Professional Documents
Culture Documents
A Novel Receptor-Binding Domain (RBD) - Based Mrna Vaccine Against Sars-Cov-2
A Novel Receptor-Binding Domain (RBD) - Based Mrna Vaccine Against Sars-Cov-2
Uploaded by
zainab.a.m.bualiCopyright:
Available Formats
You might also like
- A Thermostable mRNA Vaccine Against COVID-19 Zhang Et - Al. 2020Document30 pagesA Thermostable mRNA Vaccine Against COVID-19 Zhang Et - Al. 2020SY LodhiNo ratings yet
- H μελέτη με θέμα: "Το RIG-I πυροδοτεί μια υπεράσπιση κατά της σηματοδότησης-άμβλυνσης αντι-SARS-CoV-2 σε ανθρώπινα πνευμονικά κύτταρα"Document60 pagesH μελέτη με θέμα: "Το RIG-I πυροδοτεί μια υπεράσπιση κατά της σηματοδότησης-άμβλυνσης αντι-SARS-CoV-2 σε ανθρώπινα πνευμονικά κύτταρα"ARISTEIDIS VIKETOSNo ratings yet
- An Antibody That Neutralizes Sars-Cov-1 and Sars-Cov-2 by Binding To A Conserved Spike Epitope Outside The Receptor Binding MotifDocument19 pagesAn Antibody That Neutralizes Sars-Cov-1 and Sars-Cov-2 by Binding To A Conserved Spike Epitope Outside The Receptor Binding Motifchato law officeNo ratings yet
- 1 s2.0 S0021925821006189 MainDocument16 pages1 s2.0 S0021925821006189 MainAngelica Maria Torregroza EspinosaNo ratings yet
- Artigo 1 - Covid-19Document3 pagesArtigo 1 - Covid-19leonardoNo ratings yet
- Versatile and Multivalent Nanobodies Efficiently Neutralize SARSCoV2ScienceDocument7 pagesVersatile and Multivalent Nanobodies Efficiently Neutralize SARSCoV2ScienceDARLENYS YADIRA MOTA MEJIANo ratings yet
- Readings On SARS-CoV-2 (Corona Virus)Document8 pagesReadings On SARS-CoV-2 (Corona Virus)negurii hearteuNo ratings yet
- JIANG 2021 (EPITOPO B) Epitope Profiling Reveals The Critical Antigenic Determinants in SARS-CoV-2 RBD-Based AntigenDocument14 pagesJIANG 2021 (EPITOPO B) Epitope Profiling Reveals The Critical Antigenic Determinants in SARS-CoV-2 RBD-Based AntigenJoão Pedro NunesNo ratings yet
- Neutralizing Antibodies Against SARS-CoV-2 CurrentDocument15 pagesNeutralizing Antibodies Against SARS-CoV-2 CurrentAntonius SimangunsongNo ratings yet
- An Alpaca Nanobody Neutralizes Sars-Cov-2 by Blocking Receptor InteractionDocument9 pagesAn Alpaca Nanobody Neutralizes Sars-Cov-2 by Blocking Receptor InteractionRicardoPeixotoNo ratings yet
- Neuropilin-1 Facilitates SARS-CoV-2 Cell Entry and Infectivity (Cantuti-Castelvetri 2020)Document7 pagesNeuropilin-1 Facilitates SARS-CoV-2 Cell Entry and Infectivity (Cantuti-Castelvetri 2020)gd_hbarNo ratings yet
- Infection Enhancing Anti Sars Cov 2 Antibodies Recognize Both The Original Wuhan D614G Strain And Delta Variants A Potential Risk For Mass Vaccination Nouara Yahi Henri Chahinian Jacques Fantini full chapter pdf docxDocument27 pagesInfection Enhancing Anti Sars Cov 2 Antibodies Recognize Both The Original Wuhan D614G Strain And Delta Variants A Potential Risk For Mass Vaccination Nouara Yahi Henri Chahinian Jacques Fantini full chapter pdf docxtolliekawann100% (7)
- Measuringtheserologic Responsetosevereacute Respiratorysyndrome Coronavirus2Document12 pagesMeasuringtheserologic Responsetosevereacute Respiratorysyndrome Coronavirus2Chris KontomarisNo ratings yet
- Lyophilization of Lipid Nanoparticle For mRNA Vaccine 1682922545Document15 pagesLyophilization of Lipid Nanoparticle For mRNA Vaccine 1682922545Piet van den OetelaarNo ratings yet
- Ab Serum CovidDocument12 pagesAb Serum CovidTania CortázarNo ratings yet
- Jurnal Sinovac 2Document6 pagesJurnal Sinovac 2Delapan SembilanNo ratings yet
- Studio Olandese Su CoronavirusDocument24 pagesStudio Olandese Su CoronavirusantiochioNo ratings yet
- Viruses: Sars-Cov-2 Spike Impairs Dna Damage Repair and Inhibits V (D) J Recombination in VitroDocument10 pagesViruses: Sars-Cov-2 Spike Impairs Dna Damage Repair and Inhibits V (D) J Recombination in Vitromuun yayo100% (1)
- Viruses: Sars-Cov-2 Spike Impairs Dna Damage Repair and Inhibits V (D) J Recombination in VitroDocument10 pagesViruses: Sars-Cov-2 Spike Impairs Dna Damage Repair and Inhibits V (D) J Recombination in VitroMohamed Fawzy mahrousNo ratings yet
- Sars-Cov-2 Can Recruit A Haem Metabolite To Evade Antibody ImmunityDocument19 pagesSars-Cov-2 Can Recruit A Haem Metabolite To Evade Antibody ImmunityShreyasri SainNo ratings yet
- WissenshaftDocument11 pagesWissenshaftkacagj bajazzoNo ratings yet
- Estudio Sobre El RemdesivirDocument28 pagesEstudio Sobre El RemdesivirA24.comNo ratings yet
- ReviewDocument8 pagesReviewIvana EstechoNo ratings yet
- 3 The Architecture of SARS CoV 2 Transcriptome 8Document19 pages3 The Architecture of SARS CoV 2 Transcriptome 8escarlet zapataNo ratings yet
- 2020 05 04 075911v1 FullDocument30 pages2020 05 04 075911v1 Fullandri priantoNo ratings yet
- Talanta: Chenchen Ge, Juan Feng, Jiaming Zhang, Kai Hu, Dou Wang, Ling Zha, Xuejuan Hu, Rongsong LiDocument9 pagesTalanta: Chenchen Ge, Juan Feng, Jiaming Zhang, Kai Hu, Dou Wang, Ling Zha, Xuejuan Hu, Rongsong LipriyaNo ratings yet
- SARS-CoV-2 IgG II ArchitectDocument10 pagesSARS-CoV-2 IgG II ArchitectcalidadlabinfectoNo ratings yet
- Protocolo - 2022Document10 pagesProtocolo - 2022Carlos Alberto Salazar DuqueNo ratings yet
- PIIS016344532300600XDocument4 pagesPIIS016344532300600Xdenemebakalim72No ratings yet
- The Pathophysiology, Diagnosis and Treatment of Corona Virus Disease 2019 (COVID-19)Document12 pagesThe Pathophysiology, Diagnosis and Treatment of Corona Virus Disease 2019 (COVID-19)GabiiGabriielaNo ratings yet
- COVID Vaccine Systematic Review Dong Dai WeiDocument14 pagesCOVID Vaccine Systematic Review Dong Dai WeiJason SnapeNo ratings yet
- Antiviral Peptides As Promising Therapeutics Against SARS-CoV 2Document8 pagesAntiviral Peptides As Promising Therapeutics Against SARS-CoV 2Stalwart sheikhNo ratings yet
