Professional Documents
Culture Documents
Quercetin: Toxicology Carcinogenesis
Quercetin: Toxicology Carcinogenesis
Uploaded by
Pig NguyenOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Quercetin: Toxicology Carcinogenesis
Quercetin: Toxicology Carcinogenesis
Uploaded by
Pig NguyenCopyright:
Available Formats
NATIONAL TOXICOLDGY PROGRAM TechnkA Report series N .
409 o
TOXICOLOGY AND CARCINOGENESIS
STUDIES OF
QUERCETIN
(CAS NO. 117-39-5)
IN F344/N RATS
(FEED STUDIES)
US DEPART" ..
OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health
FOREWORD
The National Toxicology Program (NTP) is made up of four charter agencies of the U.S. Department of Health and Human Services (DHHS): the National Cancer Institute (NCI), National Institutes of Health; the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Heal* the National Center for Toxicological Research (NCTR), Food and Drug Administration; and the National Institute for Occupational Safely and Health (NIOSH), Centers for Disease Control. In July 1981, the carcinogenesis Bioassay Testing Program, NCI, was transferred to the NIEHS. The NTP coordinates the relevant programs, staff, and resources from these Public Health Service agencies relating to basic and applied research and to biological assay development and validation. The N T P develops, evaluates, and disseminates scientific information about potentially toxic and hazardous chemicals. This knowledge is used for protecting the health of the American people and for the primary prevention of disease. The studies described in this Technical Report were performed under the direction of the NIEHS and were conducted in compliance with N T P laboratory health and safely requirements and must meet or use were in exceed all applicable federal, state, and local health and safely regulations. Animal care and accordance with the Public Health SeMce Policy on Humane Care and Use of Animals. The prechronic and chronic studies were conducted in compliance with Food and Drug Administration (FDA) Good Laboratory Practice Regulations, and all aspects of the chronic studies were subjected to retrospective quality assurance audits before being presented for public review. These studies are designed and conducted to characterize and evaluate the toxicologic potential, including carcinogenic activity, of selected chemicals in laboratory animals (usually two species, rats and mice). Chemicals selected for NTP toxicology and carcinogenesis studies are chosen primarily on the bases of human exposure, level of production, and chemical structure. Selection per se is not an indicator of a chemical's carcinogenic potential.
SMe e c, These N T P Technical Reports are available for sale from the National Technical Information U.S. DepartmentofCommerce, 5285 PortRoyalRoad,Springfield, VA 2 1 1 ( 0 - 8 - 6 0 .Single 26 734745) copies of this Technical Report are available without charge while supplies last from the NTP Central DataManagement,NIEHS,P.O. Box 12233,MD AO-01, Research Triangle Park, NC 27709 (919-541-1371).
NTP TECHNICAL REPORT
ON THE
TOXICOLOGY AND CARCINOGENESIS STUDIES OF QUERCETIN
(CAS NO. 117394)
IN F344/N RATS
(FEEDSTUDIES)
NATIONAL TOXICOLOGY PROGRAM P.O. Box 12233 ResearchTrianglePark,NC27709
September 1992
NTP TR 409
N I H Publication No. 92-3140
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health
Quercetin, NTP TR 409
CONTRIBUTORS
National Toxicology Program
Evaluated and mtepreted results and reported jindings
NTP Pathology Working Group
Ev-d
s h h , prepared pathology report (27 April 1990)
C.J. Alden, Ph.D. G.A. Boorman, D.V.M., Ph.D. D.A. Bridge, B.S. J.K. Dunnick, Ph.D. S.L. Eustis, D.V.M.,Ph.D. T.J. Goehl, Ph.D. R.A. Griesemer, D.V.M., Ph.D. J.R. Hailey, D.V.M. J.K. Haseman, Ph.D. C.W. Jameson, Ph.D. G.N. Rao, D.V.M.,Ph.D. D.B. Walters, Ph.D. K.L. Witt, M.S., OakRidgeAssociatedUniversities
R.M. Kovatch, D.V.M., Chair J. Cullen, V.M.D.,Ph.D.
Pathology Associates, Inc. North Carolina State University
B. E Hamilton, D.V.M., Ph.D.
S Imoto, D.V.M., Ph.D., .
M.P. Jokinen, D.V.M. J. Mahler, D.V.M.
Consultant
Experimental Pathology Laboratories Shin Nippon Biomedical Laboratories
National Toxicology Program National Toxicology Program
E.E. McConnell, D.V.M.
EG&G Mason Research Institute
Conducted studies, evaluated pathology jindings
M.M. McDonald, D.V.M., Ph.D.
National Toxicology Program
H.S. Lilja, Ph.D., PrincipalInvestigator A.J. Block, Ph.D. H.J. Esber, Ph.D. R.W. Fleischman, D.V.M. M. Hagopian, PbD.
Biotechnical Services, Inc.
Prepared Technical Report
Experimental Pathology Laboratories, Inc.
Provided pathology qualityassessment
L.G. Cockerham, Ph.D., PrincipalInvestigator P. Chaffin, M.S. G.F. Corley, D.V.M. D.D. Lambright, PbD. W.D. Sharp, B.A.,B.S.
B. F. Hamilton, D.V.M., Ph.D, PrincipalInvestigator
Integrated Laboratory Systems
Prepared quality assurance adits
J.C. Bhandari, D.V.M., Ph.D.,
PrincipalInvestigator
CONTENTS
ABSTRACT
.............................................................. EXPLANATION OF LEVELS OF EVIDENCE OF CARCINOGENIC ACTIVITY ............. PEERREVIEWPANEL ..................................................... SUMMARY OF PEER REVIEW COMMENTS ..................................... INTRODUCTION ......................................................... MATERLALSANDMETHODS ................................................ RESULTS ...............................................................
DISCUSSION AND CONCLUSIONS REFERENCES
APPENDIX
5
8
9 10
1 1
17 23
APPENDIX
APPENDIX
B
C
APPENDIX D APPENDIX E
APPENDIX
........................................................... Summary of Lesions in Male Rats in the 2-Year Feed Study ............... Summary of Lesions in Female Rats in the 2-YearFeed Study .............. Genetic Toxicology ............................................. Organ Weights and Organ-Weight-to-Body-Weight Ratios ..................
Hematology. Clinical Chemistry. and Urinalysis Results Chemical Characterization and Dose Formulation Studies Feed and Compound Consumption in the 2-Year Feed
............................................
35
43 49 93 137 143 147 153 161 165 171
APPENDIX G
APPENDIX
................. Studies ..............
...................
H I
Ingredients. Nutrient Composition. andContaminant Levels in NIH-07 Rat and Mouse Ration Sentinel Animal Program
APPENDIX
.................................. ........................................
ABSTRACT
OH
OH
0
QUERCETIN
CAS NO. 117-39-5
Chemical Formula:
C,,H,,,07
Molecular Weight: 302.23
Synonyms: C.I. NaturalYell,ow, 10; C.I. 75670;Cyanidelonon15:2;,FlavinMeletin;Quercetine;Quercetol;Quertin;Quertine; Sophoretin;Xanthaurine; 3,3 ,4 ,5,7-Pentahydroxyfiavone;3,5,7,3 ,4 -Pentahydroxyflavone; 2-(3,4-DihydroxyphenyI)-3,5,7-trihydmxy-
4H-l-benzopn-4-one
Quercetin is a member o f agroup o f naturally occurring compounds, the flavonoids, which have a common flavone nucleus composed o f two benzene rings linked through a heterocyclicpyrone ring. Quercetin is found in various plants, food products, and dyes o f natural origin. The estimated average daily intake of quercetin by an individualin the United States is 25 mg. The and Food Drug Administration nominated quercetin for toxicity and carcinogenicity studies in the rat because it is a chemical that widely is distributed foods. in Quercetin was administered to rats by dosedfeed since human exposure is by dietary consumption. Information in the literature showed that quercetin administered in the diet to rats at levels up to approximately 4% caused a minor body weight effect, whereas higher dose levels produced greater than 10% reduction in weight body gains relative to controls. Based on this information, the NTP 2-year
studies were conducted by administering 0, l O , , O O lO,OOO, or 4O ppm quercetin (>95% pure) in OO 0 , feed to groups o f 50 male female and rats for 104 weeks. Ten additional animals per dose group were evaluated at 6 and 15 months.
Body Weigh, Survival, and Clinical Findingv in the Z Y W studies Body weights of exposed male and female rats given 1 O and 1 , O ppm were within 5% o f controls OO , 0O O throughout the studies. Reduced body weight gain 0 O O , in male and female rats receiving 4 O ppm was observed by week 15 and the final mean body weights were 87% o f controls at week 104. Survival and feed consumption were similar among exposed and control groups throughout the studies. The average amounts o f quercetin consumed per day by , O 0O O O 0 O O , the l O , 1 , O and 4 O ppm dose groups after week 52 were 40, 400, and 1,900 mg/kg of body weight.
