Professional Documents
Culture Documents
09chapter 1introduction
09chapter 1introduction
Uploaded by
varsha02jadhavCopyright:
Available Formats
You might also like
- 3-Topical and Transdermal Drug Products-Product Quality Tests PDFDocument6 pages3-Topical and Transdermal Drug Products-Product Quality Tests PDFLinh NguyenNo ratings yet
- HPLC Method Development ProtocolDocument40 pagesHPLC Method Development ProtocolDavid Torres100% (1)
- FDS StudyDocument8 pagesFDS StudyAnnisaIndahPNo ratings yet
- Forced DegradationDocument8 pagesForced DegradationBiyaya San PedroNo ratings yet
- Forced Degradation - Mass BalanceDocument8 pagesForced Degradation - Mass BalanceppiccoliniNo ratings yet
- ReviewDocument11 pagesReviewNoonNo ratings yet
- 1407 1415 1 PB PDFDocument6 pages1407 1415 1 PB PDFBenedito Alvarenga JuniorNo ratings yet
- USP Impurities of Drug (Substances and Products)Document5 pagesUSP Impurities of Drug (Substances and Products)attiah178No ratings yet
- InTech-Analytical Method ValidationDocument19 pagesInTech-Analytical Method ValidationIordache SorinNo ratings yet
- Pharmaceutical Impurities: A ReviewDocument8 pagesPharmaceutical Impurities: A ReviewparasNo ratings yet
- SYNOPSISDocument14 pagesSYNOPSISabhijit.adgube3376No ratings yet
- Forced Degradation StudyDocument4 pagesForced Degradation StudyDavor ŠestanNo ratings yet
- Method Validation in the Drug development Process-김현성Document54 pagesMethod Validation in the Drug development Process-김현성bloannnNo ratings yet
- InTech-Analytical Method ValidationDocument19 pagesInTech-Analytical Method ValidationYolby Milena Rodriguez ArizaNo ratings yet
- Materi Sstability Indicating MethodForce Degradation StudiesDocument19 pagesMateri Sstability Indicating MethodForce Degradation StudiesAtqillah IirNo ratings yet
- Journal of Drug Delivery and TherapeuticsDocument7 pagesJournal of Drug Delivery and TherapeuticsutayNo ratings yet
- A Brief Study On Forced Degradation Studies With Regulatory GuidanceDocument8 pagesA Brief Study On Forced Degradation Studies With Regulatory GuidanceInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- A Review On Step-by-Step Analytical Method ValidationDocument13 pagesA Review On Step-by-Step Analytical Method ValidationIOSR Journal of Pharmacy100% (1)
- A Review On Step-by-Step Analytical Method ValidationDocument13 pagesA Review On Step-by-Step Analytical Method ValidationMd MoinulNo ratings yet
- Analytical Method Development and ValidaDocument8 pagesAnalytical Method Development and ValidaSalman AbuzuhairaNo ratings yet
- AReviewonStep by StepAnalyticalMethodValidationDocument14 pagesAReviewonStep by StepAnalyticalMethodValidationVENESYA SARAH MATULESSYNo ratings yet
- Forced Degradation Studies-DDT June2010-Rd3Document4 pagesForced Degradation Studies-DDT June2010-Rd3Prem GoelNo ratings yet
- Analytical Method Development: April 2020Document14 pagesAnalytical Method Development: April 2020Chuyang ChenNo ratings yet
- Comparison of Various International Guidelines For Analytical Method ValidationDocument12 pagesComparison of Various International Guidelines For Analytical Method Validationeduardo3000No ratings yet
- Usp NFDocument4 pagesUsp NFlirisNo ratings yet
- R G F Who E M R: Egional Uideline or The Astern Editerranean EgionDocument30 pagesR G F Who E M R: Egional Uideline or The Astern Editerranean EgionJenner ButlongNo ratings yet
- Stability Indicating HPLC Method Development A ReviewDocument7 pagesStability Indicating HPLC Method Development A ReviewEditor IJTSRDNo ratings yet
- Definition and TerminologyDocument8 pagesDefinition and Terminologyచిమ్ముల సందీప్ రెడ్డిNo ratings yet
- Challenges in Analytical Method Development ForDocument3 pagesChallenges in Analytical Method Development ForTanuja PathareNo ratings yet
- Forced Degradation StudiesDocument9 pagesForced Degradation Studiesppiccolini100% (1)
- Lecture 5 - 6 & 7 - 2022-1Document20 pagesLecture 5 - 6 & 7 - 2022-1Koki KingNo ratings yet
- AQbD Consultation DocumentDocument15 pagesAQbD Consultation DocumentmadhubiochemNo ratings yet
- USP 1086 Impurities in Drug Substances and Drug ProductsDocument3 pagesUSP 1086 Impurities in Drug Substances and Drug ProductsMuhammad JamilNo ratings yet
- Impurity Profiling of Pharmaceuticals PDFDocument15 pagesImpurity Profiling of Pharmaceuticals PDFvikram kushwahaNo ratings yet
- Chemistry, Manufacturing and Controls Requirements: Exhibit 1: CMC Requirements For A New PharmaceuticalDocument3 pagesChemistry, Manufacturing and Controls Requirements: Exhibit 1: CMC Requirements For A New PharmaceuticalPranav Patel100% (1)
- Development of Validated Stability-Indicating Assay Methods - Critical ReviewDocument31 pagesDevelopment of Validated Stability-Indicating Assay Methods - Critical ReviewJoe Luis Villa MedinaNo ratings yet
- 476-PF OnlineDocument3 pages476-PF OnlinelizdelafuentevNo ratings yet
- 2-Oral Drug Products-Product Quality Tests PDFDocument4 pages2-Oral Drug Products-Product Quality Tests PDFLinh NguyenNo ratings yet
- Ich Q3BDocument31 pagesIch Q3BHussein Abu GhazalehNo ratings yet
- Annex 1: WHO Good Practices For Pharmaceutical Quality Control LaboratoriesDocument49 pagesAnnex 1: WHO Good Practices For Pharmaceutical Quality Control LaboratoriesFrancesca Porcelli100% (1)
- Guidance For Industry: Q3A Impurities in New Drug SubstancesDocument17 pagesGuidance For Industry: Q3A Impurities in New Drug Substances09187135911No ratings yet
- 1 - Introduction To Quality Control and Quality Assurance-RevDocument34 pages1 - Introduction To Quality Control and Quality Assurance-RevBerhanu LimenewNo ratings yet
- 1.3.2 ICH GuidelinesDocument3 pages1.3.2 ICH GuidelinesRushadiJatmikoNo ratings yet
- Analytical Method Validation - Pharmaceutical GuidelinesDocument3 pagesAnalytical Method Validation - Pharmaceutical GuidelinesMSL India100% (1)
- Seminar (Photostability)Document12 pagesSeminar (Photostability)Mr. HIMANSHU PALIWALNo ratings yet
- Ich Guidelines For Stability Testing of New Drug Substance and Drug ProductsDocument39 pagesIch Guidelines For Stability Testing of New Drug Substance and Drug ProductsRahul LakhaniNo ratings yet
- Topical and TransdermalDocument4 pagesTopical and Transdermalshamsi009No ratings yet
- 1226 Verification of Compendial Procedures PDFDocument2 pages1226 Verification of Compendial Procedures PDFmaria mercedesNo ratings yet
- Quality Control Review ArticleDocument18 pagesQuality Control Review ArticleMukesh Tiwari100% (1)
- 2013-09-18 USP Stability 2 ProceduresDocument58 pages2013-09-18 USP Stability 2 ProceduressreekanthsharmaNo ratings yet
- Forced Degradation Studies: Journal of Analytical & Pharmaceutical ResearchDocument5 pagesForced Degradation Studies: Journal of Analytical & Pharmaceutical ResearchBijeshNo ratings yet
- Are View Article On Analytical Method ValidationDocument12 pagesAre View Article On Analytical Method ValidationMelissa KadoumNo ratings yet
- Impurity Profiling Theory and PracticeDocument6 pagesImpurity Profiling Theory and PracticesrichainuluNo ratings yet
- New Trends in Forced Degradation StudiesDocument10 pagesNew Trends in Forced Degradation StudiesLina SakellariouNo ratings yet
- Guidelines For Validation of Analytical ProceduresDocument17 pagesGuidelines For Validation of Analytical ProceduresnickoRiesNo ratings yet
- 1086 Impurities in Drug Substances and Drug: ProductsDocument7 pages1086 Impurities in Drug Substances and Drug: ProductsPrathiNo ratings yet
- Patel Riddhiben M., Patel Piyushbhai M., Patel Natubhai MDocument9 pagesPatel Riddhiben M., Patel Piyushbhai M., Patel Natubhai Msandriss-2No ratings yet
- Fouadspaperin IJCS2014Document12 pagesFouadspaperin IJCS2014varsha02jadhavNo ratings yet
- Oxid 5 MinDocument2 pagesOxid 5 Minvarsha02jadhavNo ratings yet
- Mental Health Challenges in YouthDocument8 pagesMental Health Challenges in Youthvarsha02jadhavNo ratings yet
- Procure To Pay Cycle - CERTIFICATION.1Document9 pagesProcure To Pay Cycle - CERTIFICATION.1varsha02jadhavNo ratings yet
- Oxid 3Document2 pagesOxid 3varsha02jadhavNo ratings yet
- FormatDocument2 pagesFormatvarsha02jadhavNo ratings yet
- Oxid 1Document2 pagesOxid 1varsha02jadhavNo ratings yet
- Sap CVDocument2 pagesSap CVvarsha02jadhavNo ratings yet
- Oxid 2Document2 pagesOxid 2varsha02jadhavNo ratings yet
- 17 Resultant DiscussionDocument35 pages17 Resultant Discussionvarsha02jadhavNo ratings yet
- AnalyticalOutsourcingDocument6 pagesAnalyticalOutsourcingvarsha02jadhavNo ratings yet
- 00 Cover Page For Golden EmbossingDocument1 page00 Cover Page For Golden Embossingvarsha02jadhavNo ratings yet
- 15 MATERIAL AND METHODS NewDocument1 page15 MATERIAL AND METHODS Newvarsha02jadhavNo ratings yet
- 03 AcknowledementDocument2 pages03 Acknowledementvarsha02jadhavNo ratings yet
- 10 Drug Profile and Literature SurveyDocument6 pages10 Drug Profile and Literature Surveyvarsha02jadhavNo ratings yet
- 16 MethodsDocument9 pages16 Methodsvarsha02jadhavNo ratings yet
- 12chapter 3 Results and DiscussionDocument33 pages12chapter 3 Results and Discussionvarsha02jadhavNo ratings yet
- 19 ReferencesDocument4 pages19 Referencesvarsha02jadhavNo ratings yet
- 02 Certificates DinalDocument2 pages02 Certificates Dinalvarsha02jadhavNo ratings yet
- FigureDocument2 pagesFigurevarsha02jadhavNo ratings yet
- ..Experimental Study of Heat Transfer Enhancement Using Waterethylene Glycol BasedDocument8 pages..Experimental Study of Heat Transfer Enhancement Using Waterethylene Glycol BasedSyed Hassan LiaquatNo ratings yet
- Qpedia Mar07 Pressure Drop CalculationsDocument2 pagesQpedia Mar07 Pressure Drop Calculationskriengsak ruangdechNo ratings yet