- Articulo 8 CovidDocument33 pagesArticulo 8 CovidJuan OrlandoniNo ratings yet
- The SARS-CoV-2 Genome, Its Variants and Their Various Way of ImmunizationDocument7 pagesThe SARS-CoV-2 Genome, Its Variants and Their Various Way of ImmunizationInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- ZNFX1 dsRNADocument24 pagesZNFX1 dsRNAFrancesc Xavier Bofill De RosNo ratings yet
- 705 2010 Article 729Document7 pages705 2010 Article 729Lote LewaivunaNo ratings yet
- Molecular Docking Using Chimera and Autodock VinaDocument22 pagesMolecular Docking Using Chimera and Autodock VinaShisir PuriNo ratings yet
- Two Linear Epitopes On The SARS-CoV-2 SpikeDocument7 pagesTwo Linear Epitopes On The SARS-CoV-2 SpikeRheum RaponticumNo ratings yet
- Sars-Cov-2 Nsp1 Suppresses Host But Not Viral Translation Through A Bipartite MechanismDocument16 pagesSars-Cov-2 Nsp1 Suppresses Host But Not Viral Translation Through A Bipartite MechanismJanina PuenteNo ratings yet
- Journal Pre-Proof: Infection, Genetics and EvolutionDocument29 pagesJournal Pre-Proof: Infection, Genetics and EvolutionMohammed Shuaib AhmedNo ratings yet
- Crispr-Cas12 Sars Cov2Document8 pagesCrispr-Cas12 Sars Cov2Brayann InlvNo ratings yet
- Longitudinal Evaluation and Decline of Antibody Responses in Sars-Cov-2 InfectionDocument24 pagesLongitudinal Evaluation and Decline of Antibody Responses in Sars-Cov-2 InfectionCesar ValleNo ratings yet
- Novel Drugs Targeting The Sars-Cov-2/Covid-19 Machinery: Ariane Sternberg, Dwight L. Mckee and Cord NaujokatDocument11 pagesNovel Drugs Targeting The Sars-Cov-2/Covid-19 Machinery: Ariane Sternberg, Dwight L. Mckee and Cord Naujokatperi umardianaNo ratings yet
- A Prefusion SARS-CoV-2 Spike RNA Vaccine 2020.09.08.280818v1.fullDocument38 pagesA Prefusion SARS-CoV-2 Spike RNA Vaccine 2020.09.08.280818v1.fullDavid MoraNo ratings yet
- 1 s2.0 S1074761320303265 MainDocument17 pages1 s2.0 S1074761320303265 MainAleksandar DimkovskiNo ratings yet
- DiB 2019 CarvalhoDocument11 pagesDiB 2019 CarvalhoAristobolo SilvaNo ratings yet
- Processes: Trimeric Sars-Cov-2 Spike Proteins Produced From Cho Cells in Bioreactors Are High-Quality AntigensDocument11 pagesProcesses: Trimeric Sars-Cov-2 Spike Proteins Produced From Cho Cells in Bioreactors Are High-Quality AntigensHan VoNo ratings yet
- Kimia Medisinal: Oleh Kelompok 8Document16 pagesKimia Medisinal: Oleh Kelompok 8Hasryanto YantoNo ratings yet
- Zerospike Project BrochureDocument24 pagesZerospike Project Brochurestephen.cordnerNo ratings yet
- Biomedicines 10 01950Document16 pagesBiomedicines 10 01950Lija LajiNo ratings yet
- Sars-Cov-2: Phylogenetic Status, Mutations and Therapeutic Research Based On Spike ProteinDocument10 pagesSars-Cov-2: Phylogenetic Status, Mutations and Therapeutic Research Based On Spike ProteinArley GutarraNo ratings yet
- Kim Et Al., 2020Document19 pagesKim Et Al., 2020신유찬No ratings yet
- Journal Pre-Proof: Journal of Controlled ReleaseDocument17 pagesJournal Pre-Proof: Journal of Controlled ReleaseGisela GloryNo ratings yet
- Rapid Test For Detecting Neutralizing Anti RBD Antibodies PDFDocument4 pagesRapid Test For Detecting Neutralizing Anti RBD Antibodies PDFFernando GTgroupNo ratings yet
- Antigenic Characterization of The SARS-CoV-2 Omicron Subvariant BA.2.75Document26 pagesAntigenic Characterization of The SARS-CoV-2 Omicron Subvariant BA.2.75Shaviera dentiNo ratings yet
- Computers in Biology and MedicineDocument10 pagesComputers in Biology and MedicineTausif AlamNo ratings yet
- Mansfield 2004Document5 pagesMansfield 2004Nico Alexander ReyesNo ratings yet
- Sars-Cov-2 Infection Triggers Widespread Host Mrna Decay Leading To An Mrna Export BlockDocument39 pagesSars-Cov-2 Infection Triggers Widespread Host Mrna Decay Leading To An Mrna Export BlockStacey KrellerNo ratings yet
- Vaccination Versus Hybrid ImmunizationDocument9 pagesVaccination Versus Hybrid ImmunizationJovanaNo ratings yet
- Jurnal Kampen 2020Document29 pagesJurnal Kampen 2020Crosco BDLNo ratings yet
- Self-Amplifying RNA Vaccines For Infectious DiseasesDocument13 pagesSelf-Amplifying RNA Vaccines For Infectious DiseasesRicardo SousaNo ratings yet
- Adaptive Immunity To SARS-CoV-2 and COVID-19Document21 pagesAdaptive Immunity To SARS-CoV-2 and COVID-19Yuri YogyaNo ratings yet
- 089 44 Final Biologi Fasa4 DLP T4-62-84Document23 pages089 44 Final Biologi Fasa4 DLP T4-62-84BF CLNo ratings yet
- NEJMoa 2028436Document12 pagesNEJMoa 2028436arman ahdokhshNo ratings yet
- Enders 1954 - Propagation-In-Tissue-Cultures-Of-Cytopathogenic-Agents-From-Patients-With-MeaslesDocument10 pagesEnders 1954 - Propagation-In-Tissue-Cultures-Of-Cytopathogenic-Agents-From-Patients-With-MeaslesRoger TNo ratings yet
- How Effective Are COVIDDocument4 pagesHow Effective Are COVIDMARHUMA HAPSIN. ABBASNo ratings yet
- Article-2021-COVID-19-Neutralizing Antibodies Predict Disease Severity and SurvivalDocument25 pagesArticle-2021-COVID-19-Neutralizing Antibodies Predict Disease Severity and Survivalcarlos ArozamenaNo ratings yet
- Science Abm3425Document24 pagesScience Abm3425Lindomar Bonfim NobreNo ratings yet
- Corti Et Al., 2021Document38 pagesCorti Et Al., 2021LunaNo ratings yet
- Dog & Cat Import FormDocument4 pagesDog & Cat Import FormDavid Paul HensonNo ratings yet
- Anti HBeDocument6 pagesAnti HBeNaveen Kumar MNo ratings yet
- J Transci 2018 10 013Document7 pagesJ Transci 2018 10 013Clara Espinosa ArnandisNo ratings yet
- Chimeric Peptidomimetic Antibiotic Efficiently Neutralizes Lipopolysaccharides (LPS) and Bacteria-Induced Activation of RAW MacrophagesDocument9 pagesChimeric Peptidomimetic Antibiotic Efficiently Neutralizes Lipopolysaccharides (LPS) and Bacteria-Induced Activation of RAW MacrophagesKATIA PAMELA VILLAVICENCIO SANCHEZNo ratings yet