Quercetin, NTP
TR 409
Nonneoplastic and N w p h t i c E$@& in the t Y w Studies
2 years were observedin the kidney. Therewere dose-related increases in the severity of chronic nephropathy (control, 2.7; low-dose, 2.7; mid-dose, 3.0; high-dose, 3.2) and a slight increased incidence in focal hyperplasia of the renal tubule epithelium (1/50; 2/50; 3/50; 4/50). Parathyroid hyperplasia, indicative o f renal secondary hyperparathyroidism, also increased incidence indosed male rats (1/43,
6/45, 6/43, 17/43).
with the dietary administration of quercetin for
In male rats, the principal toxic effects associated
There was a statistically significant, dose-related decrease in the incidence o f mammary gland fibroadenomas in exposed female rats (29/50, 27/50, 16/50, 9/50), which may in part be attributed to lower body weight gains. There was a treatment-related accumulation o f yellow-brown granular pigment adsorbed to or absorbed by the epithelial cells of the glandular stomach, ileum, jejunum, and, to a lesser extent, the duodenum and colon. The severity o f the pigmentation in these tissues increased with increased length of exposure. Therewerenoother lesions considered to be related to chemical administration. Quercetininducedgenemutations in Salmonella zphimurium strains TAlOO and TA98 with and S) without exogenous metabolic activation ( 9 . PosiGenetic T&hgy
The evaluation o f single sections from the left and right kidneys revealed renal tubule adenomas in threemaleratsandadenocarcinomas in another 0 O O , male rat receiving 4 O ppm quercetin; none were seen in the controls. Examination of additional step sections of the male rat kidney identified additional hyperplasia and adenomas in all dose groups (hyperplasia:2/50,2/50, 6/50, 8/50; adenoma: 1/50, 6/50). The overall incidence o f renal 2/50, 7/50, tubuleadenoma or adenocarcinoma combined in male rats was 1/50 in controls and 9/50 in the highdose group. There was no apparent effect o f quercetin on the kidney o f female rats. A single renal tubule adenoma was seen in a female receiving 10,OOO ppm; this neoplasm was not considered biologically significant.
tive results were also obtained in tests with and without S9 for induction o f sister chromatid exchanges and chromosomal aberrations in Chinese hamster ovary cells.
Cl Ws h n c h Under the conditions of these 2-year feed studies there was some evidence o carcinogenic activiry* of f quercetin in male F344/N rats based on an increased incidence o f renal tubule cell adenomas. There was no evidence o carcinogenicactiviry of quercetin in f 10,O or female F344/N rats receiving l,OOO, OO 4 O ppm. The incidence o f renal tubule hyper0 O O , plasia and the severity o f nephropathy were
increased in exposed male rats.
* Explanation o f Levels o f Evidence o f Carcinogenic Activity is on page 8. Asummary
discussion on this Technical Report appear on page 10.
of peer review comments and the public
Quercetin, NTP TR 409
Summary of the 2-Year Carcinogenesis and Genetic Toxicology Studies of Quercetin
Male F W / N Rats
0, l O , lO,OOO, or 4 O ppm , O O 0O O ,
Female F W / N Rats
0, l,OOO, lO,OOO, or 4 O ppm 0O O , in feed
in feed
Final body weights (% of controls) 2-Year survival rates Nonneoplastic effects
97%, 95%, 87%
101%, 98%, 87%
30/50, 28/50, 35/50, 28/50
26/50, 29/50, 25/50, 25/50
Kidney: renal tubule hyperplasia (singlesections):1/50, 2/50, 3/50,
4/50;
8/50;
None
(stepsections):
2/50, 2/50, 6/50,
chronic nephropathy (severity grades: 2.7, 2.7, 3.0, 3.2)
Neoplastic effects
Kidney (single sections): adenoma - 0/50, 0/50, 0150, 3/50; adenocarcinoma - 0150, 0150, 0150, 1/50; (stepsections):adenoma - 1/50,
2/50, 7/50, 6/50
None
Level of evidence of carcinogenic activity
Salmonello typhimuriwn (gene mutation):
Some evidence
No evidence
Genetic toxicology
Sister chromatid exchanges Chinese hamster ovary cells in vitro: Chromosomal aberrations Chinese hamster ovary cells in vitro:
Positive with and without S9 in strains TAlOO and TA98 Positive with and without S9 Positive with and without S9
Quercetin, N T P
TR 409
EXPLANATION OF LEVELS OF EVIDENCE OF CARCINOGENIC ACTMTY
The National Touricology Program describes the results of individual experiments on a chemical agent and notes the strength of the evidence f o r conclusions regarding each study. Negative results, in which the study animals do not have a greater incidence o f s neoplasia than control animals, do not necessarily mean that a chemical i not a carcinogen, inasmuch as the experiments are conducted under a limited set o f conditions. Positive results demonstrate that a chemical is carcinogenic for laboratory animals under the conditions o f the study and indicate that exposure to the chemical has the potential for hazard to humans. Other organizations, such as the International Agency for Research on Cancer, assign a strength of evidence for conclusions based on an examination o f all available evidence, including animal studies such as those conducted by the N T P , epidemiologic studies, and estimates o f exposure. Thus, the actual determination o f risk to humans from chemicals found to be carcinogenic in laboratory animals requires a wider analysis that extends beyond the purview o f these studies.
Five categories o f evidence o f carcinogenic activity are used in the Technical Report series to summarize the strength o f the evidence obsewed in each experiment: two categories for positive results (clear evidence and some evidence); one category for n uncertain findings (equivocal evidence); one category for no observable effects ( oevidence); and one category for experiments that cannot be evaluated because o f major flaws (inadequate study). These categories o f interpretative conclusions were first adopted in June 1983 and then revised in March 1986 for use in the Technical Report series to incorporate more specifically the concept o f actual weight o f evidence o f carcinogenic activity. For each separate experiment (male rats, female rats, male mice, female mice), one of the following five categories is selected to describe the findings. These categories refer to the strength o f the experimental evidence and not to potency or mechanism.
Clear evidence o f carcinogenic activity is demonstrated by studies that are interpreted as showing a dose-related (i) increase o f malignant neoplasms, (ii) increase o f a combination o f malignant and benign neoplasms, or (iii) marked increase o f benign neoplasms if there is an indication from this or other studies of the ability of such tumors to progress to malignancy. Some evidence o f carcinogenic activity is demonstrated by studies that are interpreted as showing a chemical-related increased incidence of neoplasms (malignant, benign, or combined) in which the strength o f the response is less than that required for clear evidence. Equivocal evidence o f carcinogenic activity is demonstrated by studies that are interpreted as showing a marginal increase of neoplasms that may be chemical related. No evidence o f carcinogenic activity is demonstrated by studies that are interpreted as showing no chemical-related increases in malignant or benign neoplasms. Inadequate study o f carcinogenic activity is demonstrated by studies that, because o f major qualitative or quantitative limitations, cannot be interpreted as valid for showing either the presence or absence o f carcinogenic activity.
When a conclusion statement for a particular experiment is selected, consideration must be given to key factors that would extend the actual boundary of an individual category o f evidence. Such consideration should allow for incorporation o f scientific experience and current understanding o f long-term carcinogenesis studies in laboratory animals, especially f o r those evaluations that may be on the borderline between two adjacent levels. These considerations should include:
l
l
l l
l l l
l
l
l
l l l
adequacy o f the experimental design and conduct; Occurrence o f common versus uncommon neoplasia; progression (or lack thereof) from benign to malignant neoplasia as well as from preneoplastic to neoplastic lesions; some benign neoplasms have the capacity to regress but others (of the same morphologic t p )progress. At present, ye it is impossible to identify the difference. Therefore, where progression is known to be a possibility, the most prudent course is to assume that benign neoplasms o f those types have the potential to become malignant; combining benign and malignant tumor incidence known or thought to represent stages o f progression in the same organ or tissue; latencyin tumor induction; multiplicity in site-specificneoplasia; metastases; supporting information from proliferative lesions (hyperplasia) in the same site o f neoplasia or in other experiments (same lesion in another sex or species); presence or absence o f dose relationships; statistical significance o f the observed tumor increase; concurrent control tumor incidence as well as the historical control rate and variability for a specific neoplasm; suwival-adjusted analyses and false positive or false negative concerns; structure-activitycorrelations; and in some cases, genetic toxicology.