- Ap005 2Document1 pageAp005 2Floyd PriceNo ratings yet
- Solution Report 78-1Document44 pagesSolution Report 78-1Aryan PrakashNo ratings yet
- Experiment 2 UV-VIS - Ilyas Fikri B. Mohd SaidDocument17 pagesExperiment 2 UV-VIS - Ilyas Fikri B. Mohd Saidnn bbNo ratings yet
- Gynae ReportDocument85 pagesGynae ReportSunil SagarNo ratings yet
- NEETriumph PHYSICAL CHEMISTRYDocument133 pagesNEETriumph PHYSICAL CHEMISTRYHiral Mayank ShahNo ratings yet
- The Classification of Granitic Pegmatites RevisitedDocument22 pagesThe Classification of Granitic Pegmatites RevisitedNyemer BaruelNo ratings yet
- Inspection Test Plan: Material Clause Test Field/Lab Test MethodDocument6 pagesInspection Test Plan: Material Clause Test Field/Lab Test MethodKeshav VarpeNo ratings yet
- p2r Plastic Eom Wil 10150 eDocument28 pagesp2r Plastic Eom Wil 10150 eJOSE INESNo ratings yet
- Analysis of Microstructure and Hardness Behaviour of Grain Refined A390 Alloy Mini Project ReportDocument23 pagesAnalysis of Microstructure and Hardness Behaviour of Grain Refined A390 Alloy Mini Project ReportRahul SanjayanNo ratings yet
- Process Description1Document74 pagesProcess Description1mohsen ranjbarNo ratings yet
- Summary For Antibiotic For USMLE Exam - USMLE MaterialsDocument6 pagesSummary For Antibiotic For USMLE Exam - USMLE MaterialsAshik ThapaNo ratings yet
- Soundness of Aggregates by Use of Sodium Sulfate or Magnesium SulfateDocument5 pagesSoundness of Aggregates by Use of Sodium Sulfate or Magnesium Sulfatekordef zeminNo ratings yet
- Cell Culture MediaDocument11 pagesCell Culture MediaPratibha KushwahaNo ratings yet
- Hipertensi KomplikasiDocument15 pagesHipertensi KomplikasiGhea RofifahNo ratings yet
- Extraction of Essential Oils Present in Aniseed (Saunf), Carum (Ajwain) and Cardamom (Ilaichi)Document16 pagesExtraction of Essential Oils Present in Aniseed (Saunf), Carum (Ajwain) and Cardamom (Ilaichi)suhani bansalNo ratings yet
- MigDocument6 pagesMigPensel KoNtotNo ratings yet
- The Disadvantages of Fly AshDocument8 pagesThe Disadvantages of Fly AshShariar Masud TowhidNo ratings yet
- Chemical Analysis of Powder and Set Forms of Portland Cement Gray MTA White MTADocument7 pagesChemical Analysis of Powder and Set Forms of Portland Cement Gray MTA White MTAMuhammad Abdul Wajid RaiNo ratings yet
- Price CardDocument2 pagesPrice Cardvim patelNo ratings yet
- Advanced Welding ProcessesDocument8 pagesAdvanced Welding ProcessesbabbiNo ratings yet
- Q1.Catalase Is An EnzymeDocument53 pagesQ1.Catalase Is An EnzymeFaith-Misha ChesworthNo ratings yet
- Biofloc For Indoor Shrimp Farming - Second Edition - RAS AquacultureDocument15 pagesBiofloc For Indoor Shrimp Farming - Second Edition - RAS AquacultureJohnTizo-Pioquinto100% (1)
- The Industrial Applications of AlkenesDocument15 pagesThe Industrial Applications of Alkenesiman kashifNo ratings yet
- FDA PresentationDocument27 pagesFDA PresentationTimothy William C. Laurence100% (1)
- Frank P Incropera - Fundamentals of Heat and Mass Transfer (2007, John Wiley)Document19 pagesFrank P Incropera - Fundamentals of Heat and Mass Transfer (2007, John Wiley)shahzad aliNo ratings yet
- 02.CJ Safety and Environmental Instructions S222.328-01 en-USDocument30 pages02.CJ Safety and Environmental Instructions S222.328-01 en-USPedro OliveiraNo ratings yet
- DSA 2011 03526 - Couplings & Flange Adaptors Datasheet 1 PDFDocument4 pagesDSA 2011 03526 - Couplings & Flange Adaptors Datasheet 1 PDFMehedi HasanNo ratings yet
- Atom WorksheetsDocument4 pagesAtom Worksheetsapi-271960049100% (1)
09chapter 1introduction
09chapter 1introduction
Uploaded by
varsha02jadhavOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
09chapter 1introduction
09chapter 1introduction
Uploaded by
varsha02jadhavCopyright:
Available Formats
CHAPTER 1 INTRODUCTION
1.0 INTRODUCTION
1.1 GENERAL INTRODUCTION
Safety and efficacy of pharmaceuticals are two fundamental issues of importance in drug therapy.
The safety of a drug is determined by its pharmacological-/toxicological profile as well as the
adverse effects caused by the impurities in bulk and dosage forms. The number of drugs
introduced into the market is increasing every year. These drugs may be either new entities or
partial structural modification of the existing one. There is a scope, therefore to develop newer
analytical methods for such drugs. Analytical methods development and validation play important
roles in the discovery, development, and manufacture of pharmaceuticals. The official test
methods that result from these processes are used by quality control laboratories to ensure the
identity, purity, potency, and performance of drug products. Identification and quantification of
impurities is a crucial task in pharmaceutical process development for quality and safety. Related
components are the impurities in pharmaceuticals which are unwanted chemicals that remain with
the active pharmaceutical ingredients (APIs), or develop during stability testing, or develop
during formulation or upon aging of both API and formulated APIs to medicines. The presence of
these unwanted chemicals even in small amounts may influence the efficacy and safety of the
pharmaceutical products. Various analytical methodologies are employed for the determination of
related components in pharmaceuticals. There is a great need for development of new analytical
methods for quality evaluation of new emerging drugs.
The need to validate an analytical method is encountered by analysis in the pharmaceutical
industry on an almost daily basis, because adequately validated methods are a necessity for
approvable regulatory filings.
1.2 PHARMACEUTICAL IMPURITIES
An impurity in a drug substance as defined by the International Conference on Harmonisation
(ICH) Guidelines (ICH, 1996) is any component of the drug substance that is not the chemical
entity defined as the drug substance. The safety of a drug product is dependent not only on the
toxicological properties of the active drug substance itself, but also on the impurities that it
contains. ICH Q3A and Q3B (ICH, 2006)address issues relevant to the regulation of impurities in
the drug substance and drug product. While many of the concepts and principles outlined in these
documents are applicable to Abbreviated New Drug Applications (ANDAs), certain additional or
modified restraints need to be considered.
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 1
CHAPTER 1 INTRODUCTION
1.2.1 Classification of impurities
The nature and the quantity of these impurities is governed by a number of factors, including
synthetic route of the drug substance, reaction conditions, quality of the starting material of the
drug substance, reagents, solvents, purification steps, excipients, drug product manufacturing
processes, packaging, and storage of the end product. Based on ICH Q3A (ICH, 1996), drug
substance impurities can be classified into the following categories:
Organic impurities (process- and drug-related)
Inorganic impurities
Residual solvents
Organic impurities can arise during the manufacturing process and/or storage of the drug
substance.
They can be identified or unidentified, volatile or non-volatile, and include:
Starting materials
By-products
Intermediates
Degradation products
Reagents, ligands, and catalysts
Inorganic impurities can result from the manufacturing process. They are normally known and
identified and include:
Reagents, ligands and catalysts
Heavy metals or other residual metals
Inorganic salts
Other materials (e.g., filter aids, charcoal)
Solvents are inorganic or organic liquids used as vehicles for the preparation of solutions or
suspensions in the synthesis of the drug substance or the manufacture of the drug product. Since
these are generally of known toxicity, the selection of appropriate limits for these solvents is
easily accomplished (ICH, 1197).
1.3 TYPES OF STABILITY STUDIES
1.3.1 Stability of Active Pharmaceutical Ingredient (API)
Chemical moiety is group of specific atoms within molecule that is responsible for characteristic
chemical reaction. In case of drug Active Pharmaceutical Ingredient. (API) is basically
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 2
CHAPTER 1 INTRODUCTION
responsible for the therapeutic property of drug. API is also referred as Drug Substance (DS) in
regulatory guidelines such as International Conference on Harmonization of technical
requirements for registration of pharmaceuticals for human use (ICH) and Center for Drug
Evaluation and Research (CDER).
It is necessary to establish purity profile before starting any formulation development work to set
specifications for the allowed levels of impurities. The changes in impurity levels with storage
time must be studied by subjecting the API to various accelerated and stress conditions. This
study will provide information about storage of bulk API and its retesting duration during actual
processing. In this stage there is need to develop method for related substance as well as stability
indicating method to monitor the purity of API. If any of the impurities are increased, or if new
impurities are developed during storage, they are called as “degradation products”. Quality
specifications and limits must also be set for the degradation products as required by ICH.
1.3.2 Stability Indicating Method (SIM)
A Stability indicating method (SIM) is an analytical procedure used to quantitate the decrease in
the amount of the active pharmaceutical ingredient (API) in drug product due to degradation.