- ViruSure Brochure 2020Document20 pagesViruSure Brochure 2020joeNo ratings yet
- Aid 2011 1502Document148 pagesAid 2011 1502pasargadae_mNo ratings yet
- Jordan2017 Infectious BronchitisDocument28 pagesJordan2017 Infectious BronchitisVicente Vega SanchezNo ratings yet
- 5 Concerns About SARS-CoV2 Biology: A Call To Pause, Deliberate, and Revise Policy - by J Jay Couey - Gigaohm Biological - MediumDocument24 pages5 Concerns About SARS-CoV2 Biology: A Call To Pause, Deliberate, and Revise Policy - by J Jay Couey - Gigaohm Biological - MediumAnonymous lEJuFJMNo ratings yet
- The Journey of Antibody and Antigen Test During COVID-19 Pandemic in Indonesia, The Advantages & DisadvantagesDocument34 pagesThe Journey of Antibody and Antigen Test During COVID-19 Pandemic in Indonesia, The Advantages & DisadvantagesEldo TaufilaNo ratings yet
- Laboratory Testing For COVID-19 - Dr. Trilis Yulianti, M.kesDocument20 pagesLaboratory Testing For COVID-19 - Dr. Trilis Yulianti, M.kesYayax RakhmanNo ratings yet
- DR Robert Malone Speaks OutDocument40 pagesDR Robert Malone Speaks OutB100% (2)
- Rational Design of A Triple-Type Human Papillomavirus Vaccine by Compromising Viral-Type Speci FicityDocument15 pagesRational Design of A Triple-Type Human Papillomavirus Vaccine by Compromising Viral-Type Speci FicitySamer ShamshadNo ratings yet
- Laboratory Techniques in Rabies: Fourth EditionDocument494 pagesLaboratory Techniques in Rabies: Fourth EditionKimjhee Yang WongNo ratings yet
- Tests Krok Gen Bact VirDocument18 pagesTests Krok Gen Bact Viryasserzeddou3000No ratings yet
- Materi Pembicara Dr. TrilisDocument13 pagesMateri Pembicara Dr. TrilisDamian HamzahNo ratings yet
- Nanomaterials 11 01788 v2 5Document25 pagesNanomaterials 11 01788 v2 5Mark LaceyNo ratings yet
- jn.1 Ire CleanDocument6 pagesjn.1 Ire CleanKabakçı HüsnüNo ratings yet
- Produksi Kolostrum Antivirus Avian InfluenzaDocument11 pagesProduksi Kolostrum Antivirus Avian InfluenzaRohendi RohendiNo ratings yet
- Immune Response To HIVDocument9 pagesImmune Response To HIVTugas HeinzNo ratings yet
- Respiratory Virus InfectionsDocument9 pagesRespiratory Virus InfectionsChawki MokademNo ratings yet
A Novel Receptor-Binding Domain (RBD) - Based Mrna Vaccine Against Sars-Cov-2
A Novel Receptor-Binding Domain (RBD) - Based Mrna Vaccine Against Sars-Cov-2
Uploaded by
zainab.a.m.bualiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
A Novel Receptor-Binding Domain (RBD) - Based Mrna Vaccine Against Sars-Cov-2
A Novel Receptor-Binding Domain (RBD) - Based Mrna Vaccine Against Sars-Cov-2
Uploaded by
zainab.a.m.bualiCopyright:
Available Formats
www.nature.
com/cr
www.cell-research.com
LETTER TO THE EDITOR OPEN
A novel receptor-binding domain (RBD)-based mRNA
vaccine against SARS-CoV-2
Cell Research (2020) 30:932–935; https://doi.org/10.1038/s41422-020-0387-5
Dear Editor, significantly higher in the lymph nodes of RBD mRNA-LNP-
The pandemic of coronavirus disease 2019 (COVID-19) caused immunized mice than in those of S1 mRNA-LNP-immunized
by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mice, whereas only a background level of Tfh and GC B cells was
highlights the need to develop effective and safe vaccines. Similar shown in the LNP control-injected mice. Plasma cells were also
to SARS-CoV, SARS-CoV-2 recognizes angiotensin-converting significantly increased in splenocytes of the vaccinated mice, as
enzyme 2 (ACE2) as receptor for host cell entry.1,2 SARS-CoV-2 compared to the control group (Supplementary information,
spike (S) protein consists of S1, including receptor-binding domain Fig. S5c). These data demonstrate the recruitment of Tfh, GC B,
(RBD), and S2 subunits.3,4 We previously demonstrated that RBDs and/or plasma cells in vivo, particularly after immunization with
of SARS-CoV and MERS-CoV serve as important targets for SARS-CoV-2 RBD mRNA-LNP vaccine.
the development of effective vaccines.5,6 We further evaluated humoral immune responses and neu-
To identify an mRNA candidate vaccine, we initially designed tralizing antibodies induced by S1 and RBD mRNA-LNPs. Mice
two mRNA constructs expressing S1 and RBD, respectively, were immunized with each mRNA-LNP at three different
1234567890();,:
of SARS-CoV-2 S protein (Fig. 1a). Both culture supernatants schedules (Supplementary information, Fig. S4a–c), and sera
and lysates of cells transfected with S1 or RBD mRNA reacted were collected for detection of IgG, subtype (IgG1 and IgG2a),
strongly with a SARS-CoV-2 RBD-specific antibody (Supplemen- and neutralizing antibodies. First, ELISA results revealed that S1
tary information, Fig. S1a), demonstrating expression of the and RBD mRNA-LNPs (30 μg/mouse, I.D. prime and boost)
target proteins. induced RBD-specific IgG (Fig. 1b), IgG1 (Th2) (Supplementary
To detect whether S1 and RBD mRNAs durably express information, Fig. S5d), and IgG2a (Th1) (Supplementary informa-
antigens in multiple cell types, we constructed N-terminal tion, Fig. S5e) antibodies 10 days after boost immunization and
mCherry-tagged SARS-CoV-2 S1 and RBD mRNAs, encapsulated that IgG antibody titer induced by RBD was significantly higher
them with lipid nanoparticles (LNPs) (Supplementary information, than that by S1 (Fig. 1b). Pseudovirus neutralization assay showed
Fig. S1b), and tested mCherry expression. Relative to the control, that S1 and RBD mRNA-LNPs elicited neutralizing antibodies
both RBD- and S1-mCherry mRNAs showed robust protein against SARS-CoV-2 pseudovirus entry into human ACE2-
expression in cells for at least 160 h, with higher expression expressing 293T (hACE2/293T) cells; particularly, RBD elicited
of the RBD construct (Supplementary information, Fig. S2a). significantly higher-titer neutralizing antibodies than S1 (Fig. 1c).