Quercetin, NTP
TR
409
PEER REVIEW PANEL
The members o f the Technical Reports Review Subcommittee who evaluated the N T P draft Technical Report on quercetin on March 11, 1991 are listed below. Panel members serve as independent scientists, not as representatives o f any institution, company, or governmental agency. In this capacity, panel members have fwe major responsibilities in reviewing N T P studies:
l
l
l
l
to ascertain that all relevant literature data have been adequately cited and interpreted, to determine if the design and conditions of the N T P studies were appropriate, to ensure that the Technical Report presents the experimental results and conclusions fully and clearly, to judge the significance of the experimental results by scientific criteria, and to assess the evaluation o f the evidence o f carcinogenic activity and other observed toxic responses. Chair
Daniel S Longnecker, M.D., .
Department o f Pathology Dartmouth Medical School Hanover, NH Toxicology Division Mobil Oil Corporation Princeton, NJ
Jay I. Goodman, Ph.D.,
PrincipalReviewer Department o f Pharmacology and Toxicology Michigan State University East Lansing, MI Department o f Veterinary Pathobiology College of Veterinary Medicine University of Minnesota St. Paul, MN Department of Pharmacology and Toxicology University of Kansas Medical Center Kansas City, KS Department o f Biostatistics University o f Washington Seattle, WA
Paul T. Bailey, Ph.D.
David W.Hayden, D.V.M., Ph.D.
Louis S Beliczky, M.S., M.P.H., PrincipalReviewer .
Department of Industrial Hygiene United Rubber Workers International Union Akron, OH
Curtis D. Klaassen, Ph.D.
Gary P. Carlson, Ph.D.
Department o f Pharmacology and Toxicology Purdue University West Lafayette, IN School o f Aerospace Medicine Brooks Air Force Base, TX PrincipalReviewer Consultants in Veterinary Pathology Munysville, PA
Barbara McKnight, Ph.D.
Harold Davis, D.V.M., Ph.D.
Ellen K Silbergeld, Ph.D.* Lauren Zeise, PbD.
University o f Maryland Medical School Baltimore, MD California Department o f Health ServicesiRCHAS Berkeley, CA
Robert H. Garman, D.V.M.,
*Did not attend
10
Quercetin,
NTP TR 409
SUMMARY OF PEER REVIEW COMMENTS
On March 11, 1991, the draft Technical Report on toxicology and carcinogenesis studies o f quercetin received public review by the National Toxicology Program Board of Scientific Counselors' Technical Reports Review Subcommittee and associated Panel of Experts. The review meeting was held at the National Institute of Environmental Health Sciences, Research Triangle Park, NC.
the
Dr. J.K. Dunnick, NIEHS, introduced the toxicology and carcinogenesis studies o f quercetin by discussing the uses o f the chemical and rationale for study, describing the experimental design, reporting on survival and body weight effects, and commentingon neoplasms and nonneoplastic lesions of the kidneys in male and female rats. The proposed conclusions were some evidence of carcinogenic activity in male rats and no evidence of carcinogenic activity in female o f the low rats. Dr. Dunnickaddedthatbecause but slightly increased number o f renal neoplasms in male rats, additional step sections of residual kidneys from all control and high-dose rats were cut and evaluated. Dr. Garman, a principal reviewer, agreed with the proposed conclusions. He thought the conclusions in male rats were quite reasonable based both on the frequencies o f hyperplasia and o f benign and malignant renal tubule epithelial neoplasms and on the morphology o f these neoplasms.Dr. Garman asked whether the induction o f neoplasms was related to hyaline droplet nephropathy, and if so, he imply a decreased level of thought this might exposure to concern with regard to human quercetin. Dr. J.R.Hailey, NIEHS, said there was no evidence for the hyaline droplet nephropathy in this study and also no evidence ofkidney lesions from interim evaluations at 6 and 15 months.Dr. Garman asked for clarification o f the identity and tissue location o f the pigment found in the gastrointestinal tract. Dr. Haileysaid the identity was not determined.
Dr. Goodman, the second principal reviewer, agreed with the proposed conclusions. He inquired what effect the procedure o f step sectioning o f the kidneys has on the incidence o f kidney neoplasms in control treated and animals. Dr. J. Haseman, NIEHS, said that for the eight NTF' studies for which step sections have been evaluated, the control rate of renal tubule neoplasms in male rats is 3.7%, or slightly more than double the rate in the current historical control database o f 1.6%. Dr. Dunnick reported that the findings from the step sections in other studies have been supportive o f the original diagnoses. Dr. Goodman suggested that specific references o f studies on chemically induced azll-globulin nephropathy in maleratsshould be Dr. considered for inclusion in the discussion. Dunnick said they would be added. Mr. Beliczky, the third principal reviewer, agreed with the proposed conclusions. He commented that the increased sensitivity of detection for renal neoplasms and preneoplastic lesions resulting from step sectioning wasimpressive. He askedwhether studies on quercetin had been done inmice.Dr. Dunnick responded that several previous studies by others in mice had shown no evidence o f carcinogenic effects.
Dr. Carlson said he was not convinced that two squamous cell carcinomas of the tongue in high-dose femaleratswere unrelated to chemical administration. Dr. Dunnick said the number was within the historical control range andmicroscopic analysis indicated no supporting preneoplastic lesions. Dr. Garman moved that the Technical Report on quercetin be accepted with the revisions discussed and the conclusions as written for male rats, some evidence of carcinogenic activity, and for female rats, no evidence o carcinogenic activity. Dr. Goodman f seconded the motion, which was accepted unanimously with 10 votes.
1 1
INTRODUCTION
OH
OH
0
QUERCETIN
CAS NO.117-39-5
Chemical Formula: C,,H,,O, Molecular Weight:
302.23
Sophoretin; Xanthaurine; 3,31,4',5,7-Pentahyd~avone;3,5,7,3',4'-PentahydroJryflavone; 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy4H-l-bempyran-4-one
Synonyms: C.I. Natural Yellow 10; C.I. 75670; Cyanidelonon 1522; FlavinMeletin;Quercetine;Quercetol;Quertin;Quertine;
taste, which is insoluble in water, slightly soluble in alcohol, and soluble in glacial acetic acid and aqueous alkaline solutions (Weast, 1979; Merck Index, 1983). Quercetin is a member of a group o f naturally occurring compounds, the flavonoids, which have a common flavonenucleuscomposed of two benzene rings linked througha heterocylicpyrone ring. Animals are unable to synthesize the flavone nucleus; thus, flavonoids are found exclusively in the plant kingdom. Quercetin and more than 2 O , O O other flavonoids occur as condensation products o f p-glycosides (Herrmann, 1976; Kuhnau, 1976; Brown, 1980,IARC,1983). Quercetin is found invarious food products and plants, including fruits, seeds, vegetables, tea, coffee, bracken fern, and natural
PHYSICAL AND CHEMICAL PROPERTIES, PRODUCTION,OCCURRENCE, AND USE Quercetin is a yellow, crystalline solid with a bitter
dyes. Quercetin is usually obtained from the hydrolysis o f rutin (quercetin-3-rutinoside), a naturally occurring flavonoid glycoside (Griffith et al., 1955), although it can also be synthesized (Shakhova et a. 1962). f, Flavonoids, including quercetin, were once thought to have therapeutic applications, including induction o f smooth muscle relaxation, reduction o f capillary fragility, and as anti-inflammatory agents. However, in 1970, the Food and Drug Administration withdrew its approval o f drugs containing rutin or quercetin because there was insufficient evidence to support the reported pharmacologic effects (Brown, 1980, IARC, 1983). Thetotal flavonoid intake in the U.S. is estimated at 1 g per person per day, with an average daily intake o f the individual flavonoid, quercetin, of approximately 25 mg per person (Kuhnau, 1976).
