Professional Documents
Culture Documents
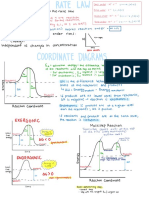
Week 6 Pre-Lab Question That I Got Wrong Explanation
Week 6 Pre-Lab Question That I Got Wrong Explanation
Uploaded by
Ashley JoshiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Week 6 Pre-Lab Question That I Got Wrong Explanation
Week 6 Pre-Lab Question That I Got Wrong Explanation
Uploaded by
Ashley JoshiCopyright:
Available Formats
A =
Ec I The reaction volume = 1 .
25mL The volume of enzyme
=
55M
= S A =
& Sc I
The information that we will use :
initial of change (A) 0 85 mint
rate of
=
we use the Absorbance
.
+ +
& =
6220M cm
The question wants us to :
0 calculate the rate of NADH Mmint Beer Lambert
in using
⑦ calculate triAl mal of NADH
appearing per minute from total volume of reaction mixture
-
> Invest into mmulment
⑦
anvest mmalment to cone unit Amount of enzyme that Immot substrate minute)
Units
catalyses
=
per
① SA =
E1C L
+ 0 . 85 mint =
(6220M/C) (AC) ( 1 CM)
a
5 min
1 c
=
1 . 367 x 10 M mint
② In this step we want to convert the M part to mot , then umol
: we use reaction mixture volume
1 c =
1 367 x 15-4 Mmin
G
.
This is the same as :
1 2 =
1 367
.
x 10 mot L" mint
Lt
Get rid of by multiplying with the reaction mixture volume
Renation mix. . Volume
=
1 .
20 mL
=
1 2.
x 10-3 L
=> 1 .
367 x 10-4 mo mint x 1 2
.
x 10-3
-
1 .
64 x 10-7 mol mint
C
3 convert to umalmint CI know instructions
say
mmul , but its easier to concert to umol)
&
S
1 64 x 10-7 mot min-
!
.
(1 mol ·
1000000 und)
. = .
0 164 umolmin-
(this is
why)
④ Now
, we need convert
to into UNITS =
Cone unit =
Amount of enzyme that catalyses Immot substrate per minute)
divide of umalmint
we well the rele
by volume of enzyme in me to
unity
get
:
: umamin
=
2 . 981 UnitemL" FINAL ANSWER
0 . 055 mL
You might also like
- Dvorak - (From Rusalka) - Song To The MoonDocument6 pagesDvorak - (From Rusalka) - Song To The MoonMichaelPraetoriusNo ratings yet
- Historical Atlas of The British Empire BookletDocument80 pagesHistorical Atlas of The British Empire BookletCraig Martin100% (4)
- Tutorial 01 CRE A186207Document33 pagesTutorial 01 CRE A186207NURUL SYAHIRAH BINTI ABDUL HALIMNo ratings yet
- Lesson 2 (Size of Reactants)Document4 pagesLesson 2 (Size of Reactants)Tan Jun hanNo ratings yet
- Upd C11 CHM EngDocument18 pagesUpd C11 CHM EngArinjoy Mervyn GomesNo ratings yet
- Week 4Document4 pagesWeek 4呂旼亮No ratings yet
- Cee 235a HWDocument21 pagesCee 235a HWNguyen DuyNo ratings yet
- เคมีเพิ่มDocument5 pagesเคมีเพิ่มPavaridNo ratings yet
- Ncert Kaksha Formula Sheets Chemistry Class 11thDocument18 pagesNcert Kaksha Formula Sheets Chemistry Class 11thABCD Play school100% (4)
- Bonus Assignment 1 - 387Document1 pageBonus Assignment 1 - 387Arundhati GuptaNo ratings yet
- COURS AstroDocument23 pagesCOURS Astro7b98gyyzf8No ratings yet
- 0223微積分A二Document9 pages0223微積分A二江品萱No ratings yet
- FINALSDocument2 pagesFINALSjrcruzpogi0242424No ratings yet
- CH 4Document26 pagesCH 4林孟群No ratings yet
- EX9 Una BombaDocument2 pagesEX9 Una BombaRoger UnoxxNo ratings yet
- Free Diseno de Maquinas T5 Gulag FreeDocument12 pagesFree Diseno de Maquinas T5 Gulag FreePablo GarciaNo ratings yet
- DC Motors FormulasDocument3 pagesDC Motors FormulasSaabierah SalieNo ratings yet
- Chemical KineticsDocument2 pagesChemical KineticsMelvin SotoNo ratings yet
- Problemario Primera Ley de Sistemas Abiertos de Flujo No EstacionarioDocument2 pagesProblemario Primera Ley de Sistemas Abiertos de Flujo No EstacionarioRulis DCNo ratings yet