According to an FDA guidance document, a stability- indicating method is validated quantitative
analytical procedure that can be used to detect how the stability of drug substance and drug
product changes with time. A stability- indicating method accurately measures the changes in
active ingredient concentration without interference from other degradation products, impurities
and excipients. Stress testing is carried out to demonstrate specificity of the developed method to
measure the changes in concentration of drug substance when little information is available about
potential degradation product. The development of suitable stability indicating method provides a
background for the preformulation studies, stability studies and the development of proper storage
requirements.
1.3.3 Importance of SIM
1. SIM helps to determine the intrinsic stability of drug molecule by establishing degradation
pathway.
2. SIM determines and identifies the likely degradation products.
3. SIM helps to design and assess the API stability requirements.
4. SIM helps to monitor the stability of a given drug in a finished product.
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 3
CHAPTER 1 INTRODUCTION
5. SIM is also used in cleaning validation.
Table 1.1: Summary of ICH guidelines and pharmacopoeia chapters referring SIAM or
forced degradation
Guideline Title of Guideline Reference to SIAM or Forced Degradation
Reference
Q 1 A (R2) Stability testing of new Page 2: Stress testing of drug substance can help
Drug Substances and to identify likely degradation products, which can
Products in turn help to establish degradation pathways
and the intrinsic stability of molecule and validate
stability indicating power of the analytical
procedure used.
Page 2: Validated stability-indicating analytical
procedure should be applied.
Page 7: Analytical procedures should be fully
validated and stabilityindicating.
Q1B Stability Testing: The forced degradation studies should be desired
Photostability to provide suitable information to develop and
Testing of new Drug validate test methods for the confirmatory
Substances studies.
and Products
Q 2 (R1) Validation of Analytical Page 7: As appropriate, this should include
procedures: Text and samples stored under relevant stress conditions:
Methodology light, heat, humidity, acid/base hydrolysis and
oxidation.
Q 3 A (R2) Impurities in new Drug Page 3: The registration application should
Substances include documented evidence that the analytical
procedures are validated and suitable for
detection and quantification of impurities.
Q 3 B (R2) Impurities in new Drug Page 3: The registration application should
Products include documented evidence that the analytical
procedures are validated and suitable for
detection and quantification of degradation
products.
1.3.4 Regulatory Requirements
From a regulatory perspective, forced degradation studies provide data to support the followings:
Identification of possible degradation products
Degradation pathways and intrinsic stability of the drug molecule
Validation of stability indicating analytical procedures.
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 4
CHAPTER 1 INTRODUCTION
Issues addressed in regulatory guidance include:
Forced degradation studies are typically carried out using one batch of material.
Forced degradation conditions are more severe than accelerated stability testing such as
50°C; ≥75% relative humidity; in excess of ICH light conditions; high and low pH,
oxidation, etc.
Photostability should be an integral part of forced degradation study design.
Degradation products that do not form in accelerated or longterm stability may not have to
be isolated or have their structure determined.
Mass balance should be considered.
Issues not specifically addressed in regulatory guidance:
Exact experimental conditions for forced degradation studies (temperatures, duration,
extent of degradation, etc.) are not specified.
Experimental design is left to the applicant's discretion.
There is guidance available from the FDA as well as from private industry on regulatory
requirements for IND and NDA filings.
1.4. Forced degradation strategy
The requirements for forced degradation testing depend on needs and the stage of development of
the compound. For example, pre-clinical through phase-II project needs dictate intense method
development (FDC guide lines, 2003), and the rate of compound attrition is high. Therefore, when
developing a rational study design, forced degradation studies should be focused on method
development activities, and not isolation and identification of degradation products. The focus of
stress testing should be directed to characterization and elucidation of degradation products.
Forced degradation process flow map is presented in Figure 1.1
Step 1 Prediction of Degradation products
Predict most likely degradation products using degradation data base from literature and
organic chemistry knowledge
chemistry knowledge
Step 2 Knowledge of Physicochemical Property of Drug Molecule
Collect information on physicochemical properties of drug molecule from literature to set
HPLC method to monitor degradation behavior
Step 3 Designing of Protocol for Stress Studies
Develop forced degradation protocol based on chemistry and sensitivity of API towards
Step 4 Development of SIAM
different stress conditions
Carry out separation of all degradation products by using HPLC and establish peak purity data
by using
Sinhgad Institute of Pharmacy, Narhe, diode 5array detector
Pune.Page
CHAPTER 1 INTRODUCTION
Step 5 Characterization of Degradation products
Utilize LC-MS, LC-NMR, preparative isolation, and column chromatography, preparative
HPLC to identify unknown degradation products
Step 6 Validation of Developed SIAM
Carry out validation of developed SIAM according to ICH guideline-Analytical procedures and
methods validation
Step 7 Document Degradation products and Mechanisms
Prepare report and establish pathways of degradation along with mechanistic approach
Figure: 1.1 Forced degradation processes flow map
1.5 FORCED DEGRDATION STUDY
Forced degradation studies of API and Drug product include appropriate solid state and solution
state stress conditions (e.g. acid/base hydrolysis, heat, oxidation, and light exposure) in
accordance with ICH guidelines. Forced degradation studies should be conducted whenever a
stability indicating method is required. Studies may need to be repeated as methods, processes, or
formulations change.
1.6.1 Active pharmaceutical ingredient
The specified stress conditions should result in approximately 5-35% degradation of the API or
represent a reasonable maximum condition achievable for the API. The specific conditions
(intensity and duration) used will depend on the chemical characteristics of the API. The stressed
sample should be compared to the unstressed sample (control) and the appropriate blank. A
compound may not necessarily degrade under a given stress condition. No further stressing is
advised in these cases (Reynolds D.,et al. 2007).
1.6.2 Acid hydrolysis
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 6
CHAPTER 1 INTRODUCTION
For an acid catalysed force degradation study for a particular API, the API is exposed to acidic
conditions. The API (at a known concentration) is usually treated with 0.1-1 M acid solution of
either hydrochloric acid or sulphuric acid. For certain APIs that are partially soluble or insoluble
in the described acidic solution, addition of an appropriate co-solvent, or adjustment of solution
pH in the acidic range may be required to achieve dissolution; or the APIs can be run as
suspensions (Reynolds D.W.,et al. 2007). Special attention to the API structure should be paid
when choosing the appropriate co-solvent (i.e. do not use alcohols for acidic conditions due to
their reactivity). Dimethylsulfoxide, acetic acid and propionic acid are useful under acidic
conditions. Additionally, the sample may be heated for a defined time/temperature to accelerate
degradation, depending on the API sensitivity to heat.
1.6.3 Base hydrolysis
For a base catalysed force degradation study for a particular API, the API is exposed to basic
conditions. The API (at a known concentration) is usually treated with 0.1-1 M base solution of
either sodium hydroxide or potassium hydroxide or lithium hydroxide. For certain APIs which are
partially soluble or insoluble in the described basic solution, addition of an appropriate co-solvent,
or adjustment of solution pH may be required to achieve dissolution; or the APIs can be run as
suspensions. Glyme and 1, 4-dioxane facilitates reactions in basic conditions. Additionally, the
sample may be heated for a defined time/temperature to acceleratedegradation, depending on the
API sensitivity to heat.
1.6.4 Oxidative degradation
Oxidation can be carried out under an oxygen atmosphere or in the presence of peroxides. The use
of oxygen is a more realistic model. Free radical initiators may be used to accelerate oxidation.
Generally, a free radical initiator and peroxide will produce all primary oxidation degradation
products observed on real-time stability. Therefore, free radical and/or hydrogen peroxide
conditions are strongly recommended at all stages of development. For peroxide conditions,
hydrogen peroxide reagent, 2, 2`-azobisisoburironitrile etc can be used. As previously indicated,
the addition of an appropriate co-solvent may be necessary, depending on API solubility.
Hydrogen peroxide stress testing can be useful in drug product studies where hydrogen peroxide
is an impurity in an excipient. The sample may be heated for a defined time/ temperature to
accelerate degradation, depending on the API sensitivity to heat.
1.6.5 Thermal degradation
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 7
CHAPTER 1 INTRODUCTION
Solid state stability can be evaluated utilizing accelerated storage temperatures in general greater
than 50°C and 75% relative humidity. The duration of exposure is dependent on the API
sensitivity. If the forced degradation thermal/humidity conditions produce a phase change, it is
recommended to also run thermal/humidity conditions below the critical thermal/ humidity that
produce the phase change.
1.6.6 Photostability
Studies are performed in accordance with ICH photostability guidelines (ICH, 1996). Option 1 or
Option 2 conditions can be used. According to the ICH guidelines, “the design of the forced
degradation experiments is left to the applicant's discretion although the exposure levels should be
justified. The recommended exposures for confirmatory stability studies are an overall
illumination of not less than 1.2 million lux hours and an integrated near ultraviolet energy of not
less than 200W-h/m2. For solution studies, acetonitrile is the co-solvent of choice. Methanol can
produce more artificial degradation products from methoxy radicals produced from light
exposure.
1.6.7 Drug product
Drug product degradation cannot be predicted solely from the stability studies of the API in the
solid state or solution. The non-active pharmaceutical ingredients can also react with the API
orcatalyze degradation reactions. Impurities in the excipients can also lead to degradation in the
drug product not originally observed in the API. For formulations, heat, light, and humidity are
often used. The stress conditions should result in approximately 5-35% degradation of the API or
represent reasonable maximum condition achievable for a given formulation. The specific
conditions used will depend on the chemical characteristics of the drug product. For a solid drug
product, the key experiments are thermal, humidity, photo stability and oxidation, if applicable.
For solution formulations, key experiments are thermal, acid/ base hydrolysis, oxidation and
photostability. It is recommended to compare stressed samples with unstressed samples and an
appropriate blank. For drug product studies, the blank sample is an appropriate placebo. The
stressed placebo sample will provide information about excipient compatibility.
1.2 EMPIRICAL APPROACH TOWARDS FOR FORCED DEGRADTION
It is necessary to carry out forced degradation (stress study) for particular drug substance or drug
product to develop stability indicating method. Generally desired range of degradation should lie
between 5-30%. This achieved by varying the stress conditions like exposure time, temperature or
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 8
CHAPTER 1 INTRODUCTION
concentration of stressing agent (acid, base, oxidizer etc.). Overstressing may destroy the
compound or may lead to further degradation of relevant primary degradants. Under stressing
may fail to generate important degradation products. The degradation studies should be
determined after the maximum recommended time? Stress conditions, even if sufficient
degradation has not at all cost as it would only increase the complexity of the unwise to try to
degrade the drug at all cost as it would only increase the complexity of the method development
with little or no benefit in the quality of the data generated by the method. Specific parameters for
stress testing of drug substance and drug product are shown in following tables.