12
Quercetin, N T P TR 409
Rats fed diets containing up to 1% quercetin for Quercetin glycosides are relativelypoorly absorbed 410 days ShOWed no decrease in M Y weight gain by the small intestine. Microflora o f the lower and compound-related no histoPathologic lesions 1952). bowel hydrolyze the flavonide-glycoside to quercetin ( b b r o s e et and the sugar, and quercetin is then absorbed into the enterohepatic system (Brown, 1980, Tamura e? a . 1980, akkenheuser et a . 1987). After oral f, f, REPRODUCTIVE TOXICOLOGY f f, administration of quercetin to rabbits (Booth e a . The reproductive toxicity of quercetin was studied in 1956) or rats (Petrakis et a . 1959), three metabf, 34 male and femaleF 4 rats fed diets containing 0.1% olites o f quercetin were identified in the urine: 3,4or 0.2% quercetin frombirth to breeding during dihydroxyphenylacetic3-methoxy-4-hydroxyacid, phenylacetic (homovanillic acid acid), and week 12or 13. During gestation and lactation, rn-hydroxyphenylaceticacid. These metabolites are animals were fed diets without quercetin. Quercetin thought to be formed in the liver after fusion o f the had no effect on mean viable litter size, live birth heterocylicpyrone ring. When Brown and Griffiths index, 3-day survival o f pups, lactation index, or weight o f pups at birth or at 21 days (Stoewsand (1983) administered quercetin to rats by intraf f, ,O peritoneal injection, they identified the 3 $-methyl- e a . 1984). When 0, 20, 200, or 2O O mgkg quercetin was administered to Sprague-Dawley rats ether of quercetin (isorhamnetin) as a metabolite in from days 6 through 15 o f gestation, no overt signs bile. o f toxicity were seen in the dams evenat the highest The distribution, metabolism andexcretion of 4-[14C] dose, but average fetal weight o f the 2,OOO mgkg quercetin in male ACI rats were studied by group was reduced relative to control fetal weight. autoradiographyandquantitation o f radioactivity No fetal abnormalities attributable to chemical f, (Ueno e f a . 1983). After oral administration, 20% administration were observed (Willhite, 1982). of the dose was absorbed from the digestive tract andthen excreted into the bile and urine within 48 hours as glucuronide or sulfate conjugates. CARCINOGENICITY Autoradiographic analysis o f arat 3 hours after receiving a single 2.3 mgkg oral dose of quercetin Quercetin has been studied in a variety o f test showed that most of 'the radioactivity remained in systems for carcinogenicity and in the majority o f the digestive tract with low levels seen in the blood, these studies there was no evidence o f neoplasms related to chemical administration (Table 1). In a liver, kidney, lung, and rib. 2-year study o f M44 rats, 0%, 1.25% or 5% querIn five human volunteers, no quercetin was detected cetin was administered in the diet for 104 weeks, in the plasma or urine after oral administration o f followed by an additional 8-weekrecovery period (It0 et a l , 1989). Major tissues and organ systems 4 g of quercetin (Gugler e f a . 1975). f, were examined histopathologically. Hyperplastic polyps of the cecum were found in males and TOXICITY females fed diets containing 5% quercetin. An The oral LD, of quercetin was reported as adenomaand two adenocarcinomas of the cecum 1 0 mgkg in the mouse and 161 mgkg in the rat; were observed in high-dose males, while two 6 the LD, in the mouse by the subcutaneous route adenomas o f the colon were observed in the highwas reported as 97 mgkg (Sullivan e al., 1951). dose females. The incidences of these neoplasms f The purity o f the compound used in this study was were not considered statistically significant and the not specified. Subsequent studies haveshown that authorsconcludedthat there was no evidence for rodents tolerate much higher doses of quercetin. any clear carcinogenic effect.
METABOLISM DISTRIBUTION AND
Introduction
13
TABLE 1
Quercetin Rodent Carcinogenicity Studies
Strain of Rodent Route of Administration and Dose'
Diet
Length of
Dosing
Histopathologic Findingsb
Reference
Male and female F344DuCj rats Dietand female Male albino rats
0, 1.25, 5.0%
0, 0.1% quercetin
104 weeks 58 weeks
Negative Intestinal and urinary bladder neoplasms in treated group Negative Negative Negative Negative
It0 et a L , 1989 Pamukcu et a L ,
1980
Male and female ACI rats Male and female
Diet 1, 5 , 10% Diet
0, 0.1% 0, 2%
850 days
Hirono et aL,
1981 1983
F344 rats
540 days
842 days 351 to 709 days
Takanashi et a L , Saito et a L , 1980 Morino et aL,
1982
Male and female ddY mice Male and female golden hamstels Female I C W Swiss mice
Diet Diet
0, 1, 4%
DMBA as initiator on skin, 25 mg quercetin applied to the skin 3 times per week for 25 Weeks Diet
0, 5%
Negative (no skin neoplasm induction)
Van Duuren and Goldschmidt, 1976
Male F344 rats (effects on initiation/ promotion in urinary bladder)
a) Quercetin given for 25 weeks after initiation with 0.01% BHBN, b) 5% quercetin given as an initiator for 4 weeks followed by 0.001% BHBN for 29 weeks
No effects on initiation/ promotion in urinary bladder
Hirose et a L , 1983
Female ICR mice
Skin initiated with DMBA, promoted with telocidin twice per week, quercetin ( 0 3 bmol) treatment applied topically with telocidin
20 weeks
Suppressed skin neoplasm formation
Nishino et a L ,
1984a
(continued)
14
Quercetin,
NTP TR 409
TABLE 1 Quercetin Rodent Carcinogenicity Studies (continued)
Strain of Rodent Route of Administration and Dose Length of Dosing Histopathologic Findings' Reference
Diet Male female and A(MJms) mice' Female CD-1 mice
0, 5%
23 weeks
Negative (no increase or decrease in lung neoplasms) Suppressed Kat0 skin neoplasm formation
Hosaka and Hirono, 1981
Skin-initiated with DMBA, promoted with TPA, quercetin ( 0pmol) applied 3 topically after each TPA treatment
18 weeks
et a L , 1983
DMBA = 7,12dimethyl[a]anthracene;TPA = 12-0-tetradecanoylphorbol-13-acetate; BHBN = N-butyl-N-(4-hydroxyIbutyl)nitrosamine Negative = no evidence for neoplasms related to administration of quercetin This strain develops lung neoplasms at a low incidence by week 23.
Quercetin has been shown to inhibit the promotion of skin neoplasms in the mouse (Kato et al., 1983; Nishino et aL, 1984a) and to suppress the formation o f urinary bladder neoplasms (Hirose et aL, 1983). Quercetin had no effect on the formation o f lung neoplasms in strain A mice (Hosaka and Hirono, 1981) and did not induce preneoplastic glutathione S-transferase placental form-positive foci in F344 rats(It0 et aL, 1988). When quercetin was given intraperitoneally for 6 days at a dose of 500 mgkg per day to hepatectomized rats followed by phenobarbital treatment, there was no increase in liver neoplasms compared to rats treated in the same manner without quercetin (Kato et al., 1985).
.
quercetin can inhibit the promotion of neoplasms n (Kato et aL, 1983). Follow-up studies i vitro suggest that quercetin can inhibit cell proliferation. Quercetin has been shown to have pleiotropic effects on the transformation o f BALB 3T3 cells. Ata concentration o f 0.5 pg/mL, quercetin suppresses the promoting action of 12stetradecanoylphorbol-13-acetate on cells initiated with 20-methylcholanthrene (MCA), but at higher concentrations (5.0 pg/mL), quercetin enhanced cell transformation by MCA (Tanaka et al., 1987). Quercetin has been shown to inhibit cell proliferation in Ehrlich ascites neoplasms cells and to inhibit thymidine incorporation (Graziani and Chayoth, 1W9). Quercetin also inhibits the growth of squamous cell carcinoma lines in vitro (Castillo et al., 1989). Bracken fern, which contains quercetin and many other chemicals, causes mononuclear cell leukemia, intestinal neoplasms, urinary bladder carcinomas, and mammary adenocarcinomas in rats; urinary bladder neoplasms in guinea pigs; alimentary tract and urinary bladder cancers in cattle; and intestinal
Pamukcu et al. (1980) reported that albino rats (Norwegian strain) fed a diet containing 0.1% showed an increased quercetin for 58 weeks incidence o f intestinal and urinary bladder neoplasms in dosedanimals. Other long-term rat studies have not confirmed this carcinogenic effect (Hirono et al., 1981; Takanashi et al., 1983; Ito et al., 1989). Long-term studies in mice also showed no carcinogenic effects from quercetin (Saito et al., 1980). Some of these animal studies showed that
Introduction
15
carcinomasandhepatomas in toads (IARC, 1986, appear to be caused by 1987). The neoplasms known carcinogens, such as shikimic acid and tannin, also found in bracken fern (Evans, 1984, Hirono, 1986).
