Professional Documents
Culture Documents
Analytical Chemistry Expt 5
Analytical Chemistry Expt 5
Uploaded by
Class recordingOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Analytical Chemistry Expt 5
Analytical Chemistry Expt 5
Uploaded by
Class recordingCopyright:
Available Formats
ANALYTICAL CHEMISTRY
Name: Date Submitted:
Program/Year & Section: Instructor:
Experiment 5
RATE OF A REACTION
I. OVERVIEW
The speed at which a reaction proceeds is called the rate of a reaction. During a
chemical reaction, the amount of reactants decreases with time, and simultaneously
the amount of products increases. The reaction rate can be determined by
measuring the change in concentration of any of the products or reactants over a
specific length of time.
II. OBJECTIVE
To determine the factors that affect reaction rates
III. MATERIALS
One empty 500 mL soda plastic bottle or a 500 mL plastic water bottle
3% hydrogen peroxide (½ cup)
Dishwashing liquid solution or any soap solution
Baker’s active yeast (available in supermarkets or bakery supply stores)
Food coloring (optional)
IV. PROCEDURES
1. Dissolve one teaspoon or one packet of active yeast in a small amount of
warm water. Keep still for about 5 minutes.
2. Dilute a small amount of dishwashing liquid in about ¼ cup of water, or
dissolve soap in water.
3. Place about ¼ cup of the dishwashing liquid solution or soap solution into the
plastic bottle. Two to three drops of food color can be added and mixed.
4. Add ½ cup of hydrogen peroxide to the soap solution.
5. Record your observation.
6. Add the yeast to the mixture in the bottle.
7. Describe what happens. Identify which reagent caused the reaction to
proceed.
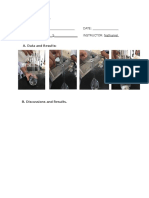
V. DATA AND RESULTS
VI. QUESTIONS
1. What makes the reaction proceed?
2. Is the reaction exothermic or endothermic?
3. How will you know if it endothermic or exothermic?
VII. CONCLUSION
You might also like
- To Study The Digestion of Starch by Salivary AmylaseDocument14 pagesTo Study The Digestion of Starch by Salivary AmylasePratham Parashar89% (9)
- Experiments To Show How Concentration and Surface Area Affect Rate of ReactionDocument7 pagesExperiments To Show How Concentration and Surface Area Affect Rate of ReactionArnav MahajanNo ratings yet
- Chemistry Investigatory File - 063557Document21 pagesChemistry Investigatory File - 063557goelvinayak406No ratings yet
- PAG4.2 Student The Effect of Enzyme Conc On The Rate of A Reaction - v0.1Document2 pagesPAG4.2 Student The Effect of Enzyme Conc On The Rate of A Reaction - v0.1Zeeshan FarooqNo ratings yet
- StoichiometryDocument34 pagesStoichiometryvanditNo ratings yet
- Laboratory Manuan Lab 2Document4 pagesLaboratory Manuan Lab 2Oka DeeNo ratings yet
- Science10 Q4 M7ADocument15 pagesScience10 Q4 M7AKunyubunani BilatNo ratings yet
- PAG4.2 Student The Effect of Enzyme ConcDocument2 pagesPAG4.2 Student The Effect of Enzyme ConcAmaniNo ratings yet
- Investagotry 2023Document17 pagesInvestagotry 2023ritik thakurNo ratings yet
- Factors That Affect Reaction RatesDocument4 pagesFactors That Affect Reaction RatesenieynazNo ratings yet
- Enzymes Lab ReportDocument13 pagesEnzymes Lab ReportIsland VitalNo ratings yet
- ChemimanualDocument55 pagesChemimanualAnil SaiNo ratings yet
- Jawahar Navodaya Vidyalaya - 20231114 - 074405 - 0000Document16 pagesJawahar Navodaya Vidyalaya - 20231114 - 074405 - 0000lifep7417No ratings yet
- Analytical Chemistr Expt 6Document2 pagesAnalytical Chemistr Expt 6Class recordingNo ratings yet
- Experiment 3Document3 pagesExperiment 3Lemon AdeNo ratings yet
- Chemistry Project For Class 12Document2 pagesChemistry Project For Class 12GSI BHUBANESWARNo ratings yet
- For Exer 3Document16 pagesFor Exer 3Louiegi AlvarezNo ratings yet
- Chemistry Project Class 12 PDFDocument13 pagesChemistry Project Class 12 PDFtechnical beatNo ratings yet
- Investigating The Effect of PH On Amylase Activity: ProcedureDocument4 pagesInvestigating The Effect of PH On Amylase Activity: ProcedureJohanna Marie GantalaoNo ratings yet
- To Study The Digestion of Starch by Salivary AmylaseDocument15 pagesTo Study The Digestion of Starch by Salivary AmylaseRaHuL KuMaR75% (4)
- Ma. Jade R. Agres - Full Report No. 2Document7 pagesMa. Jade R. Agres - Full Report No. 2Ma. Jade AgresNo ratings yet
- 201 - Unit 1 QBDocument9 pages201 - Unit 1 QBPrachi BhoirNo ratings yet