Table 1.2 General protocols for stress testing of drug substance and drug products
Stress condition Drug substance Drug product
As solid As solution Or Solid Liquidb
suspension dosage
forma
Hydrolysis (Acid, Base and + +c
Thermal)
Oxidative + +
Photo- degradation + + + +
Thermal + + +
Thermal/ Humidity + +
a
For tablets, capsules or powder blend.
b
For oral solutions, oral suspensions, or parenteral.
c
Not required for buffered formulation.
Table No. Recommended stress conditions for drug substance
Stress type Conditions Time
Acid hydrolysis 1 mg/ml in 0.1N (up to 1-7 days
1N)HCL; RT or higher
Base hydrolysis 1 mg/ml in 0.1N (up to 1N) 1-7 days
NaOH; RT or higher
Oxidative/ solution 0.3% (up to 3 %)H2 O2; RT; Few hours to 7 days
protected from light
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 9
CHAPTER 1 INTRODUCTION
Thermala 70ºC Up to 2 weeks
Thermal/ Humiditya 70ºC / 75% RH Up to 2 weeks
Photo-degradation Fluorescent and UV light Up to 2 weeks
a
If the solid drug substance is unstable to thermal stress at high temperature due to melting,
decomposition, etc., use a lower temperature with longer stress time.
b
ICH guidelines for appropriate light exposure: Fluorescent = 1.2 million lux hours,
UV= 200 Wh/ m2
Table. Recommended stress conditions for drug product
1.7 STABILITY-INDICATING METHOD DEVELOPMENT
A stability-indicating method is defined as an analytical method that accurately quantitates the
active ingredients without interference from degradation products, process impurities, excipients,
or other potential impurities. A method that accurately quantitates significant degradation
products may also be considered stability-indicating. A proactive approach to developing a
stability indicating HPLC method should involve forced degradation at the early stages of
development with the key degradation samples used in the method development process. Forced
degradation should be the first step in method development. If forced degradation studies are
performed early, method development and identification of primary degradation products and
unknown impurities can be run in parallel. Using this process, a validated HPLC analytical assay,
mechanisms of degradation, and the impurity/degradation product information for filing can all be
generated without delays in the project timeline.
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 10
CHAPTER 1 INTRODUCTION
1.7.5 Identification of degradation products
Degradation products structure elucidation is a collaborative effort involving the analytical
chemist, processchemist and/or formulator, as well as the degradation, mass spectrometry and
NMR experts.Typically, the focus will be on collecting LC/MS data onlythrough the Phase 1
clinical stage. At the Phase 2 clinical stage and beyond, more time is investedin isolation,
synthesis and structural identity using NMR characterization of forced degradationproducts of
concern.
1.8CHROMATOGRAPHY METHOD DEVELOPMENT
Method development should be based on several considerations. It is preferable to have
maximumsample information to make development fast and desired for intended analytical
methodapplication, physical and chemical properties are most preferable as primary
information.Moreover, separation goal needs to define at beginning so; appropriate method can be
developedfor the purpose. An LC method development is very huge area for even
pharmaceuticals withregulatory requirement of international standards. So, prior to method
validation and usage atquality control many aspects need to focus as per ICH guidelines. Method
development can bebased on a sample and goals as well as available resources for
chromatography but few basic stepsfor method development are can be discussed as given below
(Snyder L., et al.2010).
Steps in method development
1. Sample information, define separation goals
2. Sample pre-treatment, need of special HPLC procedure
3. Selection of detector and detector settings
4. Selection of LC method; preliminary run; estimate best separation conditions
5. Optimize separation conditions
6. Check for problems or requirement for special procedure
7. Method validation
Sample information
1. Number of compounds present
2. Chemical structure of compounds
3. Chemical nature
4. Molecular weight of compounds
5. pKa Value(s) of compounds
6. Sample solubility
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 11
CHAPTER 1 INTRODUCTION
7. Sample stability and storage
8. Concentration range of compounds in sample
9. UV spectra of compounds or properties for detection of compounds
Solvent consumption
Minimum mobile phase use per run is desirable
Determine sample concentration which gives LOQ below the identification threshold in case of
related substancesSample may require sample pre-treatment to remove interferences and/or
protect thecolumn and equipmentAs a part of sample pre-treatment; filter compatibility study is
required
1.8.1 Chromatographic detection
Before the first sample is injected during the HPLC method development we must bereasonably
sure that the detector selected will sense all sample components of interest. Normallyvariable
wavelength UV detector is the first choice of the chromatographers, because of theirconvenience
and applicability for most organic samples. UV spectra can be obtained by PDAdetector. When
the UV response of the sample is inadequate, other detector or derivativeHPLC method can be
used.
1.8.2 Selection of LC method and mobile phase selection in partition chromatography
Chromatography requires a proper balance of the intermolecular forces between the analyte,
themobile phase, and the stationary phase for effective analysis. The important criteria to consider
formethod development are resolution, sensitivity, precision, accuracy, limit of detection, limit
ofquantitation, linearity, reproducibility, and time of analysis and robustness of the method. In all
ofthese, the column quality plays an important role since the peak shape affects all criteria
requiredfor optimum separation. The factors that affect the column efficiency have already been
describedabove. Column dimensions and particle size affect the speed of analysis, resolution,
columnbackpressure, detection limit, and solvent consumption. The future of reversed-phase
HPLC method development will involve a significant increase in the use of use narrow-bore and
micro-bore columns. Often inchoosing a column for partition chromatography, the polarity of the
stationary phase is matchedroughly to that of the analytes in the sample; a mobile phase of
different polarity is used for elution.The analytes must be soluble in the mobile phase and the
solvent must be compatible with theanalytical method. As a general guide, use normal phase
chromatography for the separation of polarcompounds and reversed-phase chromatography for
components that are in the moderately polar tonon-polar range.Normal phase chromatography
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 12
CHAPTER 1 INTRODUCTION
commonly involves the use of silica, aminopropyl, diol, andcyanopropyl stationary phases. These
columns may be used to separate polar compounds such asamines, anilines, nitroaromatics,
phenols, and pesticides. Isocratic elution in reversed-phase chromatography is typically
accomplished using a mobile phase mixture of water and another solvent of lower eluting strength
(acetonitrile, methanol). In cases where the time of analysis is compromised or when the
resolution is poor, gradient elution using 2 or 3 different solvents is recommended. The relative
polarity of a solvent is a useful guide to solvent selection in partitionchromatography. The relative
polarities of the listed solvents may differ slightly depending on theliterature source, since the
scale used to measure polarity may be different. The following shouldsuffice as a general
reference for relative solvent polarity. There is a strong dependence of the retention time on the
mobile phase composition, and theretention parameter may be easily altered by variation of
solvent polarity. This is the easiest way toimprove chromatographic resolution of two overlapping
species or to decrease overall separationtime for components with widely differing retention
values. A good starting point is a mixture ofwater and a polar organic solvent (methanol or
acetonitrile). The effect of mobile phase polarity onelution time can be tested at a few different
solvent proportions. If greater selectivity is required, amobile phase comprising of 3-4 solvents
may be used. Theoretical calculations have indicated thata mobile phase mixture of water, THF,
methanol, and acetonitrile may be used to resolve mostreversed-phase applications within a
reasonable length of time. The various analytes to be separatedmay also be arranged based on the
polarities of their functional groups.
1.8.3 Stationary phases
Column is heart of the chromatography which can be selected depending upon their pore size,
surface area, carbon load, particle size, length, chemistry of column. The degree of retention of a
neutral hydrophobic analyte on a wholly alkyl phase (C18 or C8) can be inferred from the carbon
load value. Maximum C18 and C8 columns were used, 62% and 9%, respectively, for impurity
and forced degradation profiling. Amino, C2, C3, phenyl and cyano columns with variation in
pore size, particle size and dimension were used for the study (Jain D.,et al. 2013).
Table 1.2 Types of column
Type of Column Remark
C-18 ("octadecyl", "ODS") Rugged; retentive; widely used
C-8 ("octyl") Similar to C18, but slightly less retentive
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 13
CHAPTER 1 INTRODUCTION
C-3 and C-4 Less retentive; less stable; used mainly for peptides and
proteins
C-1 ("trimethylsilyl") Least retentive; least stable
Phenyl and phenethyl Moderately retentive; selectivity change
CN ("cyano") Moderately retentive; also normal-phase
NH2 ("amino") Weakly retentive; more often used for normal-phase; less
stable
Polystyrene Stable for 1 < pH < 13; good peak shape and lifetime ;
selectivity change; selectivity change; often less efficient
The most widely used HPLC packing are the long-chain alkyls such as C18 or C8. Thesediffer
somewhat in their overall retentivity, although most separations can be carried out on
eithermaterial. Shorter-chain alkyl packings are less retentive, but are also less stable. The silyl
etherbonds are labile to hydrolysis at low pH. With long-chain packing, the hydrophobicity of the
chainlimits the rate of hydrolysis as well as protecting the underlying silica from dissolution in
basicsolution. Aromatic bonded phases typically have an overall retentivity comparable to that of
C8material, but with an added selectivity for samples which can differ in their interactions
witharomatic groups. More polar groups, such as cyano or amino can also be bonded to silica to
provideselectivity differences compared to the alkyl phases. The resulting packings are more
commonlyused for normal- phase than for reversed-phase LC. Finally, polystyrene based packing
provide aviable alternative to silica for applications in which silanol interactions must be avoided
altogether or for which high pH operation is required. Because such packing have no silanol
groups, their selectivity can be quite different from that of silica-based materials. Beyond the very
tenuous guidelines given above, there is no way to make sweeping generalizations concerning
initial column choices for particular samples. Because selectivity is based on differences is
molecular structure and depends on secondary interactions, the only effective way to establish
suitability of aparticular column for a particular sample is empirical and based on trial-and-error
method.The most common type of HPLC column is the C18 or C8 bonded phase silica.
Theseprovide a good compromise among retention, selectivity, lifetime, operating pressure, etc.