Quercetin (maximum dose of 1 O mgkg) did not OO , induce micronuclei in bone marrow erythrocytes o f mice exposed either by intraperitoneal injection or gavage (Aeschbacher et al., 1982; MacGregor et al., 1983); feed studies (5% and 10% in chow for 8 days) also yielded negative results for microGENETIC TOXICITY nucleus induction (MacGregor et al., 1983). No The mutagenicity o f quercetin and other flavonoids increase in the frequency of sister chromatid has been reviewed by Sugimura et al. (1977), Brown exchanges in rabbit lymphocytes was observed 1 or (1980), and Nagao et al. (1981). Structural require- 7 days after intraperitoneal (i.p.) injection o f 250 to 1,OOO mgkg quercetin (MacGregor et al., 1983). in ments for mutagenic activity o f flavonoids Salmonella are discussed in detail by MacGregor and Results o f dominant lethal assays with quercetin in Jurd (1978) and Nagao et al. (1981). Mutagenic male Swiss mice (200 to 400 mgkg i.p.) and Wistar flavonoids (primarily the 3-hydroxyflavones) rats (200 and 300 mgkg i.p.) were also negative generally contain a free hydroxyl group at the (Aravindakshan et al., 1985). 3 position, a double bond between positions 2 ee and 3, anda keto group atthe 4 position. The F c s or fecal extracts from laboratory rats fed quercetin showed mutagenic activityin Salmonella presence o f exogenous metabolic activating systems may render some of these structural requirements typhimurium and urine from treated rats showed a nonessential. Of the flavonoids, quercetin exhibits small amount o f mutagenic activity (in proportion to the strongest mutagenic activity. Quercetin induces administered dose) (Stoewsand et al., 1984, Crebelli gene mutations in base substitution as well as et al., 1987). Bacterial-mediated degradation o f frameshift strains of Salmonellatyphimurium, with quercetin in the gut and lack o f absorption may be and exogenous without metabolic activation, contributing factors to the observed lack of in vivo Additionally, bacterial gene although activation increases the magnitude o f the genetic effects. mutation studies with known metabolites of mutagenic response (Bjeldanes and Chang, 1977; shown little effect: 3-hydroxyHardigreeandEpler, 1978; Brown and Dietrich, quercetin have phenylacetic acid, 3,4-dihydroxyphenylacetic acid, 1979; Bartholomew and Ryan, 1980, McCoy et al., 1983; Stoewsand et al., 1984, Kuroda, K. et al., 1985; homovanillic acid,and phloroglucinol carvxylic acid duction in Kuroda, M. et al., 1985; Busch et al., 1986, Ldfroth were all negative for gene reversion i1 S typhimurhm strains TA98 and TAl00 (Bjeldanes . et al., 1986, Rueff et al., 1986, Brams et al., 1987; 1977; MacGregor and Jurd, 1978; Marzin et al., 1987; MacGregor and Wilson, 1988). and Chang, Hatcher et al., 1981). However, positive results were It has also been reported to induce sex-linked obtained in S typhimurium with dihydroquercetin . recessive lethal mutations in cells male germ of (TA98) isorhamnetin and (TA98, TA100) Drosophilamelanogaster (Watson, 1982). Tests for (MacGregorand Jurd, 1978;Nagao et al., 1981). chromosomal effectsmammalian in cell cultures have also been positivewith quercetin: it induced 3,4-Dihydroxyphenylacetic acid (100 pg/mL) caused chromosomal aberrations and sister chromatid chromosomal aberrations in Chinese hamster ovary exchanges, in the absence o f S9 metabolic activation, cells treated for 3 hours with or without S9 activain ChinesehamsterDon-6and B-131 fibroblasts tion (Stich et al., 1981). (Yoshida et al., 1980), in Chinesehamster ovary cells (Stich et al., 1981; Carver et al., 1983), and in TUDY RATIONALE human HE2144 fibroblasts and leukocytes (Yoshida Quercetin was nominated by The Food and Drug et al., 1980). In addition, sister chromatid exchanges Administration for toxicity and carcinogenicity (Rueff et al., 1986) andchromosomal aberrations studies in the rat because it is widely distributed in (Marzin et al., 1987) were induced by quercetin in natural foods, and although long-term studies had the presence, as well as the absence, o f S9 in human beenconducted previously in both rats and mice, peripheral lymphocyte cultures. Despite the there was conflicting information on the carcinoconsistently positive results for quercetin in vino genicity of quercetin in the rat. Quercetin was assays for genotoxic activity, most o f the in vivo test administered to rats by dosed feed because human data were negative. exposure is by dietary consumption.
17
MATERIALS AND METHODS
PROCUREMENT AND
CHARACTERIZATION
Quercetin was obtainedfromFreeman Industries (Tuckahoe, NY) in two lots. It was prepared by hydrolyzing rutin (quercetin-3-rutinoside), a naturally occurring flavonoid glycoside. Both lots (lot no. 969-3790-05, anhydrous form, and lot no. 969-048318BL, dihydrate form)were used throughout the studies. Identity, purity, and stability analyses were conducted by the analytical chemistry laboratory, Midwest Research Institute (Kansas City, Missouri) (Appendix F). The study chemical, a yellow crystalline powder,was identified as quercetin by infrared, ultravioletfisible, and nuclear magnetic resonance spectroscopy. Both lots were greater than 95% pure, as determined by titration, Karl Fischer water analysis, weight loss on drying, chromatographic analysis, nuclear magnetic resonance spectroscopy, and elemental analyses. The largest impurity was identified by spectroscopy and mass spectrometry as ellagicacid (2.6% in lot 369-3790-05 and 1.1% in lot 969-0483-18BL). Stability studies performed by high-performance liquid chromatography indicated that quercetin was stable as a bulk chemical for at least 2 weeks at temperatures to 60" C when protected from light in a nitrogen atmosphere. Based on the results of a stability study, the bulk chemical was stored at 0" f 5" Cthroughout the study period. The bulk chemical was monitored highperiodically by the study laboratory using performance liquid chromatography and infrared spectroscopy. No change in the study material was
detected.
homogeneity and stability of l0,OOO ppm quercetin in feed. Homogeneity was confirmed by ultraviolet spectroscopy. Stability of dose formulations stored at temperatures up to 25" C for at least 14 days was confirmed by high-performance liquid chromatography. During the studies, the dose formulations were stored in opaque plastic bags (because of reported light sensitivity) at approximately 4" C for no longer than 2 weeks. The study laboratory conducted periodic analyses o f the quercetin dose formulations using ultraviolet spectrophotometry (AppendixF). During the 2-year studies, the dose formulations were analyzed at approximately 8-week intervals and all formulations were within 10% o f the target concentrations (Table F ) Results o f periodic referee analyses o f 2. the dose formulations performed by the analytical chemistry laboratory were in agreement with the 3. results from the study laboratory (Table F )
PREPARATION AND ANALYSIS OF DOSEFORMULATIONS The dose formulations wereprepared by mixing appropriate amounts o f quercetin and feed (Table Fl). Studies were conducted by the analytical chemistry laboratory to determine the
Groups of 70 rats o f each sex were administered 0, l O , lO,OOO, or 4O ppm quercetin. These OO , OO 0 , doses were selected based on the literature reports whichshowed that quercetin administered in the diet at levels up to approximately 4% (0 O ppm) 4O O , caused a minor body weight decrement, and thatthis effect was more severe at doses higher than 4%. Since 1 O ppm was the dose level used in the one , O O study reporting carcinogenic results in rats, this concentration was selected as the low dose for these dose studies. Tenmaleandtenfemaleratsper group wererandomly selected and necropsied for interim evaluation after 6 months and 15 months of chemical administration.
Study Design
Y YEAR STUDIES
Male and female F344/N rats were obtained at 4 to 5 weeks o f age from Charles River Breeding Laboratories (Portage, MI) for use in the 2-year
Source and Specification of Animals
18
Quercetin,
NTP TR 409
studies.Males werequarantined for 15 days and females were quarantined for 21 days. Five animals o f each sex were randomly selected and killed for parasite evaluation and gross observation of disease. The rats were placed on study at about 7 weeks o f age. The health o f the animals was monitored during the course of the studies according to the protocols of the N T P Sentinel Animal Program (Appendix I).
Animal Maintenance
Rats werehoused five per cage. Feed andwater were available ad libitum. Racks were rotated in the room every 2 weeks, and cages were rotated from top to bottom within eachgroup every 2 weeks. Further details o f animal maintenance are given in Table 2.
performed on all animals. At necropsy, all organs and tissues were examined for gross lesions. All major tissues were fixed and preserved in 10% neutral buffered formalin, processed andtrimmed, embedded in paraffin, sectioned, and stained with hematoxylin and eosin for microscopic examination. Histopathology examinations o the tissues were f performed according to an "inverse pyramid" design (McCoMell, 1983a,b). Complete histopathologic examinations were performed on all grossly visible lesions in all dose groups, on all control animals, OO 0 , and on animals receiving 4O ppm. Selected histopathology examinations performed were on 1 O and 1 , O ppm dose groupanimals dying OO , 0O O before the end o f the study period. The tissues, tissue groups, andorgans examined are listed in Table 2. Upon completion of the microscopic evaluation by the laboratory pathologist, the slides, paraffin blocks, and residual wet tissues were sent to the N T P Archives for inventory, slideblock match, and wet tissue audit. The slides, individual animal data records, and pathology tables were sent to an independent pathology quality assessment laboratory. The individual animal records and pathology tables were compared for accuracy, slide and tissue counts were verified, and histotechnique was evaluated. The kidney from all male and rats the kidney, uterus, and thyroid gland from all female rats were reevaluated microscopically by a quality assessment pathologist. Additionally, the duodenum, ileum, jejunum, and glandular stomach were reviewed from all animals in all groups for pigmentation. All diagnoses o f primary mammary gland tumorand squamous cell carcinoma o f the tongue in females were examined. Since the urinary bladder had been affected in a previous study, 20% of the control and 4O ppm dose groups were randomlyselected for OO 0 , microscopic review o f the urinary bladder. The quality assessment and report slides were submitted to the N T P PathologyWorking Group (PWG) chair, who reviewed all kidneys and all segments o f duodenum, ileum, jejunum and glandular stomach with a diagnosis of pigmentation. All uteri and mammary glands with tumor a diagnosis and all tongues with the diagnosis of squamous cell carcinoma from female rats were also reviewed. Representative examples o f potential chemical-related nonneoplastic lesions and
All animals were observed twice daily and clinical findings wererecorded weekly for 13 weeks and
Clinical Examinations and Pathology
monthly thereafter. Rats were weighed at study initiation, onceper week for 14 weeks, andonce every 4 weeks thereafter. Feed consumption was measured weekly.