- 12 May Handout (Physics)Document17 pages12 May Handout (Physics)AmiteshNo ratings yet
- Quantum Mechanics Notes - Ii: Amit Kumar Jha (Iitian)Document15 pagesQuantum Mechanics Notes - Ii: Amit Kumar Jha (Iitian)Tiasha DevNo ratings yet
- Práctica Chávez Física (1) 2Document11 pagesPráctica Chávez Física (1) 2daniela sdenkaNo ratings yet
- Physical Chemistry Formula (2) 3Document6 pagesPhysical Chemistry Formula (2) 3Anand RockyNo ratings yet
- Seminario 13 - Pregunta 5 (Grupo 5)Document1 pageSeminario 13 - Pregunta 5 (Grupo 5)stefanyvaldivia6No ratings yet
- RegletedDocument3 pagesRegletedYulia YuanNo ratings yet
- Hifiuibrii: GivenDocument43 pagesHifiuibrii: GivenShreyas PrabhuNo ratings yet
- Chemical Kinetics Mind MapDocument1 pageChemical Kinetics Mind MapSamridhi MoudgilNo ratings yet
- Example Problem Solutions - Chapter 8Document18 pagesExample Problem Solutions - Chapter 8Nguyen Tien DungNo ratings yet
- Vector 3Document12 pagesVector 3editorNo ratings yet
- Cycle and Engine Analysis of An Ideal Rankine Cycle 3Document11 pagesCycle and Engine Analysis of An Ideal Rankine Cycle 3Sean GoNo ratings yet
- TrigonometryDocument21 pagesTrigonometryAbhijeet AdhikaryNo ratings yet
- Cap PDFDocument1 pageCap PDFharshitnayak22No ratings yet
- Week 3Document21 pagesWeek 38dzkyfxkn5No ratings yet
- Physics 3Document78 pagesPhysics 3alexandragochianNo ratings yet
- Control 3 MecFluidosDocument3 pagesControl 3 MecFluidosBarbara OlmosNo ratings yet
- 650191d7ca4bac0018beec42 - ## - STRUCTURE OF ATOM Mind - 240412 - 202134Document1 page650191d7ca4bac0018beec42 - ## - STRUCTURE OF ATOM Mind - 240412 - 202134raulomprakash2006No ratings yet
- 9P 1.602xto": ProtonDocument5 pages9P 1.602xto": ProtonZoha FaisalNo ratings yet
- PDF Updated Class 11 Physics Formula Sheet CompressDocument22 pagesPDF Updated Class 11 Physics Formula Sheet CompressdrjbjpNo ratings yet
- PPPTHĐDocument12 pagesPPPTHĐNHI NGUYỄN TRẦN THẢONo ratings yet
- Equivalent Computers: Lambda CalculusDocument8 pagesEquivalent Computers: Lambda Calculusemanuel mayaNo ratings yet
- Dead Load and Live LoadDocument4 pagesDead Load and Live LoadKimberly Anne NgoNo ratings yet
- Strengths of Materials TutorialDocument2 pagesStrengths of Materials TutorialCharl CronjeNo ratings yet
- Tema 8 FísicaDocument4 pagesTema 8 FísicaNaviroNo ratings yet
- Latti: Qeare inDocument1 pageLatti: Qeare inzyzy6527No ratings yet
- Taller #5 Matefin MCSVDocument2 pagesTaller #5 Matefin MCSVsuarezvillamilmariacamilaNo ratings yet
- (9024SOD2) C15) : All MyDocument1 page(9024SOD2) C15) : All Myqxpvgd66mmNo ratings yet
- ch 1 pw formulaDocument4 pagesch 1 pw formulaAkshita SinghNo ratings yet
- แบบฝึกหัดเรื่องoscillatory Motion 65011058Document4 pagesแบบฝึกหัดเรื่องoscillatory Motion 6501105865011058No ratings yet
- Beam Deflection PDFDocument7 pagesBeam Deflection PDFHaikal HakimNo ratings yet
- Capilaridad 2Document5 pagesCapilaridad 2matiassantiago0309No ratings yet
- Wuolah Free Ejercicios T7 Gulag FreeDocument4 pagesWuolah Free Ejercicios T7 Gulag FreeuserwuolahNo ratings yet
- Chap 1 NotesDocument3 pagesChap 1 NotesKe ShaneNo ratings yet
- Waves 2Document1 pageWaves 2fghhfgfNo ratings yet
- Dynamic 3Document2 pagesDynamic 3คน. ดีNo ratings yet
- Inax 為: chomgeDocument1 pageInax 為: chomge9872315No ratings yet
- Gunawanaprisaputri 864820 24222928 APSC 182Document1 pageGunawanaprisaputri 864820 24222928 APSC 182Aprisa PutriNo ratings yet