In mostapplications, either a C8 or C18 will do equally well. There is often more selectivity
differencebetween the “same” columns from different manufacturers than between “different”
columns fromthe same source. Silica columns are the most commonly used type because they
tend to be moreefficient and reproducible than their polymeric equivalents. Thelatter can be quite
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 14
CHAPTER 1 INTRODUCTION
useful when anextended pH range is required, or when silanol interactions give rise to excessive
tailing on silicabased column.
1.8.4 Choosing HPLC mobile phase buffers
Buffers are used in HPLC mobile phase preparations in order to achieve
reproduciblechromatography. They are needed when an analyst is dealing with an ionisable
sample species. Inreversed phrase chromatography samples are separated based on their
hydrophobicity. The lesspolar a sample is the longer it is retained on the column. When an analyte
is ionized it becomesmore polar and subsequently less retained on the column. Acids become
ionized as the pHincreases, conversely bases become ionized as the pH decreases. To develop a
rugged methodbuffers should be employed that are at least 2 pH units away from the analytepKa.
This is drawnfrom the Henderson-Hasselbach Equation:
pH = pKa + log([A ]/[HA])
-
Essentially, operating at a pH near to the pKa of the sample analyte means that it will be in
apartially dissociated state, the analyte will partially in its weak acid or base form and partially in
itsconjugate form. This will cause peak distortion in the chromatography are poor
peakreproducibility. Operating with a mobile phase at least 2 pH units away from the
analytepKaensures that in excess of 99% of the sample will be in a single state. Once the proper
pH range forthe mobile phase is determined choosing the correct buffer can begin. Buffer capacity
is optimizedat or near a pH equal to the pKa of the buffer. As a rule of thumb, most buffers work
suitably wellwithin ±1 pH unit of their pKa.
Another consideration when choosing a buffer is the type of detector being used. Citrate may not
besuitable for some UV/Vis applications due to its high UV cut off limit. Likewise, if
massspectroscopy detection is used, a volatile buffer such as TEA or acetate should be employed
whilenon-volatile buffers such as phosphate or citrate should be avoided. Once a buffer range and
typeare identified the proper concentration must be used. Ideally, unless using the buffer as ion-
pairreagent, the buffer should have negligible effect on the overall separation and retention of
thesample analytes. The concentration should be set just highenough to control the mobile phase
pH.But still low enough to avoid possible precipitation of the buffer salts in the presence of
organicsolvents. Typically a buffer concentration in the range of 20-50mM is suitable.It is better
to check the miscibility of an aqueous buffer solution in the highest concentration oforganic
mobile phase that will be present during the course of a gradient HPLC run beforeputting it on
chromatographic system. This can easily be checked by mixing the aqueous portionwith the
correct amount of organic in a beaker and observe the presence of any salting out. Also,recall that
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 15
CHAPTER 1 INTRODUCTION
pH is only defined in an aqueous system. So, when adjusting the pH of the mobile phase, it must
be done prior to the addition of the organic solvent. Proper selection and preparation of the mobile
phase will help ensure good peak shape in a chromatogram. An understanding of pKa for
ananalyte and applying the recommendations outlined above will help in choosing and preparing
the right buffer for the required application.Typically organic analytes are analyzed at a mobile
phase pH either two units greater or less thanpKa of the analyte to avoid any secondary
equilibrium effects that might compromise the chromatography. The pH shift of the mobile phase
lead to faster methoddevelopment, rugged methods and an accurate description of the analyte
retention as a function ofpH at varying organic compositions. The pH of the mobile phase affects
also the analyte UVresponse. Understanding the effects of charge delocalization and conjugation
on the UV responsewill allow the chromatographer to choose the proper pH and wavelength of
detection to obtain amethod with high sensitivity.Reversed-phase HPLC has become the dominant
chromatography technique for separationsand analysis. This is due to the subtle ways in which
molecules interact with the reversed-phasechromatographic surface, offering the chromatographer
remarkable control over the separationprocess through manipulation of the separation conditions.
Optimizing separations may consist ofenhancing resolution in order to better quantitate the key
components or reducing analysis time inorder to increase analytical throughput. In order
tooptimize a separation, the chromatographerneeds to understand the underlying factors that affect
resolution and analysis time.
1.8.5 Variables that affect plate number
Flow rate, column length, particle size, column quality and operating temperature, these
variablesalso affect the run time and pressure. Because HPLC hardware is limited to operating
pressures ofabout 5,000 psi, it is rarely feasible to generate extremely large column plate numbers.
HPLCcolumns today provide 5,000 - 15,000 plates for well-behaved samples. This number has
remainedroughly constant for over twenty years, but the column length needed to achieve it has
decreased bya factor of about 5, with a corresponding decrease in run time. A rough
approximation of the platenumber expected from a given column is estimated by the following
expression:Where, L is the column length in cm and is the packing particle size in μm.The
column in current routine practice consists of a 5-μm bonded-phase silica in a 10 or 15-
cmcolumn. 3-μm packing in shorter columns gives faster analysis but is not as rugged as their 5-
μmcounterparts. Either configuration can generate the 10,000 or so plates required for general-
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 16
CHAPTER 1 INTRODUCTION
purpose HPLC method development. Column dimensions can be arbitrarily divided into the
followingcategories based on internal diameter (this terminology is not universal):
A) Preparative ≥10 mm
B) Analytical 3-5 mm
C) Micro bore ≤ 1 mm
D) Semi-preparative 5-10 mm
E) Narrow bore 2-3 mm
Standard analytical scale columns are typically available in 5, 10, 15, and 25-cm lengths.
Thechromatographic peaks are assumed to be symmetrical, that is chromatogram is Gaussian
shape. Inpractice the chromatographic peaks, however, are rarely symmetrical. Although
mathematicallyelegant general expressions for quantifying peak symmetry can be developed,
practical difficultieshave led chromatographers to the “rough and ready” definition of asymmetry
shown here.10% ofpeak height is typically but not universally used for the measurement. The
asymmetry factorgenerally increases as the measurement is made further down the peak. USP
(pharmacopoeial)methods commonly specify a tailing factor of the peak measured by formula:
Tailing factor =2CB/AB
Tailing factor is measured at 5% of peak height (see Figure 1.2). Tailing factor at 5%
andasymmetry factor at 10% give very roughly equivalent numbers overall, but may be quite
differentin specific cases (depending on the exact shape of the peak).
Figure 1.2 Tailing and asymmetry factors
A bad column can lead to tailing bands in one of two ways. A plugged frit or a void will
causetailing for all bands in the chromatogram. A column packed with “acidic silica” particles
will causetailing of basic (amine) components of the sample. In the Figure 1.3 compound-3 is
basic(dimethylaniline), and it is seen to tail in the top chromatogram, but notthe bottom. The
reason isthat the top separation is carried out with an acidic silica packing. By far the major
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 17
CHAPTER 1 INTRODUCTION
contributor topeak tailing is the existence of secondary retention effects. On silica based columns,
these comeprimarily from interactions with un-derivatizedsilanolgroups.Some silanols are quite
acidic (pKa can range down to 3.5 or lower) which means that they caninteract via ion exchange
at most reasonable pH values. Even at low pH (or with neutral silanols), asufficiently aggressive
base can remove the proton to generate an ion-exchange interaction. Just tomake life interesting,
there is a non-bonding electron pair on the oxygen that can also interact withacids via hydrogen
bonding. Actually, the problem is not interactions with silanols, but ratherhindered interactions
with silanols such that some sample molecules become strongly held via atwo-point interaction
(ion exchange + hydrophobic). The characteristics required for tailing are theexistence of a low
concentration of highly retentive active sites. As a result, these sites are quicklyoverloaded and
attachment / release from these sites may be slow. Each of these latter effects canresult in peak
tailing. Unfortunately, theoretical predictions of mobile phase and stationary phase interactions
with a given set of sample components are not always accurate, but they do help tonarrow down
the choices for method development. The analyst must usually perform a series of trial-and error
experiments with different mobile phase compositions until a satisfactory separationis achieved.
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 18
CHAPTER 1 INTRODUCTION
Figure 1.3 Asymmetric and symmetric chromatograms
1.9 METHOD VALIDATION
Once an analytical method is developed for its intended use, it must be validated. The extent
ofvalidation evolves with the drug development phase. Usually, a limited validation is carried out
tosupport an Investigational New Drug (IND) application and a more extensive validation for
NewDrug Application (NDA) and Marketing Authorization Application (MAA). Typical
parametersrecommended by FDA, USP, and ICH are as follow (ICH, 2005):
1. Specificity
2. Linearity & Range
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 19
CHAPTER 1 INTRODUCTION
3. Precision
(A) Method precision (Repeatability)
(B) Intermediate precision (Ruggedness)
4. Accuracy (Recovery)
5. Solution stability
6. Limit of Detection (LOD)
7. Limit of Quantification (LOQ)
8. Robustness
Method validation is vast area which includes many validation parameters with
differentapproaches for different level of requirement based on intended use of analytical method,
criticalityand regulatory requirements. Validated method also can give the unpredicted or
unknown problemduring the course of routine usage, because validated method has also limited
level of confidence,as method was validated for known or predicted variable parameters or every
method can failsooner or later. But still after method development it needs to be validated as per
requirementwhich gives certain level of confidence for its intended use. A common method
validation protocolis followed for all the method developed during the research project (FDA,
ICH Q2A & Q2B,2005).
1.9.1 Specificity
Specificity is the ability of the method to measure the analyte in the presence of other
relevantcomponents those are expected to be present in a sample. The relevant components might
includeimpurities, degradation products, matrix, etc. Lack of specificity of an individual
procedure may becompensated by other supporting analytical procedure(s).Specificity can also be
demonstrated by verification of the result with an independent analytical procedure. In the case of
chromatographic separation, resolution factors should be obtained for critical separation. Tests for
peak homogeneity, for example, by diode array detection (DAD) ormass spectrometry (MS) are
recommended. The evaluation of the specificity of the method wasdetermined against placebo.
The interference of the excipients of the claimed placebo present inpharmaceutical dosage form is
derived from placebo solution. Further the specificity of the methodtoward the drug is established
by means of checking the interference of the degradation products inthe drug quantification for
assay during the forced degradation study. The peak purity of analytepeak was evaluated in each
degraded sample with respect to total peak purity and three point peakpurity. The peak purity
value must be more than 0.999 in every case.