After 6 months, 10 male and 10 female rats from each dose group werekilled for interim evaluations. An additional 10 rats from each dose group were randomly selected and killed f o r 15-month interim evaluations. Blood was drawn from the tails of rats to measure the following hematology parameters: erythrocytes, total leukocyte count, leukocyte differential counts, and nucleated erythrocytes. Blood collected from the jugular vein was analyzed for concentrations of blood urea nitrogen, creatinine, sodium, potassium, chloride, alanine aminotransferase, aspartate aminotransferase, and sorbitol dehydrogenase. One week prior to the 6- or 15-month interim evaluations, urine was collected overa24-hour period, the volume was measured and the concentrations o f chloride, potassium, and sodium were determined. The brain, liver, and right kidney o f eachanimalwere weighed at necropsy. Further details o f the interim evaluations are presented in Table 2. Animalsfoundmoribund, selected for the 6- or 15-month interim evaluations, or surviving to the end of the 2-year studies were killed. Necropsy was
Materials and Methods
19
neoplasms, lesions for which there was a difference in diagnosis between the study pathologist and reviewing pathologist, and lesions o f general interest were selected by the chair for review by the PWG. All renal neoplasms andhyperplasias were examined by the PWG. The PWG consisted o f the quality assessment pathologist and other pathologists experienced in rodent toxicologicpathology. This group examined the tissues without knowledge of dose groups or previously rendered diagnoses. When the consensus opinion o f the PWG differed from that of the laboratory pathologist, the diagnosis was changed. Thus,the final diagnoses represent a consensuso f contractor pathologists and the PWG. Details of these review procedures have been described by Maronpot and Boorman (1982) and Boorman et aL (1985). For subsequent analysis of pathology data, the diagnosed lesions for each tissue type are evaluated separately or combined according tothe guidelines o f McConnell et aL (1986).
However, when macroscopic examination was required to detect lesions (e.g., skin or mammary glandneoplasms) prior to histologic sampling, or when lesions had multiple potential sites o f Occurrence (e.g., mononuclear cell leukemia), the denominators consist o f the number o f animals on which a necropsy was performed.
Analysis o Neoplasm Incidence f
Statistical Methods Survival Analyses
The probability of survival was estimated by the product-limit procedure of Kaplan and Meier (1958) and is presented in the form of graphs.Animals were censored from the survival analyses at the time they were found dead o f other than natural causes; animals dying from natural causes were not censored. Statistical analyses for a possibledoserelated effect on survival used the method of Cox (1972) for testing two groups for equality and Tarones (1975) life table test to identify doserelated trends. All reported P values for the survival analyses are two sided.
The majority o f neoplasms in these studies were considered to be incidental to the cause o f death or not rapidly lethal. Thus, the primary statistical method used was a logistic regression analysis, which assumed that the diagnosed neoplasms were discovered as the result o f death from an unrelated cause and thus did not affect the risk o f death. In this approach, neoplasm prevalence was modeled as a logistic function o f chemical exposure and time. Both linear and quadratic terms in time were incorporated initially, and the quadratic term was eliminated if it did not significantly enhance the fit of the model. The dosed and control groups were compared on the basis o f the likelihood score test for the regression coefficient o f dose. This method of adjusting for intercurrent mortality is the prevalenceanalysis o f Dinseand Lagakos(1983), further described and illustrated by Dinse and Haseman (1986). When neoplasms are incidental, this comparison o f the time-specific neoplasm prevalence also provides a comparison o f the timespecific neoplasm incidences (McKnight and Crowley,1984). In addition to logistic regression, alternative methods o f statistical analysis were used, and the results o f these tests are summarized in the appendixes. These include the life table test (Cox, 1972; Tarone, 1975), appropriate for rapidly lethal neoplasms, and the Fisher exact test and the Cochran-Armitage trend test (Armitage, 1971; Gart et aL, 1979), procedures based on the overall proportion o f tumor-bearing animals.
Calculation o Incidence f
Tables A1 and B1 summarize the incidence of neoplasms in male and female rats. Tables A5 and B5 summarize the incidence of nonneoplastic lesions in male and female rats. The incidence o f neoplasms or nonneoplastic lesions is given as the ratio o f the number of animals bearing such lesions at a specific anatomic site to the number o f animals inwhich that site was examined. In most instances, the denominators include only those animals for which the site was examined histopathologically.
Tss o f significance included pairwise comparisons et
o f each dosed group with controls and a test for an overall dose-response trend. Continuity-corrected tests were used in the analysis o f tumor incidence, The and reported P values are one sided. procedures described above also were used to evaluate selected nonneoplastic lesions. (For further
20
Quercetin, NTP
TR 409
dose-response discussion o f these statistical methods, see Haseman, that does not assume a monotonic 1984). trend (Dunnetts or Dunns test). Average nephropathy severity values for the 2-year studies were analyzed for significance using the MannWhitney U test (Hollander and Wolfe, 1973). Historical Control Data Although the concurrent control group is always the first and most appropriate control group used for QUALITY METHODS evaluation, there are certain instances in which historical control data can be helpful in the overall The 2-year studies wereconducted in compliance with FDA Good Laboratory Practice Regulations assessment o f neoplasm incidence. Consequently, control tumor incidences from the N T P historical (21 CFR Part 58). In addition, as study records they were control database (Haseman et aL, 1984,1985) are weresubmitted tothe NTP Archives, audited retrospectively by an independent quality included in the NTP reports for neoplasms assurance contractor. Separate audits covering appearing to show compound-related effects. completenessand accuracy o f the pathology data, pathology specimens, final pathology tables, and preliminary review draft o f this NTP Technical Analysis of Continuous Variables Report were conducted. Audit procedures and Two approaches were employed to assess the findings are presented in the reports, which are on file at the NIEHS. The audit findings were significance o f pairwise comparisons between dosed andcontrolgroups in the analysis o f continuous reviewed and assessed by N T P staff so that all discrepancies had been resolved or were otherwise variables. Organ and body weight data, which have approximatelynormal distributions, were analyzed addressed during the preparation o f this Technical using the parametric multiple comparison Report. procedures o f Williams (1971,1972) andDunnett (1955). Clinical chemistry, urinalysis, and GENETIC TOXICITY hematology data, which typically skewed have distributions, were analyzed using the nonparametric The genetic toxicity of quercetin was assessed by multiple comparison methods of Shirley (1977) and testing the ability o f the chemical to induce mutaDunn (1964). Jonckheeres test (Jonckheere, 1954) tions in various strains of Salmonellalyphimurium was used to assess the significance of dose-response and to induce sister chromatid exchanges and chrotrends and to determine whether a trend-sensitive mosomal aberrations in Chinese hamsterovary cells. test (Williams or Shirleys test) was more The protocols for these studies and tabularpresentaappropriate for pairwise comparisons than a test tions of their findings are given in Appendix C.