- Invertigatory Project ChemistryDocument12 pagesInvertigatory Project ChemistryTanishq GargNo ratings yet
- Substrate Substrate Substrate Substrate SubstrateDocument3 pagesSubstrate Substrate Substrate Substrate Substratecondoleeza smithNo ratings yet
- Practical 2Document8 pagesPractical 2Ibrahim Muhamad0% (1)
- Yeast GrowthDocument13 pagesYeast GrowthSmartPurdyNo ratings yet
- Chemistryproject - 12Document14 pagesChemistryproject - 12Hitesh MendirattaNo ratings yet
- Chemistry Project 1Document23 pagesChemistry Project 1Apurav RanaNo ratings yet
- Report Sheet: Name: Date: GROUP NO.: - 5 - INSTRUCTOR: Nathaniel PeraltaDocument4 pagesReport Sheet: Name: Date: GROUP NO.: - 5 - INSTRUCTOR: Nathaniel PeraltaRodneyDelaCruzNo ratings yet
- Preparation Practical 8 With AnswersDocument3 pagesPreparation Practical 8 With AnswersMaría Sánchez BlancoNo ratings yet
- Chemistry Investigatory ProjectDocument14 pagesChemistry Investigatory ProjectNeba Khan100% (2)
- Practical 2Document26 pagesPractical 2vs322794No ratings yet
- Chemistry Project On Salivary AmylaseDocument16 pagesChemistry Project On Salivary AmylaseSurjyasnata Rath100% (2)
- To Study The Digestion of Starch by Salivary AmylaseDocument15 pagesTo Study The Digestion of Starch by Salivary AmylaseNarender KumarNo ratings yet
- Submitted By: Investigatory Project WorkDocument14 pagesSubmitted By: Investigatory Project WorkVikram BarapatreNo ratings yet
- Pink Wachirapaet - Temperature and Rate of ReactionDocument4 pagesPink Wachirapaet - Temperature and Rate of Reactionapi-480730245No ratings yet
- SppoDocument14 pagesSppoSamer Samer100% (2)
- Solubility Enhancement of Ibuprofen Using Hydrotropic AgentsDocument4 pagesSolubility Enhancement of Ibuprofen Using Hydrotropic AgentsDianAhmadNo ratings yet
- Digestion of Salivary GlandDocument12 pagesDigestion of Salivary GlandJatin suthar100% (1)
- Chemistry Project Report On To Study The Digestion of Starch by Salivary Amylase and Effect of PH and Temperature On ItDocument5 pagesChemistry Project Report On To Study The Digestion of Starch by Salivary Amylase and Effect of PH and Temperature On ItGSI BHUBANESWARNo ratings yet
- EnzymeDocument5 pagesEnzymeBinnie KaurNo ratings yet
- Enzyme Activity Lab Report - IB BiologyDocument15 pagesEnzyme Activity Lab Report - IB BiologyNada SalmanNo ratings yet
- My FileDocument12 pagesMy FilekratimNo ratings yet
- 12TH Chem.Document13 pages12TH Chem.Tanu SinghNo ratings yet
- Catalysts and Oxygen ExperimentDocument12 pagesCatalysts and Oxygen ExperimentMUHAMMAD AKRAMNo ratings yet
- Unit 1 - Activity 4 - Factors Affecting Enzyme LabDocument2 pagesUnit 1 - Activity 4 - Factors Affecting Enzyme LabThin HtetNo ratings yet
- MMMJ PDFDocument16 pagesMMMJ PDFmansurimuaaj099No ratings yet
- M I Technology: Department of Biology TW 2014 BiofuelDocument9 pagesM I Technology: Department of Biology TW 2014 Biofuel6A(24) Marsh WongNo ratings yet
- Submitted By: Investigatory Project WorkDocument16 pagesSubmitted By: Investigatory Project Workshivam yadavNo ratings yet
- PHA6115 Lab Experiment 1Document3 pagesPHA6115 Lab Experiment 1The Dededo NativeNo ratings yet
- Investigating The Effect of PH On Amylase Activity Ss 34Document4 pagesInvestigating The Effect of PH On Amylase Activity Ss 34Abhishek KurreyNo ratings yet
- To - Study - The - Digestion - Ofby - S Alivary - AmylaseDocument14 pagesTo - Study - The - Digestion - Ofby - S Alivary - AmylaseshouryaNo ratings yet
- Chemistry - Investigatory - Project (1) .PPTX (Read-Only)Document14 pagesChemistry - Investigatory - Project (1) .PPTX (Read-Only)djjeena619No ratings yet
- Investigating The Effect of PH On Amylase Activity Ss 34Document4 pagesInvestigating The Effect of PH On Amylase Activity Ss 34darren boesonoNo ratings yet
- Chemistry ProectDocument4 pagesChemistry ProectTanishq SainiNo ratings yet
- Chemistry Project Report OnDocument14 pagesChemistry Project Report OnAditya MaviNo ratings yet
- LABORATORY MANUAL FOR A MINI PROJECT: MSCB 1113 BIOCHEMISTRY & MICROBIAL PHYSIOLOGYFrom EverandLABORATORY MANUAL FOR A MINI PROJECT: MSCB 1113 BIOCHEMISTRY & MICROBIAL PHYSIOLOGYNo ratings yet
- A Comprehensive Book on Experimental PharmaceuticsFrom EverandA Comprehensive Book on Experimental PharmaceuticsRating: 5 out of 5 stars5/5 (1)
- AQA Biology Unit 1: Revision Notes: myrevisionnotes, #1From EverandAQA Biology Unit 1: Revision Notes: myrevisionnotes, #1Rating: 5 out of 5 stars5/5 (2)