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 20
CHAPTER 1 INTRODUCTION
1.9.2 Linearity and Range
The linearity of an analytical procedure is its ability (within a given range) to obtain test
results,which are directly proportional to the concentration (amount) of analyte in the sample. A
linearrelationship should be evaluated across the range of the analytical procedure. It is
demonstrateddirectly on the drug substance by dilution of a standard stock solution of the drug
productcomponents, using the proposed procedure. For the establishment of linearity, minimum
of fiveconcentrations are recommended by ICH guideline. The value of correlation co-efficient
(r2) shouldfall around 0.99.
1.9.3 Precision
The precision of an analytical procedure expresses the closeness of agreement (degree of
scatter)between a series of measurements obtained from multiple sampling of the same
homogeneoussample. Precision may be considered at two levels: repeatability and intermediate
precision. Theprecision of an analytical procedure is usually expressed as the variance, standard
deviation orcoefficient of variation of a series of measurements.
Repeatability: Repeatability study is performed by preparing a minimum of 6 determinations
at100% of the test concentration and analyzed as per the respective methodology.
Intermediate Precision: The extent to which intermediate precision should be
establisheddependson the circumstances under which the procedure is intended to be used. The
analyst should establishthe effects of random events on the precision of the analytical procedure.
Typical variations to bestudied include days, analysts, equipment, etc. It is not considered
necessary to study these effectsindividually. Here, intermediate precision of the method is
checked by carrying out six independentassays of test sample preparation on the different day by
another person under the sameexperimental condition and calculated the % RSD of assays.
1.9.4 Accuracy
The accuracy of an analytical procedure expresses the closeness of agreement between the
valuewhich is accepted either as a conventional true value or an accepted reference value and the
valuefound. The evaluation of accuracy has got very prime importance as it deliberately force the
methodto extract the drug and impurities at higher and lower level.
1.9.5 Solution stability
Drug stability in pharmaceutical formulations/active pharmaceutical ingredients is a function
ofstorage conditions and chemical properties of the drug, preservative and its impurities.
Conditionused in stability experiments should reflect situations likely to be encountered during
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 21
CHAPTER 1 INTRODUCTION
actual samplehandling and analysis. Stability data is required to show that the concentration and
purity of analytein the sample at the time of analysis corresponds to the concentration and purity
of analyte at thetime of sampling. Stability of sample solution was established by storage of
sample solution atambient temperature (25°C) for 24h.
1.9.6 Limit of detection
The limit of detection (LOD) for an individual analytical procedure is the lowest amount of
analytein a sample, which can be detected but not necessarily quantitated as an exact value.
Determinationof the signal-to-noise ratio is performed by comparing measured signals from
samples with knownlow concentrations of analytewith those of blank samples and establishing the
minimumconcentration at which the analyte can be reliably detected. A signal-to-noise ratio
between 3 or 2:1is generally considered acceptable for estimating the detection limit. The limit of
detection isevaluated by serial dilutions of analyte stock solution in order to obtain signal to noise
ratios of 3:1.
1.9.7 Limit of quantitation
The quantitation limit of an individual analytical procedure is the lowest amount of analyte in
asample, which can be quantitatively determined with suitable precision and accuracy. The limit
ofquantitation (LOQ) is a parameter of quantitative assays for low levels of compounds in
samplematrices. Determination of the signal-to-noise ratio is performed by comparing measured
signalsfrom samples with known low concentrations of analyte with those of blank samples and
byestablishing the minimum concentration at which the analyte can be reliably quantified. A
typicalsignal-to-noise ratio is 10:1. The limit of quantification was evaluated by serial dilutions of
analytestock solution in order to obtain signal to noise ratios of 10:1.
1.9.8 Robustness
The robustness of an analytical procedure is a measure of its capacity to remain unaffected by
smallbut deliberate variations in method parameters and provides an indication of its reliability
duringnormal usage.
In the case of liquid chromatography, examples of typical variations are:
Influence of variations of pH in a mobile phase
Influence of variations in mobile phase composition
Different columns (different lots and/or suppliers)
Temperature
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 22
CHAPTER 1 INTRODUCTION
Flow rate
The factors chosen for all the drugs under investigation were the flow rate, mobile
phasecomposition, pH of a mobile phase and using different lot of LC column. The observation
shall besummarized and critical parameters shall be listed out in the validation report. System
suitabilityparameter must be within the limit of acceptance criteria as mentioned in the method.
1.9.9 Validation characteristics of the tests
Validation characteristics of the various types of tests are listed in Table 1.3
Table 1.3 Validation characteristics of the tests
1.9.10 Advantages of analytical method validation
The advantages of the analytical method validation are as follow:
The biggest advantage of method validation is that it builds a degree of confidence, not
onlyfor the developer but also to the user.
Although the validation exercise may appear costly and time consuming, it results
inexpensive, eliminates frustrating repetitions and leads to better time management in the
end.
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 23
CHAPTER 1 INTRODUCTION
Minor changes in the conditions such as reagent supplier or grade, analytical setup are
unavoidable due to obvious reasons but the method validation absorbs the shock of such
conditions and pays for more than invested on the process.
1.10 DEGRADATION KINETICS:
In general, reaction kinetics is the study of rate of chemical change and the way in which this rate
is influenced by conditions of concentration of reactants, products and other chemical species
which may be present, and the factors such as solvent, pressure and temperature. The rate of
reaction can be understood by studying the time course of change in the concentration.The rate of
the reaction is accepted only when the reactants are present either in a solution phase or in a
gaseous phase.It provides a rational approach to stabilization of drug products and prediction of
shelf- life and optimum storage conditions. e.g. thiamine HCl is most stable at pH 2-3 and is
unstable at pH above 6. If this is combined with a buffered vehicle of say pH 8 or 9 the vitamin is
rapidly inactivated. The rate of reaction is faster in solution dosage form than in suspension.
1.10.1 Rate, Order of reaction:
The rate, velocity, or speed of reaction is given by the expression dc/dt, where dc is the increase
or decrease of concentration over an time interval dt. According to law of mass action, the rate of
chemical reaction is proportional to the product of the molar rate concentration of the reactants
each rise to a power usually equal to the number of molecules, a and b of the substances A and B,
respectively, undergoing reaction. In the reaction.
aA+bB+.........=Products
the rate of reaction is
1 d [ A]
Rate = -
a dt
1 d [b]
- = .... k[A]a [B]b
b dt
Where k is the rate constant
The overall order of reaction is the sum of the exponents (a+b) of the concentration terms, A and
B. The order with respect to one of the reactants, A or B, is the exponent of a or b of which
particular concentration term.
1.10.2Rate constant, Half life, Shelf life:
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 24
CHAPTER 1 INTRODUCTION
1.10.2.1 Specific rate constant
The constant, k appearing in the rate law associated with a single step reaction is called the
specific rate constant for the reaction. Any change in the condition of the reaction i.e. in
temperature or solvent, or a slight change in one of the reacting species, will lead to rate law
having a different value for the specific rate constant. Experimentally, a change of specific rate
constant corresponds simply to a change in the slope of the line given by rate equation.
1.10.2.2 Half life
It is the time required for the concentration of the reactant to reduce to half of its initial
concentration. It is represented by t1/2.
1.10.2.3 Shelf life:
It is the time required for the concentration of the reactant to reduce to 90% of its initial
concentration. It is represented by t90.
1.10.3 Order of reaction:
Order of reaction is defined as the number of concentration terms on which the rate of reaction
depends when determined experimentally.
The orders which represent chemical reaction are as follows
1. Zero order
2. First order
3. pseudo first order
4. Second order
1.10.3.1 Zero order:
A reaction in which the rate of reaction does not depend on the concentration terms of the
reactants.
It is mathematically expressed as,
dc
- = ko
dt
Where ko- specific rate rate constant for zero order
Eg.Oxidation of vitamin A in an oily solution.
1.10.3.2 first order:
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 25
CHAPTER 1 INTRODUCTION
A reaction in which the rate of reaction does depends on the concentration of onereactants.
The first order rate equation can be written as
dc
- = k1 c
dt
Where k1 -specific rate rate constant for first order
C - concentration of the reactant
Eg.Decomposition of the hydrogen peroxide catalyzed by 0.02N potassium iodide.
1.10.3.3Pseudo first order:
A reaction which is originally a second order but behave like a first order reaction.
According to the definition the rate depends on the concentration terms of two reactant.
Pseudo first order rate equation can be written as
dc
- = k2 [A][B]
dt
Where A&B - reactant in reaction
k2 - Second order constant
Thus the rate depend upon the concentration of one reactant (A)i.e. first order reaction.
This type of reaction termed as “Apparent first order”.
1.10.3.4 Second order:
A reaction in which the rate of reaction does depends on the concentration term of two reactants
which rise to the power one.
A + B -----------------» Product
The first order rate equation can be written as
−da −db
= = k2 [A][B]
dt dt
Where [A]&[B]- concentration of A and B respectively
k2 - rate constant for second order
Eg.Hydrolysis of chlorbutanol on presence of sodium hydroxide.
1.10.4 Determination of order:
The order of reaction can be determined by several methods.
1.10.4.1 Substitution method:
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 26
CHAPTER 1 INTRODUCTION
The data accumulated in a kineic study can be substituted in the integrated form of the equation
which describe he various orders. When the equation is found in which the calculated k value
remain constant within the limit of experimental variation, the reaction is considered to be of that
order.
Figure 1.4A linear plot of log C vs time for a first order
1.10.4.2 Graphical method:
A plot of the data in the form of a graph as shown in figure can also be used to ascertain the order.
If a straight line results when concentration is plotted against t, the reaction is zero order. The
reaction is first order if log(a-x) vs tyield a straight line, and it is second order if 1/(a-x) vs tgives a
straight line.
1.10.4.3 Half life method:
In a zero order reaction half life is proportional to the initial concentration, a, as observed in table
no. 1.4. The half life of a first order reaction is independent of a; t1/2 for a second order reaction in
which a = b, is proportional to 1/a.