In addition, these mRNAs expressed proteins efficiently in a Neutralizing antibodies, particularly those induced by RBD mRNA-
variety of human (A549, Hep-2, HEP-G2, Caco-2, HeLa, 293 T), LNP, also potently neutralized live SARS-CoV-2 infection (Fig. 1d).
monkey (Vero E6), and bat (Tb1-Lu) cell lines (Supplementary Next, both S1 and RBD mRNA-LNPs (10 μg, I.D. prime and boost)
information, Fig. S2b). Particularly, the expression of RBD-mCherry induced SARS-CoV-2 RBD-specific IgG (Supplementary informa-
protein was higher than that of S1-mCherry protein in all cell lines tion, Fig. S6a) and neutralizing antibodies against SARS-CoV-2
tested (Supplementary information, Fig. S2b). These data indicate pseudovirus infection (Fig. 1e) 10 days after boost dose, and
long-term and broad expression of mRNA-encoding proteins, maintained at similarly high levels for 40 and 70 days post-boost
particularly RBD, in target cells. immunization, while the titer of neutralizing antibodies elicited by
We then characterized LNP-encapsulated S1 and RBD mRNAs RBD mRNA-LNP was always significantly higher than that by
for stability and subcellular localization. The mCherry-tagged S1 S1 mRNA-LNP (Fig. 1f, g; Supplementary information, S6b, c).
and RBD showed strong and stronger fluorescence intensity, Importantly, RBD mRNA-LNP induced antibody levels that
respectively, irrespective of incubation temperature (4 or 25 °C) potently neutralized live SARS-CoV-2 infection, reaching peak
and culture time (0, 24, or 72 h) (Supplementary information, titer at 70 days post-2nd immunization and being significantly
Fig. S3a). S1- and RBD-mCherry proteins were not colocalized with more potent than SARS-CoV-2 S1 mRNA-LNP-induced antibodies
nuclei but associated with lysosomes (Supplementary information, (Fig. 1h–j). Finally, RBD mRNA-LNP (10 μg, I.D. prime and
Fig. S3b). These results suggest that LNP-encapsulated SARS-CoV-2 intramuscular (I.M.) boost) also elicited significantly higher-titer
S1 and RBD mRNAs are stable at various temperatures and may be RBD-specific IgG (Supplementary information, Fig. S6d) or
resistant to lysosomal degradation. neutralizing antibodies than S1 mRNA-LNP against SARS-CoV-2
We next evaluated T follicular helper (Tfh), germinal center pseudovirus (Supplementary information, Fig. S6g) and live SARS-
(GC) B, and plasma cell responses induced by SARS-CoV-2 S1 and CoV-2 (Supplementary information, Fig. S6j) infection 10 days
RBD mRNA-LNPs in BALB/c mice. Mice were intradermally (I.D.) after boost immunization, and such antibodies maintained at
prime and boost immunized with each mRNA-LNP (30 μg/mouse) similar or even higher levels for at least 70 days post-boost dose
or empty LNP control, and draining lymph nodes or spleens (Supplementary information, Fig. S6e, f, h, i, k, l). In contrast,
were tested for Tfh, GC B, or plasma cells 10 days post-2nd empty LNP control only elicited a background, or undetectable,
immunization (Supplementary information, Fig. S4a). The per- level of antibodies incapable of neutralizing SARS-CoV-2 infection
centages of Tfh cells (Supplementary information, Fig. S5a) and (Fig. 1b–j; Supplementary information, Fig. S6). These data suggest
GC B cells (Supplementary information, Fig. S5b) were higher or that RBD mRNA-LNP vaccine immunized at different immunogen
Received: 9 June 2020 Accepted: 23 July 2020
Published online: 5 August 2020
© The Author(s) 2020
Letter to the Editor
933
doses and variant routes induced strong RBD-specific antibody antibodies can potently block binding between SARS-CoV-2 RBD
responses and potent neutralizing antibodies against pseudo- and its ACE2 receptor.
typed and live SARS-CoV-2 infection. Since SARS-CoV-2 RBD shares about 70% sequence identity with
To substantiate antiviral activity, we found the binding of SARS- SARS-CoV RBD,7 we evaluated whether serum antibodies from
CoV-2 RBD to ACE2 receptor in hACE2/293T cells was inhibited by SARS-CoV-2 mRNA-LNPs may cross-react with SARS-CoV RBD and
serum antibodies produced from RBD or S1 mRNA-LNP-vaccinated neutralize SARS-CoV infection. ELISA results showed that the titer
mice. Specifically, anti-RBD antibodies potently inhibited, in a of IgG (Fig. 1o), IgG1 (Supplementary information, Fig. S5f), and
dose-dependent manner, RBD-ACE2 receptor binding, which was IgG2a (Supplementary information, Fig. S5g) antibodies induced
much stronger than anti-S1 antibodies (Fig. 1k–m), while the by SARS-CoV-2 RBD mRNA-LNP was higher, or significantly higher,
control LNP-induced mouse sera did not inhibit RBD-ACE2 binding than those by SARS-CoV-2 S1 mRNA-LNP in cross-reacting with
(Fig. 1k, n). These data suggest that RBD mRNA-LNP-induced SARS-CoV RBD and cross-neutralizing infection by three SARS-CoV
Cell Research (2020) 30:932 – 935
Letter to the Editor
934
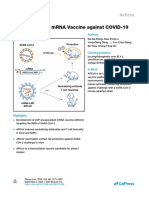
Fig. 1 Design and evaluation of SARS-CoV-2 S1 and RBD mRNA vaccines. a Schematic diagram of SARS-CoV-2 S1 and RBD mRNA
construction. The synthesized nucleoside-modified S1 and RBD mRNAs were encapsulated with LNPs to form mRNA-LNPs. b–j IgG and
neutralizing antibodies induced in immunized BALB/c mice at different immunogen doses via intradermal (I.D.) prime and boost at 4 weeks.