ASSURANCE
Materials and Methods
21
TABLE 2
Experimental Design and Materials and Methods in the 2-Year Feed Studies of Quercetin
Study Laboratory EG&G Mason Research Institute, Worcester, MA Strain and Species F344m rats Animal Source Charles River Breeding Laboratories, Portage, MI Date of Birth Males: 3 - 10 May 1982 Females: 10 - 17 May 1982 Time Held Before Study Males: 15 days Females: 21 days Average Age When Placed on Study 7 weeks Date of First Dose Males: 23 June 1982 Females: 6 July 1982 Duration of Dosing 104 weeks (7 daysbeek) Date of Last Dose Males: 15 - 21 June 1984 Females: 27 June - 6 July 1984 Necropsy Dates 6-month interim evaluation: Males: 28 - 30 December 1982; Females: 12 - 14 January 1983 15-month interim evaluation: Males: 28 - 30 September 1983; Females: 12 - 14 October 1983 2-year studies: Males: 15 - 21 June 1984; Females: 28 June - 5 July 1984 Average Age When Killed 111 weeks
70 males and 70 females
Size of Study Groups
Method of Animal Distribution Animals of each sex randomized into cage groups, and then cages randomized to treatment groups using appropriate table o f random numbers. Animals per Cage 5 Method of Animal Identification Ear punch Diet NIH47 Rat and Mouse Ration, Open formula, pellets (Zeigler Bros., Inc., Gardners, PA), available ad Iibinun
22
Quercetin, N T P
TR 409
TABLE 2
Experimental Design and Materials and Methods in the 2-Year Feed Studies of Quercetin (continued)
Feeders Stainless steel, gang style (Scientific Cages, Inc., Bryan, TX), changed once weekly
Water Tap water (City of Worcester Water Supply) via outside-thecage automatic watering system (Edstrom Industries, Inc., Waterford, WI), available ad libh caw Solid-bottom polycarbonate (Lab Products, Inc., Rochelle Park, NJ)
Aspen bed, heat-treated hardwood chips (American Excelsior Co., Baltimore, MD), changed twice weekly
Cage Filters Non-woven fiber filters (Snow Filtration, Cincinnati, OH) Animal Room Environment Temperature: 22.5" & 1.5" C Relative humidity:47.6% 2 5.8% Fluorescent light: 12 hours/day Room air changes: l2hour
Bedding
DOSS
0, l O , lO,OOO, or 40,OOO ppm quercetin in f e e d , O O
T p and Frequency of Observation ye Necropsy and Histopathology
Nmopy:
H i wi@&
Observed twice/day; body weight initially, oncehveek for 14 weeks, once/month thereafter; clinical observations onceheek for 13 weeks, oncelmonth thereafter; f e e d consumption measured onceheek. Recorded for brain,rightkidney,andliver of allanimalssacrificed at 6 and 15 months Performed on all animals Complete histopathologicexaminationsperformed on all grosslyvisiblelesionsinall dose groups and on all control and 40,OOO ppm dose animals; histopathologic examinations performed on the following tissues: At 6 months: 10,OOO ppm dose groups (large intestine, small intestine, and uterus) At 15 months: 1,OOO ppm dose groups (large intestine); 10,OOO ppm dose group (large intestine, small intestine, and stomach) OO , Animals dying early and at study termination (for 1 O and 10,OOO ppm groups): (kidney, liver, pancreas, parathyroid gland, pituitary gland, small intestine, tongue, urinary bladder, and uterus)
. i
Clinical Pathology Blood and urine samples were collected from males and females at the 6- and 15-month interim evaluations. f I Erythrocytes, leukocytes, leukocyte differential count, and nucleated erythrocytes Chiad & m i s t y Blood urea nitrogen, creatinine, sodium, potassium, chloride, alanine aminotransferase, aspartate aminotransferase, and sorbitol dehydrogenase Urinary sodium, urinary potassium, and urinary chloride
23
RESULTS
2-YEARSTUDIES
6- and 15-Month Interim Evaluations
neoplasms or nonneoplastic lesions related to quercetin administration in male or female rats at 6 or 15 months.
The relative kidney and liver weights of male and female rats that received 40,OOO ppm were significantly greater than those o f the controls at both 6 and 15 months (Tables D l and D2). For females these differences primarily reflected the reduced body weights observed inhighdose animals. No biologically significant changes in hematology or clinical chemistry parameters were observed (Tables E l and E2). The only abnormality noted in the urinalyses was the presence of calcium oxalate crystals in 7 o f 10 high-dose males at 15 months. Yellow-brown pigmentation occurred in several tissues and was most prevalent in the glandular stomach and the distal segments of the small intestine. The incidence and severity o f pigmentation increased with dose concentration and duration. At 15 months all high-dose males had pigmentation in the glandular stomach, as did 5 o f 10 high-dose females. Epithelial staining o f the small intestine was present in all high-dose males and in nine high-dosefemales at 15 months. One high-dose male also had pigmentation in the lamina propria o f the jejunum and ileum and two mid-dose femaleshad pigmentation in the jejunal and ileal submucosa. Furthermore, at 15 months eight highdose males and four high-dose females had pigmentation o f the skulls or teeth. There were no
Estimates of the probabilities of survival for male and female rats are shown i Table 3 and in the n Kaplan-Meier survival curves in Figure 1. Exposure to quercetin had no significant effect on survival.
Survival
Body Weights, Feed Consumption, and Clinical Findings in the 2-Year Studies 0 O O , Male and female rats given 4 O ppm quercetin
had lower weight body gains than those of the controls. In males, the difference was 5% at week 25, and in females, 10% at week 25 (Tables 4 and 5 and Figure 2). From 65 weeks to the end o f the study, the difference among males ranged from 6% to 13%;in females, the difference ranged from 13% to 15%. Feedconsumption by exposed males and o f the controls females was similar to that (Tables G1 and G2). The decreased bodyweight gains relative to controls were attributed to quercetin toxicity. A yellowish discoloration o f the hair coat, especially in the perineal area, was present in all mid- and high-dose animals, presumably due to the urinary and/or fecal excretion o f quercetin and/or its metabolites.
24
Quercetin, NTP TR 409
TABLE 3
Survival of Rats in the 2-Year Feed Studies of Quercetin
Male
Animals initially in study 6-Month interim evaluation' 15-Month interim evaluation' Natural deaths Moribund kills Animals surviving until end of the study Percent survival at end of studyb Mean survival (as' dy) survival analysesd 70 10 10 3 21 26 52 576 P=O.603 70 10 10 7 15 70 10 10 3 70 10 10 6 21 47 576 P=O.824
23
56 581 P =0.838N
28
22 25
577 P=O.940
50
Female
Animals initially in study 6-Month interim evaluation' 15-Month interim evaluationa Natural deaths Moribund kills Animals surviving until end of the study Percent survival at end of studyb Mean survival (as' dy) survival analysesd 70 10 10 1 19 30 60 590 P=O.709 70 10 10 4 18 28 56 574 P=O.612 70 10 10 2 13 35 71 586 P=0.445N 70
10 10 3 19 28
576 P=O.656
56
' '
Censoredfromsurvivalanalyses Kaplan-Meier determinations. Survival rates adjusted for interim evaluations. Meanofall deaths (uncensored, censored, terminal sacrifice). The result of the life table trend test (Tarone, 1975) is in the control column, and the results of the life table pairwise comparisons (Cox, 1972) with the controls are in the dosed columns. A lower mortality in a dose group is indicated by N.
Results
25
0 9 ...................
p0
15
1. .
0
10.000 PPY
30
WEEKS ON STUDY
45
60
75
90
j ..................
i ...................
i .................. I
i ..................
LL
A m
Q
CK
0.7-
...................
..................
m 0 0 . 6 - .........
m
0.5-
OPPY
1 , 0 0 0 PPY
.........
