67 / Monday, April 7, 2008 / NoticesDocument11 pagesFederal Register / Vol. 73, No. 67 / Monday, April 7, 2008 / NoticesJustia.comNo ratings yet
- Menu Journal Food Hospitality Research1Document105 pagesMenu Journal Food Hospitality Research1Juliede AlvesNo ratings yet
- Amazing Plants Teachers ManualDocument27 pagesAmazing Plants Teachers ManualSandy Hernandez100% (1)
- Co Health 10 Quarter 1Document147 pagesCo Health 10 Quarter 1Erlinda FabiaNo ratings yet
- Narrative and Pictorial Report On Gulayan Sa PaaralanDocument2 pagesNarrative and Pictorial Report On Gulayan Sa PaaralanJomarie Quirante Ü96% (50)
- Food Safety Inspection Report: Florida Department of Agriculture and Consumer Services Division of Food SafetyDocument15 pagesFood Safety Inspection Report: Florida Department of Agriculture and Consumer Services Division of Food SafetyDavid DworkNo ratings yet
- BOOK Fresh-Cut Tropical Fruits and VegetablesDocument102 pagesBOOK Fresh-Cut Tropical Fruits and VegetablesCléia Barros100% (1)
- Food Preservation MethodsDocument1 pageFood Preservation MethodsBarbara MassetteNo ratings yet
- Aboriginal Bush FoodsDocument23 pagesAboriginal Bush FoodsBeecroft Philip Adam100% (1)
- IES General Studies 2011 PDFDocument28 pagesIES General Studies 2011 PDFKshitij28No ratings yet
- Annotated BibliographyDocument4 pagesAnnotated Bibliographyapi-495053316No ratings yet
- Green Nature Triangles Project General ProposalDocument3 pagesGreen Nature Triangles Project General Proposalapi-540658681No ratings yet
- Food Adulteration: Prodyut Kumar Paul Professor, Uttar Banga Krishi ViswavidyalayaDocument25 pagesFood Adulteration: Prodyut Kumar Paul Professor, Uttar Banga Krishi ViswavidyalayaProdyut K PaulNo ratings yet
- Introduction Contains The Topics: Introduction of Seed ProductionDocument37 pagesIntroduction Contains The Topics: Introduction of Seed ProductionSheelendra Mangal BhattNo ratings yet
- Zhang 2018Document204 pagesZhang 2018Marcelo Jara FalconiNo ratings yet
- Asking and Giving OpinionsDocument6 pagesAsking and Giving OpinionsDora SilvaNo ratings yet
- Establishing Sales TargetDocument10 pagesEstablishing Sales TargetErich VirayNo ratings yet
- Synthesis: Condition and Trends in Systems and Services, Trade-Offs For Human Well-Being, and Implications For The FutureDocument12 pagesSynthesis: Condition and Trends in Systems and Services, Trade-Offs For Human Well-Being, and Implications For The FuturenmohammaNo ratings yet
- RBI Assistant Prelims 2020 The Selection PLAN 2Document75 pagesRBI Assistant Prelims 2020 The Selection PLAN 2Maddy MystroNo ratings yet
- Unhealthy BreakfastDocument2 pagesUnhealthy BreakfastOreillerNo ratings yet
- Alpaka Company Profile 2020Document25 pagesAlpaka Company Profile 2020dreamup indoNo ratings yet
- Section 5 - Students WorksheetDocument4 pagesSection 5 - Students WorksheetEsraa AhmedNo ratings yet
- FSSC AUDIT REPORT (Internal)Document3 pagesFSSC AUDIT REPORT (Internal)Md Kamruzzaman MonirNo ratings yet