Sera at 10 days post-2nd immunization with SARS-CoV-2 S1 or RBD mRNA-LNP (e.g., S1-LNP or RBD-LNP) (30 μg/mouse), or empty LNP
(control), were detected for SARS-CoV-2 RBD-specific IgG antibodies by ELISA (b) or neutralizing antibodies against pseudotyped (c) and live
(d) SARS-CoV-2 infection. Sera at 10, 40, and 70 days post-2nd immunization with above mRNA-LNPs (10 μg/mouse) or control were detected
for neutralizing antibodies against pseudotyped (e–g) and live (h–j) SARS-CoV-2 infection. The ELISA plates were coated with SARS-CoV-2
RBD-Fc protein (1 µg/ml), and IgG antibody (Ab) titer was calculated. Overall, 50% neutralizing antibody titer (nAb NT50) was calculated against
SARS-CoV-2 pseudovirus infection in hACE2/293T cells, or against live SARS-CoV-2 infection by a cytopathic effect (CPE)-based
microneutralization assay in Vero E6 cells. The dotted lines indicate detection limit. k Dose-dependent inhibition of sera of mice receiving
a vaccine (30 μg/mouse) on SARS-CoV-2 RBD-hACE2 receptor binding in hACE2/293T cells by flow cytometry analysis. Percent (%) inhibition
was calculated based on relative fluorescence intensity with or without respective serum at indicated dilutions. l–n Representative images of
such inhibition by sera (1:5) of mice immunized with SARS-CoV-2 S1 mRNA-LNP (S1-LNP) (l), RBD mRNA-LNP (RBD-LNP) (m), or empty LNP
control (n) are shown in blue lines with respective median fluorescence intensity (MFI) values. The binding between SARS-CoV-2 RBD-Fc
protein (5 µg/mL) and hACE2 is shown in red lines. Gray shades indicate Fc-hACE2 binding. o Cross-reactivity of immunized mouse sera
against SARS-CoV RBD by ELISA. SARS-CoV RBD-Fc protein-coated plates (1 µg/mL) were used to detect IgG Ab titer. p–r Cross nAb NT50 of
above sera (twofold serial dilutions from 1:5) against infection of SARS-CoV pseudovirus expressing S protein of human SARS-CoV strains Tor2
(p) and GD03 (q), or palm civet SARS-CoV strain SZ3 (r) in hACE2/293T cells. Data (b, c, e–g, k–r) are presented as means ± SEM of mice (n = 5);
data (d, h–j) are presented as means ± SEM of duplicate wells of pooled sera from five mice per group. Significant differences are shown as
*P < 0.05; **P < 0.01; ***P < 0.001. Experiments were repeated twice with similar results.
pseudoviruses expressing S proteins of human strains Tor2 immunization with this vaccine at a lower immunogen dose
(Fig. 1p), GD03 (Fig. 1q), and palm civet strain SZ3 (Fig. 1r), (10 μg) via variant immunization routes (I.D. prime and I.D. or
respectively. These results suggest that SARS-CoV-2 RBD mRNA I.M. boost) also induced high-titer IgG antibodies with neutraliz-
vaccine can elicit antibodies cross-reacting with SARS-CoV RBD ing activity against pseudotyped and live SARS-CoV-2 infection
and cross-neutralizing SARS-CoV infection. that persisted for at least 70 days during the detection period.
We also investigated SARS-CoV-2 RBD-specific T cell responses Thus the IgG and neutralizing antibody titers induced by the
induced by S1 and RBD mRNA-LNPs in immunized mice. RBD-based mRNA vaccine were higher than those reported,
Splenocytes collected 10 days post-2nd immunization were suggesting beneficial protection against SARS-CoV-2 challenge
stimulated with SARS-CoV-2 RBD overlapping peptides (Supplemen- in vivo. Future studies warrant evaluation of protective efficacy
tary information, Table S1), and detected for secretion of IFN-γ (Th1), using mRNA vaccine against other reported vaccines under
TNF-α (Th1), and IL-4 (Th2) in CD45+CD4+ T cells, as well as IFN-γ, development. Previous studies showed that SARS-CoV full-length
TNF-α, and IL-4 in CD45+CD8+ T cells by flow cytometry analysis. S protein induced harmful immune responses with enhanced
Compared with the LNP control, immunization with RBD mRNA-LNP infection or liver damage after virus challenge, raising safety
could significantly increase the frequency of IFN-γ-, TNF-α- or IL-4- concerns.6 In contrast, RBD-based SARS-CoV and MERS-CoV
producing CD45+CD4+ (Supplementary information, Fig. S7a–c) or vaccines had no evidence to cause harmful immune responses,
CD45+CD8+ (Supplementary information, Fig. S7d–f) T cells, respec- including eosinophilic immune enhancement.5,6 Although no
tively. However, S1 mRNA-LNP could only significantly increase the obvious adverse effects have been reported in currently
frequency of TNF-α-producing CD45+CD4+ (Supplementary infor- developed COVID-19 vaccines, cautions need to be paid
mation, Fig. S7b) and IFN-γ- or IL-4-producing CD45+-CD8+ regarding their safety. More studies will be needed to investigate
(Supplementary information, Fig. S7d, f) T cells, respectively. vaccine-associated immunopathology in addition to evaluate
Therefore, RBD mRNA vaccine can effectively elicit RBD-specific their protective efficacy.
CD45+CD4+ (Th1) and CD45+CD8+ T cell responses. Overall, this study identifies RBD as a key antigen to design
As opposed to DNA, mRNA does not enter the nucleus and is effective vaccines against SARS-CoV-2, indicating great potential
not lysed by lysosomal enzymes (Supplementary information, of RBD-based mRNA vaccine for mitigation of the COVID-19
Fig. S8),8 contributing to its high stability and translation pandemic and possible SARS-related epidemics in the future. The
efficiency. GC, where GC B cells interact with Tfh and B cells, is strategy of developing RBD-based mRNA COVID-19 vaccine, as
the major site for production of high-affinity antibodies.9 Here, we described herein, can also be applied to develop vaccines against
showed that RBD mRNA-LNP elicited strong Tfh and GC B cell other emerging and reemerging coronavirus diseases in the
responses and potent neutralizing antibodies able to inhibit future.
the binding between SARS-CoV-2 RBD and ACE2 receptor
(Supplementary information, Fig. S8), demonstrating its high
potency against SARS-CoV-2 infection. ACKNOWLEDGEMENTS
The repertoire of COVID-19 vaccines currently in clinical trials This work was supported by National Institutes of Health (NIH) grants (R01AI137472
include mRNA, adenovirus, and DNA-based vaccines, most of and R01AI139092) and New York Blood Center funds (VIM-NYB616 and VIM-NYB673).
which encode SARS-CoV-2 full-length S protein.10–12 It has been
shown that adenovirus-based ChAdOx1 nCoV-19 vaccine elicits
specific IgG antibody titer of 1:400–6400 and neutralizing AUTHOR CONTRIBUTIONS
antibody titer of 1:5–40, whereas the DNA vaccine induces a W.T. and X.Z. conducted the study and analyzed the data. A.D., J.S., J.C.H. and C.K.T.
performed the experiments. L.L., C.D.H. and S.J. reviewed and revised the paper. L.D.
neutralizing antibody titer of 1:74–170, against live virus
designed and supervised the study, wrote and revised the manuscript.
infection in immunized monkeys.10,11 In addition, neutralizing
antibody titer against pseudotyped SARS-CoV-2 infection
ranged from 1:89–1115 in mice immunized with a full-length ADDITIONAL INFORMATION
S-based mRNA vaccine.12 Here we found that a SARS-CoV-2 Supplementary information accompanies this paper at https://doi.org/10.1038/
RBD-based mRNA vaccine at 30 μg/mouse elicited SARS-CoV-2 s41422-020-0387-5.