Table 1.4 Rate and Half life equation
Order Integrated rate equation Half-life equation
o x = kt a
t1/2 =
2k
1 a k 0.693
log = t t1/2 =
a−x 2.303 k
2 x 1
= kt t1/2 =
a(a−x ) ak
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 27
CHAPTER 1 INTRODUCTION
1.11DRUG PROFILE:
Table 1.4: Drug profile of Tenofovir
Name of Drug: Tenofovir
Official in: British Pharmacopoeia 2012
European Pharmacopoeia 7.0
Structure:
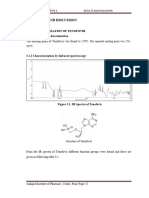
Figure 1.5: Structure of Tenofovir
Molecular formula: C9H14N5O4P
Molecular weight: 287.2123
Chemical name: ({[(2R)-1-(6-amino-9H-purin-9-yl)propan-2-
yl]oxy}methyl)phosphonic acid
Description: white to off-white crystalline powder
Solubility: Slightly soluble in water, soluble in methanol, very
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 28
CHAPTER 1 INTRODUCTION
slightly soluble in dichloromethane
Category: Antiretroviral drugs known as nucleotide analogue reverse
transcriptase inhibitors (nRTIs)
pKa 3.75
logP 1.25 at 25 °C
Melting point 276-280 °C
pH 6.5
1.12 NEED OF WORK
Literature survey reveals that there are very few optimized and validated stability indicating assay
method using factorial design reported for Tenofovir. Also stress studies are not reported as per
ICH guidelines, hence there was need to develop optimized stability indicating method for
Tenofovir API by RP-HPLC by systematic forced degradation studies.
1.12.1 AIM AND OBJECTIVE OF PRESENT WORK
The aim of present work was “To Develop a Validated Stability Indicating RP HPLC method to
investigate degradation profile and kinetics of degradation of Tenofovir”.
The objectives of present work were:-
1. To develop the RP-HPLC method for estimation of Tenofovir
2. To carry out the stress degradation of Etravirine
3. To carry out the development and validation of stability indicating assay method
4. To carry out the degradation kinetics of Etravirine
1.13 PLAN OF WORK
Selection and procurement of Drug and other chemicals
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 29
CHAPTER 1 INTRODUCTION
Literature survey
Development of RP-HPLC method of drug
Stress degradation studies
Development of stability indicating RP-HPLC method
Validation of developed method
Thesis writing and publication
1.14 LITERATURE SURVEY
Table No.1.6Literature survey for analytical methods of Etravirine
Type of Column Mobile Phase Flow Detector/
Method rate wavelength
Title :-
DEVELOPMENT AND VALIDATION OF STABILITY INDICATING RP-HPLC METHOD FOR
TENOFOVIR NANOPARTICLE FORMULATION
SYED SAJJAD HUSSEN*a, PREMNATH SHENOYb, UDUPAc, MUDDU KRISHNAa
Lichrocart (C18) (250mm × 4.6mm, 5 μm particle size) column and a mobile phase composed of acetonitrile and 0.025M potassium
di hydrogen phosphate buffer (pH 3.0 adjusted by using 10% v/v Orthophosphoric acid) in the ratio 35:65 (v/v) was used, and the
detection wavelength of 260 nm.
HPLC C18 Potassium dihydrogen phosphate pH 3.45 1ml/ PDA/308
with 0.2 ml triethylamine and Acetonitrile min nm
25:75 v/v
Title :- Stability indicating RP-HPLC method development and validation of
Tenofovir in Bulk and Pharmaceutical formulation
Sivaram Manavarthi*and Gurmeet Singh Chhabra
Der Pharma Chemica, 2014, 6(2):401-409
Perkin Elmer HPLC using C18(Phenomenex 100 x 4.6 mm x 5 micron) Column and
optimized mobile phase consists of Methanol as Solvent-A,10 mM potassium di hydrogen ortho phosphate buffer
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 30
CHAPTER 1 INTRODUCTION
of
pH -3 as a Solvent-B in the ratio of 30:70 % v/vand UV detection was carried out at 260 nm.
UPLC C18 10 mM ammonium formate and methanol PDA/308
80:20 v/v
Stability Indicating RP-UPLC Method for
Title :-
Assay of Emtricitabine and Tenofovir
Disoproxil Fumarate in Bulk and
Dosage Forms
Bommakanti Valli Purnima1,2, Tummala Vijaya Bhaskara Reddy1,
Yadlapalli Srinivas Rao3, Golkonda Ramu4, Dittakavi Ramachandran1*
HPLC C18 0 .1% acetic acid in water and 100% 1.3ml ESI
acetonitrile 60:40 v/v /min
Title :- LC-MS/MS determination of etravirine in rat plasma and its application in
pharmacokinetic studies
HPLC C18 2 mM ammonium acetate aqueous solution 0.3ml ESI
containing 0.1% formic acid and a 0.1 % /min
formic acid in methanol 65:35 v/v
Title :- Stability Indicating RP–HPLC Method for The Estimation of Etravirine in Pure and
Tablet Dosage Form
RP-HPLC C18 0.02M potassium dihydrogen phosphate & 1ml/ UV/285nm
0.003M di potassiumhydrogen phosphate min
buffer pH adjusted to 3.5 with 0.1%
orthophosphoric acid and acetonitrile
45:55v/v
Sinhgad Institute of Pharmacy, Narhe, Pune.Page 31
You might also like
- 3-Topical and Transdermal Drug Products-Product Quality Tests PDFDocument6 pages3-Topical and Transdermal Drug Products-Product Quality Tests PDFLinh NguyenNo ratings yet
- HPLC Method Development ProtocolDocument40 pagesHPLC Method Development ProtocolDavid Torres100% (1)
- FDS StudyDocument8 pagesFDS StudyAnnisaIndahPNo ratings yet
- Forced DegradationDocument8 pagesForced DegradationBiyaya San PedroNo ratings yet
- Forced Degradation - Mass BalanceDocument8 pagesForced Degradation - Mass BalanceppiccoliniNo ratings yet
- ReviewDocument11 pagesReviewNoonNo ratings yet
- 1407 1415 1 PB PDFDocument6 pages1407 1415 1 PB PDFBenedito Alvarenga JuniorNo ratings yet
- USP Impurities of Drug (Substances and Products)Document5 pagesUSP Impurities of Drug (Substances and Products)attiah178No ratings yet
- InTech-Analytical Method ValidationDocument19 pagesInTech-Analytical Method ValidationIordache SorinNo ratings yet
- Pharmaceutical Impurities: A ReviewDocument8 pagesPharmaceutical Impurities: A ReviewparasNo ratings yet
- SYNOPSISDocument14 pagesSYNOPSISabhijit.adgube3376No ratings yet
- Forced Degradation StudyDocument4 pagesForced Degradation StudyDavor ŠestanNo ratings yet
- Method Validation in the Drug development Process-김현성Document54 pagesMethod Validation in the Drug development Process-김현성bloannnNo ratings yet
- InTech-Analytical Method ValidationDocument19 pagesInTech-Analytical Method ValidationYolby Milena Rodriguez ArizaNo ratings yet
- Materi Sstability Indicating MethodForce Degradation StudiesDocument19 pagesMateri Sstability Indicating MethodForce Degradation StudiesAtqillah IirNo ratings yet
- Journal of Drug Delivery and TherapeuticsDocument7 pagesJournal of Drug Delivery and TherapeuticsutayNo ratings yet
- A Brief Study On Forced Degradation Studies With Regulatory GuidanceDocument8 pagesA Brief Study On Forced Degradation Studies With Regulatory GuidanceInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- A Review On Step-by-Step Analytical Method ValidationDocument13 pagesA Review On Step-by-Step Analytical Method ValidationIOSR Journal of Pharmacy100% (1)
- A Review On Step-by-Step Analytical Method ValidationDocument13 pagesA Review On Step-by-Step Analytical Method ValidationMd MoinulNo ratings yet
- Analytical Method Development and ValidaDocument8 pagesAnalytical Method Development and ValidaSalman AbuzuhairaNo ratings yet
- AReviewonStep by StepAnalyticalMethodValidationDocument14 pagesAReviewonStep by StepAnalyticalMethodValidationVENESYA SARAH MATULESSYNo ratings yet
- Forced Degradation Studies-DDT June2010-Rd3Document4 pagesForced Degradation Studies-DDT June2010-Rd3Prem GoelNo ratings yet
- Analytical Method Development: April 2020Document14 pagesAnalytical Method Development: April 2020Chuyang ChenNo ratings yet
- Comparison of Various International Guidelines For Analytical Method ValidationDocument12 pagesComparison of Various International Guidelines For Analytical Method Validationeduardo3000No ratings yet
- Usp NFDocument4 pagesUsp NFlirisNo ratings yet
- R G F Who E M R: Egional Uideline or The Astern Editerranean EgionDocument30 pagesR G F Who E M R: Egional Uideline or The Astern Editerranean EgionJenner ButlongNo ratings yet
- Stability Indicating HPLC Method Development A ReviewDocument7 pagesStability Indicating HPLC Method Development A ReviewEditor IJTSRDNo ratings yet
- Definition and TerminologyDocument8 pagesDefinition and Terminologyచిమ్ముల సందీప్ రెడ్డిNo ratings yet
- Challenges in Analytical Method Development ForDocument3 pagesChallenges in Analytical Method Development ForTanuja PathareNo ratings yet
- Forced Degradation StudiesDocument9 pagesForced Degradation Studiesppiccolini100% (1)
- Lecture 5 - 6 & 7 - 2022-1Document20 pagesLecture 5 - 6 & 7 - 2022-1Koki KingNo ratings yet
- AQbD Consultation DocumentDocument15 pagesAQbD Consultation DocumentmadhubiochemNo ratings yet
- USP 1086 Impurities in Drug Substances and Drug ProductsDocument3 pagesUSP 1086 Impurities in Drug Substances and Drug ProductsMuhammad JamilNo ratings yet
- Impurity Profiling of Pharmaceuticals PDFDocument15 pagesImpurity Profiling of Pharmaceuticals PDFvikram kushwahaNo ratings yet