- 期末題目Document3 pages期末題目WNo ratings yet
- Physics Report Chapter4 - 20245159Document1 pagePhysics Report Chapter4 - 20245159rzd6zh7kp7No ratings yet
- Mmmlrorpnur: ImurlDocument1 pageMmmlrorpnur: Imurl范閔堯No ratings yet
- สมุดโน้ต PDFDocument3 pagesสมุดโน้ต PDFsssssNo ratings yet
- Sheet 5Document5 pagesSheet 5fatima.alansari55No ratings yet
- The Planets in The SignsDocument12 pagesThe Planets in The SignsPedro FilipeNo ratings yet
- 5 Min FlipDocument76 pages5 Min FlipAnonymous JrCVpuNo ratings yet
- Codificación Nacional Colombia. T. XIX. 1860-1861. ÍndicesDocument28 pagesCodificación Nacional Colombia. T. XIX. 1860-1861. ÍndicesLINA MARCELA GONZALEZ GOMEZNo ratings yet
- Bed Bath & BeyondDocument5 pagesBed Bath & BeyondExquisite WriterNo ratings yet
- Fans and Drive Concepts For Rail TechnologyDocument220 pagesFans and Drive Concepts For Rail Technology다원시스No ratings yet
- Topic 1: Before You Begin Determine Personal Support RequirementsDocument20 pagesTopic 1: Before You Begin Determine Personal Support Requirementsdefa reyNo ratings yet
- Financial Accounting: Volume 3 Summary ValixDocument10 pagesFinancial Accounting: Volume 3 Summary ValixPrincess KayNo ratings yet
- Ai Mental HealthDocument11 pagesAi Mental HealthRajaNo ratings yet
- Brochure - Digital Banking - New DelhiDocument4 pagesBrochure - Digital Banking - New Delhiankitgarg13No ratings yet
- LOT1 CONCRETE Mixes 27-03-2019 - REV 9.1Document7 pagesLOT1 CONCRETE Mixes 27-03-2019 - REV 9.1Soundar PachiappanNo ratings yet
- I-Day 32Document4 pagesI-Day 32Ivan Rey Porras VerdeflorNo ratings yet
- TrimbleDocument7 pagesTrimbleSebastian Eduardo Zúñiga LeytonNo ratings yet
- EmtlDocument40 pagesEmtlammu0100% (1)
- Shani Sadhe Sati and DhaiyaDocument2 pagesShani Sadhe Sati and DhaiyaSanjay DhimanNo ratings yet
- AGENCY AGREEMENT NeptuneDocument8 pagesAGENCY AGREEMENT NeptuneFiza HairunaNo ratings yet
- Paints - : Methods of Test ForDocument22 pagesPaints - : Methods of Test ForKinToneNo ratings yet
- Pilot SpeakUP1 Learners EngDocument80 pagesPilot SpeakUP1 Learners EngPham Le Viet VuNo ratings yet
- Criminal Law 2 ReviewerDocument176 pagesCriminal Law 2 Reviewershapeeyah100% (2)
- The Origin of Elements and The Separation of Galaxies - Gamow - 1948Document2 pagesThe Origin of Elements and The Separation of Galaxies - Gamow - 1948stickygreenmanNo ratings yet
- Ae112 NotesDocument3 pagesAe112 NotesJillian Shaindy BuyaganNo ratings yet
- Neptune III Rel 2.0 - ENDocument44 pagesNeptune III Rel 2.0 - ENShawn DownloaderNo ratings yet
- Distosia BahuDocument185 pagesDistosia BahuAdith Fileanugraha100% (1)
- SaswnotesDocument11 pagesSaswnotesChuck_YoungNo ratings yet
- Chap 4. Absorption by Roots Chap 5. Transpiration Chap 6. PhotosynthesisDocument193 pagesChap 4. Absorption by Roots Chap 5. Transpiration Chap 6. Photosynthesisankurbiology83% (6)
- Peripheral Vascular DiseaseDocument47 pagesPeripheral Vascular Diseaseusmle prepNo ratings yet
- End Sem Problem Numbers EE-313 Modern Control EngineeringDocument3 pagesEnd Sem Problem Numbers EE-313 Modern Control EngineeringAyush Gupta 4-Year B.Tech. Electrical EngineeringNo ratings yet
- Chem 101e 2020 Fall mt1Document2 pagesChem 101e 2020 Fall mt1Sefa Ceren KANDEMİRNo ratings yet
- Dwnload Full Juvenile Delinquency The Core 4th Edition Siegel Test Bank PDFDocument35 pagesDwnload Full Juvenile Delinquency The Core 4th Edition Siegel Test Bank PDFscullionegrette15ek0100% (13)