RBD-specific IgG antibody titer (~1:230,000) and neutralizing
antibody titer in mice against pseudotyped and live SARS-CoV-
2 infection at ~1:10,000 and 1:540, respectively. Moreover, Competing interests: The authors declare no competing interests.
Cell Research (2020) 30:932 – 935
Letter to the Editor
935
Wanbo Tai1, Xiujuan Zhang1, Aleksandra Drelich2, Juan Shi1, 8. Geall, A. J. et al. Proc. Natl. Acad. Sci. USA 109, 14604–14609 (2012).
Jason C. Hsu2, Larry Luchsinger1, Christopher D. Hillyer1, 9. Allen, C. D., Okada, T. & Cyster, J. G. Immunity 27, 190–202 (2007).
Chien-Te K. Tseng2, Shibo Jiang1 and Lanying Du1 10. van Doremalen, N. et al. bioRxiv https://doi.org/10.1101/2020.05.13.093195
1 (2020).
Lindsley F. Kimball Research Institute, New York Blood Center, New
11. Yu, J. et al. Science. eabc6284 https://doi.org/10.1126/science.abc6284 (2020).
York, NY 10065, USA and 2Department of Microbiology and
12. Corbett, K. S. et al. bioRxiv https://doi.org/10.1101/2020.06.11.145920 (2020).
Immunology, University of Texas Medical Branch, Galveston, TX
77555, USA
These authors contributed equally: Wanbo Tai, Xiujuan Zhang Open Access This article is licensed under a Creative Commons
Correspondence: Shibo Jiang (sjiang@nybc.org) or Attribution 4.0 International License, which permits use, sharing,
Lanying Du (ldu@nybc.org) adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative
Commons license, and indicate if changes were made. The images or other third party
REFERENCES material in this article are included in the article’s Creative Commons license, unless
1. Zhou, P. et al. Nature 579, 270–273 (2020). indicated otherwise in a credit line to the material. If material is not included in the
2. Li, W. et al. Nature 426, 450–454 (2003). article’s Creative Commons license and your intended use is not permitted by statutory
3. Shang, J. et al. Nature 581, 221–224 (2020). regulation or exceeds the permitted use, you will need to obtain permission directly
4. Xia, S. et al. Cell Res. 30, 343–355 (2020). from the copyright holder. To view a copy of this license, visit http://creativecommons.
5. Wang, N., Shang, J., Jiang, S. & Du, L. Front. Microbiol. 11, 298 (2020). org/licenses/by/4.0/.
6. Du, L. et al. Nat. Rev. Microbiol. 7, 226–236 (2009).
7. Tai, W. et al. Cell. Mol. Immunol. 17, 613–620 (2020).
© The Author(s) 2020
Cell Research (2020) 30:932 – 935
You might also like
- A Thermostable mRNA Vaccine Against COVID-19 Zhang Et - Al. 2020Document30 pagesA Thermostable mRNA Vaccine Against COVID-19 Zhang Et - Al. 2020SY LodhiNo ratings yet
- H μελέτη με θέμα: "Το RIG-I πυροδοτεί μια υπεράσπιση κατά της σηματοδότησης-άμβλυνσης αντι-SARS-CoV-2 σε ανθρώπινα πνευμονικά κύτταρα"Document60 pagesH μελέτη με θέμα: "Το RIG-I πυροδοτεί μια υπεράσπιση κατά της σηματοδότησης-άμβλυνσης αντι-SARS-CoV-2 σε ανθρώπινα πνευμονικά κύτταρα"ARISTEIDIS VIKETOSNo ratings yet
- An Antibody That Neutralizes Sars-Cov-1 and Sars-Cov-2 by Binding To A Conserved Spike Epitope Outside The Receptor Binding MotifDocument19 pagesAn Antibody That Neutralizes Sars-Cov-1 and Sars-Cov-2 by Binding To A Conserved Spike Epitope Outside The Receptor Binding Motifchato law officeNo ratings yet
- 1 s2.0 S0021925821006189 MainDocument16 pages1 s2.0 S0021925821006189 MainAngelica Maria Torregroza EspinosaNo ratings yet
- Artigo 1 - Covid-19Document3 pagesArtigo 1 - Covid-19leonardoNo ratings yet
- Versatile and Multivalent Nanobodies Efficiently Neutralize SARSCoV2ScienceDocument7 pagesVersatile and Multivalent Nanobodies Efficiently Neutralize SARSCoV2ScienceDARLENYS YADIRA MOTA MEJIANo ratings yet
- Readings On SARS-CoV-2 (Corona Virus)Document8 pagesReadings On SARS-CoV-2 (Corona Virus)negurii hearteuNo ratings yet
- JIANG 2021 (EPITOPO B) Epitope Profiling Reveals The Critical Antigenic Determinants in SARS-CoV-2 RBD-Based AntigenDocument14 pagesJIANG 2021 (EPITOPO B) Epitope Profiling Reveals The Critical Antigenic Determinants in SARS-CoV-2 RBD-Based AntigenJoão Pedro NunesNo ratings yet
- Neutralizing Antibodies Against SARS-CoV-2 CurrentDocument15 pagesNeutralizing Antibodies Against SARS-CoV-2 CurrentAntonius SimangunsongNo ratings yet
- An Alpaca Nanobody Neutralizes Sars-Cov-2 by Blocking Receptor InteractionDocument9 pagesAn Alpaca Nanobody Neutralizes Sars-Cov-2 by Blocking Receptor InteractionRicardoPeixotoNo ratings yet
- Neuropilin-1 Facilitates SARS-CoV-2 Cell Entry and Infectivity (Cantuti-Castelvetri 2020)Document7 pagesNeuropilin-1 Facilitates SARS-CoV-2 Cell Entry and Infectivity (Cantuti-Castelvetri 2020)gd_hbarNo ratings yet
- Infection Enhancing Anti Sars Cov 2 Antibodies Recognize Both The Original Wuhan D614G Strain And Delta Variants A Potential Risk For Mass Vaccination Nouara Yahi Henri Chahinian Jacques Fantini full chapter pdf docxDocument27 pagesInfection Enhancing Anti Sars Cov 2 Antibodies Recognize Both The Original Wuhan D614G Strain And Delta Variants A Potential Risk For Mass Vaccination Nouara Yahi Henri Chahinian Jacques Fantini full chapter pdf docxtolliekawann100% (7)
- Measuringtheserologic Responsetosevereacute Respiratorysyndrome Coronavirus2Document12 pagesMeasuringtheserologic Responsetosevereacute Respiratorysyndrome Coronavirus2Chris KontomarisNo ratings yet