- Chemistry, Manufacturing and Controls Requirements: Exhibit 1: CMC Requirements For A New PharmaceuticalDocument3 pagesChemistry, Manufacturing and Controls Requirements: Exhibit 1: CMC Requirements For A New PharmaceuticalPranav Patel100% (1)
- Development of Validated Stability-Indicating Assay Methods - Critical ReviewDocument31 pagesDevelopment of Validated Stability-Indicating Assay Methods - Critical ReviewJoe Luis Villa MedinaNo ratings yet
- 476-PF OnlineDocument3 pages476-PF OnlinelizdelafuentevNo ratings yet
- 2-Oral Drug Products-Product Quality Tests PDFDocument4 pages2-Oral Drug Products-Product Quality Tests PDFLinh NguyenNo ratings yet
- Ich Q3BDocument31 pagesIch Q3BHussein Abu GhazalehNo ratings yet
- Annex 1: WHO Good Practices For Pharmaceutical Quality Control LaboratoriesDocument49 pagesAnnex 1: WHO Good Practices For Pharmaceutical Quality Control LaboratoriesFrancesca Porcelli100% (1)
- Guidance For Industry: Q3A Impurities in New Drug SubstancesDocument17 pagesGuidance For Industry: Q3A Impurities in New Drug Substances09187135911No ratings yet
- 1 - Introduction To Quality Control and Quality Assurance-RevDocument34 pages1 - Introduction To Quality Control and Quality Assurance-RevBerhanu LimenewNo ratings yet
- 1.3.2 ICH GuidelinesDocument3 pages1.3.2 ICH GuidelinesRushadiJatmikoNo ratings yet
- Analytical Method Validation - Pharmaceutical GuidelinesDocument3 pagesAnalytical Method Validation - Pharmaceutical GuidelinesMSL India100% (1)
- Seminar (Photostability)Document12 pagesSeminar (Photostability)Mr. HIMANSHU PALIWALNo ratings yet
- Ich Guidelines For Stability Testing of New Drug Substance and Drug ProductsDocument39 pagesIch Guidelines For Stability Testing of New Drug Substance and Drug ProductsRahul LakhaniNo ratings yet
- Topical and TransdermalDocument4 pagesTopical and Transdermalshamsi009No ratings yet
- 1226 Verification of Compendial Procedures PDFDocument2 pages1226 Verification of Compendial Procedures PDFmaria mercedesNo ratings yet
- Quality Control Review ArticleDocument18 pagesQuality Control Review ArticleMukesh Tiwari100% (1)
- 2013-09-18 USP Stability 2 ProceduresDocument58 pages2013-09-18 USP Stability 2 ProceduressreekanthsharmaNo ratings yet
- Forced Degradation Studies: Journal of Analytical & Pharmaceutical ResearchDocument5 pagesForced Degradation Studies: Journal of Analytical & Pharmaceutical ResearchBijeshNo ratings yet
- Are View Article On Analytical Method ValidationDocument12 pagesAre View Article On Analytical Method ValidationMelissa KadoumNo ratings yet
- Impurity Profiling Theory and PracticeDocument6 pagesImpurity Profiling Theory and PracticesrichainuluNo ratings yet
- New Trends in Forced Degradation StudiesDocument10 pagesNew Trends in Forced Degradation StudiesLina SakellariouNo ratings yet
- Guidelines For Validation of Analytical ProceduresDocument17 pagesGuidelines For Validation of Analytical ProceduresnickoRiesNo ratings yet
- 1086 Impurities in Drug Substances and Drug: ProductsDocument7 pages1086 Impurities in Drug Substances and Drug: ProductsPrathiNo ratings yet
- Patel Riddhiben M., Patel Piyushbhai M., Patel Natubhai MDocument9 pagesPatel Riddhiben M., Patel Piyushbhai M., Patel Natubhai Msandriss-2No ratings yet
- Fouadspaperin IJCS2014Document12 pagesFouadspaperin IJCS2014varsha02jadhavNo ratings yet
- Oxid 5 MinDocument2 pagesOxid 5 Minvarsha02jadhavNo ratings yet
- Mental Health Challenges in YouthDocument8 pagesMental Health Challenges in Youthvarsha02jadhavNo ratings yet
- Procure To Pay Cycle - CERTIFICATION.1Document9 pagesProcure To Pay Cycle - CERTIFICATION.1varsha02jadhavNo ratings yet
- Oxid 3Document2 pagesOxid 3varsha02jadhavNo ratings yet
- FormatDocument2 pagesFormatvarsha02jadhavNo ratings yet
- Oxid 1Document2 pagesOxid 1varsha02jadhavNo ratings yet
- Sap CVDocument2 pagesSap CVvarsha02jadhavNo ratings yet
- Oxid 2Document2 pagesOxid 2varsha02jadhavNo ratings yet
- 17 Resultant DiscussionDocument35 pages17 Resultant Discussionvarsha02jadhavNo ratings yet
- AnalyticalOutsourcingDocument6 pagesAnalyticalOutsourcingvarsha02jadhavNo ratings yet
- 00 Cover Page For Golden EmbossingDocument1 page00 Cover Page For Golden Embossingvarsha02jadhavNo ratings yet
- 15 MATERIAL AND METHODS NewDocument1 page15 MATERIAL AND METHODS Newvarsha02jadhavNo ratings yet
- 03 AcknowledementDocument2 pages03 Acknowledementvarsha02jadhavNo ratings yet
- 10 Drug Profile and Literature SurveyDocument6 pages10 Drug Profile and Literature Surveyvarsha02jadhavNo ratings yet
- 16 MethodsDocument9 pages16 Methodsvarsha02jadhavNo ratings yet
- 12chapter 3 Results and DiscussionDocument33 pages12chapter 3 Results and Discussionvarsha02jadhavNo ratings yet
- 19 ReferencesDocument4 pages19 Referencesvarsha02jadhavNo ratings yet
- 02 Certificates DinalDocument2 pages02 Certificates Dinalvarsha02jadhavNo ratings yet
- FigureDocument2 pagesFigurevarsha02jadhavNo ratings yet
- ..Experimental Study of Heat Transfer Enhancement Using Waterethylene Glycol BasedDocument8 pages..Experimental Study of Heat Transfer Enhancement Using Waterethylene Glycol BasedSyed Hassan LiaquatNo ratings yet
- Qpedia Mar07 Pressure Drop CalculationsDocument2 pagesQpedia Mar07 Pressure Drop Calculationskriengsak ruangdechNo ratings yet
- Ap005 2Document1 pageAp005 2Floyd PriceNo ratings yet
- Solution Report 78-1Document44 pagesSolution Report 78-1Aryan PrakashNo ratings yet
- Experiment 2 UV-VIS - Ilyas Fikri B. Mohd SaidDocument17 pagesExperiment 2 UV-VIS - Ilyas Fikri B. Mohd Saidnn bbNo ratings yet
- Gynae ReportDocument85 pagesGynae ReportSunil SagarNo ratings yet
- NEETriumph PHYSICAL CHEMISTRYDocument133 pagesNEETriumph PHYSICAL CHEMISTRYHiral Mayank ShahNo ratings yet
- The Classification of Granitic Pegmatites RevisitedDocument22 pagesThe Classification of Granitic Pegmatites RevisitedNyemer BaruelNo ratings yet
- Inspection Test Plan: Material Clause Test Field/Lab Test MethodDocument6 pagesInspection Test Plan: Material Clause Test Field/Lab Test MethodKeshav VarpeNo ratings yet
- p2r Plastic Eom Wil 10150 eDocument28 pagesp2r Plastic Eom Wil 10150 eJOSE INESNo ratings yet
- Analysis of Microstructure and Hardness Behaviour of Grain Refined A390 Alloy Mini Project ReportDocument23 pagesAnalysis of Microstructure and Hardness Behaviour of Grain Refined A390 Alloy Mini Project ReportRahul SanjayanNo ratings yet
- Process Description1Document74 pagesProcess Description1mohsen ranjbarNo ratings yet
- Summary For Antibiotic For USMLE Exam - USMLE MaterialsDocument6 pagesSummary For Antibiotic For USMLE Exam - USMLE MaterialsAshik ThapaNo ratings yet
- Soundness of Aggregates by Use of Sodium Sulfate or Magnesium SulfateDocument5 pagesSoundness of Aggregates by Use of Sodium Sulfate or Magnesium Sulfatekordef zeminNo ratings yet
- Cell Culture MediaDocument11 pagesCell Culture MediaPratibha KushwahaNo ratings yet
- Hipertensi KomplikasiDocument15 pagesHipertensi KomplikasiGhea RofifahNo ratings yet
- Extraction of Essential Oils Present in Aniseed (Saunf), Carum (Ajwain) and Cardamom (Ilaichi)Document16 pagesExtraction of Essential Oils Present in Aniseed (Saunf), Carum (Ajwain) and Cardamom (Ilaichi)suhani bansalNo ratings yet
- MigDocument6 pagesMigPensel KoNtotNo ratings yet
- The Disadvantages of Fly AshDocument8 pagesThe Disadvantages of Fly AshShariar Masud TowhidNo ratings yet
- Chemical Analysis of Powder and Set Forms of Portland Cement Gray MTA White MTADocument7 pagesChemical Analysis of Powder and Set Forms of Portland Cement Gray MTA White MTAMuhammad Abdul Wajid RaiNo ratings yet
- Price CardDocument2 pagesPrice Cardvim patelNo ratings yet
- Advanced Welding ProcessesDocument8 pagesAdvanced Welding ProcessesbabbiNo ratings yet
- Q1.Catalase Is An EnzymeDocument53 pagesQ1.Catalase Is An EnzymeFaith-Misha ChesworthNo ratings yet
- Biofloc For Indoor Shrimp Farming - Second Edition - RAS AquacultureDocument15 pagesBiofloc For Indoor Shrimp Farming - Second Edition - RAS AquacultureJohnTizo-Pioquinto100% (1)
- The Industrial Applications of AlkenesDocument15 pagesThe Industrial Applications of Alkenesiman kashifNo ratings yet
- FDA PresentationDocument27 pagesFDA PresentationTimothy William C. Laurence100% (1)
- Frank P Incropera - Fundamentals of Heat and Mass Transfer (2007, John Wiley)Document19 pagesFrank P Incropera - Fundamentals of Heat and Mass Transfer (2007, John Wiley)shahzad aliNo ratings yet
- 02.CJ Safety and Environmental Instructions S222.328-01 en-USDocument30 pages02.CJ Safety and Environmental Instructions S222.328-01 en-USPedro OliveiraNo ratings yet
- DSA 2011 03526 - Couplings & Flange Adaptors Datasheet 1 PDFDocument4 pagesDSA 2011 03526 - Couplings & Flange Adaptors Datasheet 1 PDFMehedi HasanNo ratings yet
- Atom WorksheetsDocument4 pagesAtom Worksheetsapi-271960049100% (1)