- Lyophilization of Lipid Nanoparticle For mRNA Vaccine 1682922545Document15 pagesLyophilization of Lipid Nanoparticle For mRNA Vaccine 1682922545Piet van den OetelaarNo ratings yet
- Ab Serum CovidDocument12 pagesAb Serum CovidTania CortázarNo ratings yet
- Jurnal Sinovac 2Document6 pagesJurnal Sinovac 2Delapan SembilanNo ratings yet
- Studio Olandese Su CoronavirusDocument24 pagesStudio Olandese Su CoronavirusantiochioNo ratings yet
- Viruses: Sars-Cov-2 Spike Impairs Dna Damage Repair and Inhibits V (D) J Recombination in VitroDocument10 pagesViruses: Sars-Cov-2 Spike Impairs Dna Damage Repair and Inhibits V (D) J Recombination in Vitromuun yayo100% (1)
- Viruses: Sars-Cov-2 Spike Impairs Dna Damage Repair and Inhibits V (D) J Recombination in VitroDocument10 pagesViruses: Sars-Cov-2 Spike Impairs Dna Damage Repair and Inhibits V (D) J Recombination in VitroMohamed Fawzy mahrousNo ratings yet
- Sars-Cov-2 Can Recruit A Haem Metabolite To Evade Antibody ImmunityDocument19 pagesSars-Cov-2 Can Recruit A Haem Metabolite To Evade Antibody ImmunityShreyasri SainNo ratings yet
- WissenshaftDocument11 pagesWissenshaftkacagj bajazzoNo ratings yet
- Estudio Sobre El RemdesivirDocument28 pagesEstudio Sobre El RemdesivirA24.comNo ratings yet
- ReviewDocument8 pagesReviewIvana EstechoNo ratings yet
- 3 The Architecture of SARS CoV 2 Transcriptome 8Document19 pages3 The Architecture of SARS CoV 2 Transcriptome 8escarlet zapataNo ratings yet
- 2020 05 04 075911v1 FullDocument30 pages2020 05 04 075911v1 Fullandri priantoNo ratings yet
- Talanta: Chenchen Ge, Juan Feng, Jiaming Zhang, Kai Hu, Dou Wang, Ling Zha, Xuejuan Hu, Rongsong LiDocument9 pagesTalanta: Chenchen Ge, Juan Feng, Jiaming Zhang, Kai Hu, Dou Wang, Ling Zha, Xuejuan Hu, Rongsong LipriyaNo ratings yet
- SARS-CoV-2 IgG II ArchitectDocument10 pagesSARS-CoV-2 IgG II ArchitectcalidadlabinfectoNo ratings yet
- Protocolo - 2022Document10 pagesProtocolo - 2022Carlos Alberto Salazar DuqueNo ratings yet
- PIIS016344532300600XDocument4 pagesPIIS016344532300600Xdenemebakalim72No ratings yet
- The Pathophysiology, Diagnosis and Treatment of Corona Virus Disease 2019 (COVID-19)Document12 pagesThe Pathophysiology, Diagnosis and Treatment of Corona Virus Disease 2019 (COVID-19)GabiiGabriielaNo ratings yet
- COVID Vaccine Systematic Review Dong Dai WeiDocument14 pagesCOVID Vaccine Systematic Review Dong Dai WeiJason SnapeNo ratings yet
- Antiviral Peptides As Promising Therapeutics Against SARS-CoV 2Document8 pagesAntiviral Peptides As Promising Therapeutics Against SARS-CoV 2Stalwart sheikhNo ratings yet
- Articulo 8 CovidDocument33 pagesArticulo 8 CovidJuan OrlandoniNo ratings yet
- The SARS-CoV-2 Genome, Its Variants and Their Various Way of ImmunizationDocument7 pagesThe SARS-CoV-2 Genome, Its Variants and Their Various Way of ImmunizationInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- ZNFX1 dsRNADocument24 pagesZNFX1 dsRNAFrancesc Xavier Bofill De RosNo ratings yet
- 705 2010 Article 729Document7 pages705 2010 Article 729Lote LewaivunaNo ratings yet
- Molecular Docking Using Chimera and Autodock VinaDocument22 pagesMolecular Docking Using Chimera and Autodock VinaShisir PuriNo ratings yet
- Two Linear Epitopes On The SARS-CoV-2 SpikeDocument7 pagesTwo Linear Epitopes On The SARS-CoV-2 SpikeRheum RaponticumNo ratings yet
- Sars-Cov-2 Nsp1 Suppresses Host But Not Viral Translation Through A Bipartite MechanismDocument16 pagesSars-Cov-2 Nsp1 Suppresses Host But Not Viral Translation Through A Bipartite MechanismJanina PuenteNo ratings yet
- Journal Pre-Proof: Infection, Genetics and EvolutionDocument29 pagesJournal Pre-Proof: Infection, Genetics and EvolutionMohammed Shuaib AhmedNo ratings yet
- Crispr-Cas12 Sars Cov2Document8 pagesCrispr-Cas12 Sars Cov2Brayann InlvNo ratings yet
- Longitudinal Evaluation and Decline of Antibody Responses in Sars-Cov-2 InfectionDocument24 pagesLongitudinal Evaluation and Decline of Antibody Responses in Sars-Cov-2 InfectionCesar ValleNo ratings yet
- Novel Drugs Targeting The Sars-Cov-2/Covid-19 Machinery: Ariane Sternberg, Dwight L. Mckee and Cord NaujokatDocument11 pagesNovel Drugs Targeting The Sars-Cov-2/Covid-19 Machinery: Ariane Sternberg, Dwight L. Mckee and Cord Naujokatperi umardianaNo ratings yet
- A Prefusion SARS-CoV-2 Spike RNA Vaccine 2020.09.08.280818v1.fullDocument38 pagesA Prefusion SARS-CoV-2 Spike RNA Vaccine 2020.09.08.280818v1.fullDavid MoraNo ratings yet
- 1 s2.0 S1074761320303265 MainDocument17 pages1 s2.0 S1074761320303265 MainAleksandar DimkovskiNo ratings yet
- DiB 2019 CarvalhoDocument11 pagesDiB 2019 CarvalhoAristobolo SilvaNo ratings yet
- Processes: Trimeric Sars-Cov-2 Spike Proteins Produced From Cho Cells in Bioreactors Are High-Quality AntigensDocument11 pagesProcesses: Trimeric Sars-Cov-2 Spike Proteins Produced From Cho Cells in Bioreactors Are High-Quality AntigensHan VoNo ratings yet
- Kimia Medisinal: Oleh Kelompok 8Document16 pagesKimia Medisinal: Oleh Kelompok 8Hasryanto YantoNo ratings yet
- Zerospike Project BrochureDocument24 pagesZerospike Project Brochurestephen.cordnerNo ratings yet
- Biomedicines 10 01950Document16 pagesBiomedicines 10 01950Lija LajiNo ratings yet
- Sars-Cov-2: Phylogenetic Status, Mutations and Therapeutic Research Based On Spike ProteinDocument10 pagesSars-Cov-2: Phylogenetic Status, Mutations and Therapeutic Research Based On Spike ProteinArley GutarraNo ratings yet
- Kim Et Al., 2020Document19 pagesKim Et Al., 2020신유찬No ratings yet
- Journal Pre-Proof: Journal of Controlled ReleaseDocument17 pagesJournal Pre-Proof: Journal of Controlled ReleaseGisela GloryNo ratings yet
- Rapid Test For Detecting Neutralizing Anti RBD Antibodies PDFDocument4 pagesRapid Test For Detecting Neutralizing Anti RBD Antibodies PDFFernando GTgroupNo ratings yet
- Antigenic Characterization of The SARS-CoV-2 Omicron Subvariant BA.2.75Document26 pagesAntigenic Characterization of The SARS-CoV-2 Omicron Subvariant BA.2.75Shaviera dentiNo ratings yet
- Computers in Biology and MedicineDocument10 pagesComputers in Biology and MedicineTausif AlamNo ratings yet
- Mansfield 2004Document5 pagesMansfield 2004Nico Alexander ReyesNo ratings yet
- Sars-Cov-2 Infection Triggers Widespread Host Mrna Decay Leading To An Mrna Export BlockDocument39 pagesSars-Cov-2 Infection Triggers Widespread Host Mrna Decay Leading To An Mrna Export BlockStacey KrellerNo ratings yet
- Vaccination Versus Hybrid ImmunizationDocument9 pagesVaccination Versus Hybrid ImmunizationJovanaNo ratings yet
- Jurnal Kampen 2020Document29 pagesJurnal Kampen 2020Crosco BDLNo ratings yet
- Self-Amplifying RNA Vaccines For Infectious DiseasesDocument13 pagesSelf-Amplifying RNA Vaccines For Infectious DiseasesRicardo SousaNo ratings yet
- Adaptive Immunity To SARS-CoV-2 and COVID-19Document21 pagesAdaptive Immunity To SARS-CoV-2 and COVID-19Yuri YogyaNo ratings yet
- 089 44 Final Biologi Fasa4 DLP T4-62-84Document23 pages089 44 Final Biologi Fasa4 DLP T4-62-84BF CLNo ratings yet
- NEJMoa 2028436Document12 pagesNEJMoa 2028436arman ahdokhshNo ratings yet
- Enders 1954 - Propagation-In-Tissue-Cultures-Of-Cytopathogenic-Agents-From-Patients-With-MeaslesDocument10 pagesEnders 1954 - Propagation-In-Tissue-Cultures-Of-Cytopathogenic-Agents-From-Patients-With-MeaslesRoger TNo ratings yet
- How Effective Are COVIDDocument4 pagesHow Effective Are COVIDMARHUMA HAPSIN. ABBASNo ratings yet
- Article-2021-COVID-19-Neutralizing Antibodies Predict Disease Severity and SurvivalDocument25 pagesArticle-2021-COVID-19-Neutralizing Antibodies Predict Disease Severity and Survivalcarlos ArozamenaNo ratings yet
- Science Abm3425Document24 pagesScience Abm3425Lindomar Bonfim NobreNo ratings yet
- Corti Et Al., 2021Document38 pagesCorti Et Al., 2021LunaNo ratings yet
- Dog & Cat Import FormDocument4 pagesDog & Cat Import FormDavid Paul HensonNo ratings yet
- Anti HBeDocument6 pagesAnti HBeNaveen Kumar MNo ratings yet
- J Transci 2018 10 013Document7 pagesJ Transci 2018 10 013Clara Espinosa ArnandisNo ratings yet
- Chimeric Peptidomimetic Antibiotic Efficiently Neutralizes Lipopolysaccharides (LPS) and Bacteria-Induced Activation of RAW MacrophagesDocument9 pagesChimeric Peptidomimetic Antibiotic Efficiently Neutralizes Lipopolysaccharides (LPS) and Bacteria-Induced Activation of RAW MacrophagesKATIA PAMELA VILLAVICENCIO SANCHEZNo ratings yet
- ViruSure Brochure 2020Document20 pagesViruSure Brochure 2020joeNo ratings yet
- Aid 2011 1502Document148 pagesAid 2011 1502pasargadae_mNo ratings yet
- Jordan2017 Infectious BronchitisDocument28 pagesJordan2017 Infectious BronchitisVicente Vega SanchezNo ratings yet
- 5 Concerns About SARS-CoV2 Biology: A Call To Pause, Deliberate, and Revise Policy - by J Jay Couey - Gigaohm Biological - MediumDocument24 pages5 Concerns About SARS-CoV2 Biology: A Call To Pause, Deliberate, and Revise Policy - by J Jay Couey - Gigaohm Biological - MediumAnonymous lEJuFJMNo ratings yet
- The Journey of Antibody and Antigen Test During COVID-19 Pandemic in Indonesia, The Advantages & DisadvantagesDocument34 pagesThe Journey of Antibody and Antigen Test During COVID-19 Pandemic in Indonesia, The Advantages & DisadvantagesEldo TaufilaNo ratings yet
- Laboratory Testing For COVID-19 - Dr. Trilis Yulianti, M.kesDocument20 pagesLaboratory Testing For COVID-19 - Dr. Trilis Yulianti, M.kesYayax RakhmanNo ratings yet
- DR Robert Malone Speaks OutDocument40 pagesDR Robert Malone Speaks OutB100% (2)
- Rational Design of A Triple-Type Human Papillomavirus Vaccine by Compromising Viral-Type Speci FicityDocument15 pagesRational Design of A Triple-Type Human Papillomavirus Vaccine by Compromising Viral-Type Speci FicitySamer ShamshadNo ratings yet
- Laboratory Techniques in Rabies: Fourth EditionDocument494 pagesLaboratory Techniques in Rabies: Fourth EditionKimjhee Yang WongNo ratings yet
- Tests Krok Gen Bact VirDocument18 pagesTests Krok Gen Bact Viryasserzeddou3000No ratings yet
- Materi Pembicara Dr. TrilisDocument13 pagesMateri Pembicara Dr. TrilisDamian HamzahNo ratings yet
- Nanomaterials 11 01788 v2 5Document25 pagesNanomaterials 11 01788 v2 5Mark LaceyNo ratings yet
- jn.1 Ire CleanDocument6 pagesjn.1 Ire CleanKabakçı HüsnüNo ratings yet
- Produksi Kolostrum Antivirus Avian InfluenzaDocument11 pagesProduksi Kolostrum Antivirus Avian InfluenzaRohendi RohendiNo ratings yet
- Immune Response To HIVDocument9 pagesImmune Response To HIVTugas HeinzNo ratings yet
- Respiratory Virus InfectionsDocument9 pagesRespiratory Virus InfectionsChawki MokademNo ratings yet