10,000
PPM
10.000 PPY
is
WEEKS ON STUDY
45
60
75
90
IO5
FIGURE 1 Kaplan-Meier Survival Curves for Rats Administered Quercetin in Feed for 2 Years
26
Quercetin,
NTP TR 409
TABLE 4
weeks
on
Study
Mean Body Weights and Survival of Male Rats in the 2-Year Feed Study of Quercetin
Av. W L
(9)
0 ppm No. of
Survivors
A . W L W t (46 of No. of v (9) controls) Survivors
1.000 ppm
A . W L W L (% of No. of v (9) controls) Survivors
1 , O ppm 0O O
A . W L W L of No. of v (% (g) controls) Survivors
4 , O Ppm 0 O O
1 2 3 4
6 7 8 9 10 11 12 13 14 17 2 1 2 5 29 30 33 37 41 45 49 53 57 61 65 73 81 85 89 93 97 101 104
68a
162 195 225 253 271 300 310 322 319 342 354 364 376 399 416 434 445 456 457 464 469 481 484 487 478 485 486 492 492 485 476 473 479 447 464
340
284
70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 60 60 60 59 59 59 59 59 59 59 59 58 48 47 44 43 39 3 5 3 1 26 26
160 1% 230 255 272 278 298 304 321 323 343 337 350 363 375 399 412 438 438 456 460 466 470 486 487 491 487 491 493 497 492
477 482 485 451 451
488
99 101 102 101 100 98 100 98 100 101 100 99 99 100 100 100 99 101 98 100 101 101 100 101 101 101 102 101 102 101 100 101 100 102 101 101 97
70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 60 60 60 60 60 60 60 60 60 60 60 49 49 46 43 42 38 36 33 29 29
165 203 233 257 272 287 304 312 325 336 346 334 343 359 373 395 409 430 435 448 458 464 464 482 481 484 483 490 491 483 480 473 465 450 427 440
488
102 104 103 102 100 101 102 101 101 106 101 98 97 99 99 99 98 99 98 98 100 100 99 100 100 100 101 100 101 100 98 99 99 98 94 96 95
70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 60 60 60 60 60 60 60 60 60 59 58 48 48 45 44 41 38 38 28 25 25
167 198 228 252 259 282 300 309 320 332 339 328 343 355 382 393 413 409 426 432 439 442 453 453 460 457 453 458 458 451 444 436 426 418 402 403
364
103 102 101 99 95 99 100 100 99 104 99 97 97 97 97 96 95 95 92 93 95 95 94 94 94 95
%
94 94 93 92 92 92 90 87 90 87
70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 60 60 60 60 60 60 58 58 58 58 57 47 47 46 44 43 42 40 28 25 25
Terminal sacrifice Mean for weeks 1-13 283 14-52 433 53-104 479
282 433 482
100 100 101
286 429 472
101 99 99
281 410 440
100 95 92
Results
27
TABLE 5
Weeks
01 1
Mean Body Weights and Survival
Av. W L
(9
of Female Rats in the 2-YearFeed Study of Quercetin
1 , O ppm 0O O Av..,WL W L (% of No. of (g) controls) Survivors
study
0 ppm No. of survivors
LOO0 ppm Av. W t W L (Q of No. of (g) con(mls) s w i v o r s
Av. W L L W
(9
4Oo p D m oo .
(% of
controls) survivors
No. of
1 2 3 4 5 6 7 8 9 10 11 12 13 14 17 21 25 29 33 37 41 45 49 53 57 61 65 69 73 77 81 85 89 93 97 101 104
138 153 163 165 177 187 191 199
200
210 215 215 216 225 233 244 255 257 268 279 292 301 31 1 319 327 336 343 350 355 362 365 369 369 360 365 357
208
70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 70
60 60 60 60 60 60 60 60 60
141 155 165 167 178 186 193 199 2Q4 210 21 1 214 219 218 226 233
246
102 102 101 101 101 100 101 100 102 101 101 100 102 101 101 100 101 102 103 103 102 102 102 103 103 103 102 102 102 102 101 101 102 102
101
257 263 276 299 305 317 329 337
288
101
70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 70
60 60 60
59 49 49 49 49 49 46 42 38 33 30
349 355 364 368 367 371 376 367 368 360
344
60 60 60 60 60 60 60 48 47 45 45 39 38 36 30 28
28
50
139 152 162 167 177 183 189 194 198 203 2Q5 202 207 212 217 222 232 237 242 252 273 279 290 299 310 320 331 335 340 345 348 352 355 340 351 349
261
101 99 100 101 100 98 99 98 99 98 98 94 98 97 95 95 93 94 94 94 94 93 93 94 95 95 96 95 95 95 96 94 96 98
% % %
70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 70
60 60 60 60 60 60 59 59 59 58 48 48 47 45 45 43 43 41 38 35
141 152 162 165 176 181 186 190 194 197 198 195 192
200 209
102 100 99 100 100 97 97 97 95 94 91 89 93 91 90 90 86 88 86 86 82 83 83 85 85
85 84
%
203 220 220 225 231 239
101
256 265 277 285 291 2% 303 308 31 1 314 318 312 317 311
246 248
85 85 85 85 85 86 87 87 87
70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 70 60 60 60 60 59 59 59 59 59 59 48 47 47 42 38 35 31 28
44 44
Terminal sacrifice Mean for weeks 1-13 186 14-52 257 53-104 349
a
30
188 261
35 183 243 333
99
28
179 224 297 97 88 85
355
101 103 102
95 95
Interim evaluation occurred.
28
Quercetin, NTP TR 409
..................
.........
......
:...................
....
:............
.........................
:
R...o .........................
...................
:...................
:...................
...................
...................
...................
................
................................................................................................
...................................
;
i ...................
MALE RATS
;
...
...
I .................
...................
...................
)....
15
so
WEEKS ON STUDY
;5
60
75
90
FIGURE2
Growth Curves for Rats Administered Quercetin
in Feed for 2 Y a s er
Results
29
Pathology and Statistical Analyses of Results of the 2-Year Studies
Summaries o f the incidences o f neoplasms and nonneoplastic lesions, individual animal tumor diagnoses, statistical analyses of primary neoplasms that occurred with an incidence o f at least 5% in at least one animal group, and historical control incidences for the biologically significant neoplasms mentioned in this section are presented in Appendixes A for male rats and B for female rats.
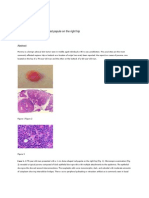
Renaltubule cellhyperplasias in malerats were focal lesions characterized by increased numbers o f tubule epithelial cells forming multiple layers which partially or totally filled the lumen and usually caused slight tubule dilation (Plate 1). The appearance o f the hyperplastic cells ranged from those of normal tubule epithelial cells to enlarged polygonal cells resembling cells o f the adenomas (Plate 2).
Kidney:
Initially, single sections o f the left and right kidneys from each rat were examined microscopically. Renaltubule neoplasms were seen in four high-dose male rats, whereas none were observed in controls (Table 6). Three of these neoplasms were adenomas, and one was an adenocarcinoma. Renaltubule neoplasms are relatively uncommon in agedrats. The combined incidence o these neoplasms in the study laboratory historical f control males for feed studies is 3/99 (3%, range 0%-6%). The combined incidence in control male rats is 8/499(1.6%, range 0%-6%) in all NTP feed 4. studies (Table A ) Additionally, there was a slight dose-related increase in the incidence o f renal tubule hyperplasia in males. Because of the low number of neoplasms in the high-dose males, the residual halves of the formalinfued kidneys from all males and females were step sectioned to provide approximately eight additional sections per rat for microscopic examination.
In general, the adenomas were small ( 0 - 0pm) 4080 and were distinguished from tubule hyperplasia by larger size and lack o f a definite tubular structure. Many adenomas had a prominent microtubular ) pattern (Plates 3 and 4. Adenomas were expansile and frequently compressed surrounding parenchyma (Plate 5). The neoplasms consisted o f large polygonal cells withabundant eosinophilic cytoplasm and large, pale-staining nuclei. The adenocarcinoma was 0 7 cm in diameter, expansile, and was composed of . variably sizedtubule-like structures which were filled with cells and contained often necrotic centers (Plate 6). Adenocarcinoma cells were clearly more anaplastic and were often characterized by marked pleomorphism, large nuclei, large nucleoli, and atypical mitotic figures (Plate 7).
The nephropathy wassignificantly more severe in 0 O O , male rats receiving 4 O ppm than in the controls ) (Table 8. There was no significant increase in the in dosed female rats. severity of nephropathy Nephropathy typical was o f the spontaneously occurring kidney lesion in aging F344/N rats. During this reevaluation, renal tubule focalhyperbased upon the extent of plasia was observed in eight high-dose males (one o f Severity grades were nephropathy and the amount o f renal parenchyma these animals had been identified in the initial evaluation), and tubule renal adenomas were affected. Nephropathy consisted o f a spectrum o f observed in six high-dose males (one o f these lesions, including varying degrees of tubule dilation, animals had been identified in the initial evaluation) distortion with occasional cyst formation, protein(Table 6). Focal hyperplasia was seen in two a m u s casts, atrophy, regeneration and hypertrophy epithelium, thickening of tubular and additional controlmales and a renal tubule adenoma of tubule glomerular basement membranes, interstitial fibrosis, wasobserved in one control male. The increased scattered foci of suppurative inflammation (primarily renal incidences o f renal tubule hyperplasia and within degenerating tubules), and a scattering o f tubule neoplasms in high-dose males is supportive varying numbers and aggregates o f mononuclear o f some evidence o f carcinogenicity. inflammatory cells within the interstitium. RegenIn the initial evaluation, a renal tubule adenomawas erating tubule epithelial cells had basophilic nuclei, seen in one mid-dose female rat, and an adenoma scant cytoplasm, and usuallyformed a single cell was found in one control female during the evalua- layer. There was also a dose-related increase in the tion o f the step sections. Thus, there was no evi- incidence o f renal pelvic transitional epithelial dence o f a chemical-related increased incidence in hyperplasia in males. This change is associated with kidney neoplasms in females (Table 7). severe nephropathy.
30
Quercetin, NTP TR 409
TABLE 6
Selected Kidney Lesions in Male Rats in the
2-Year Feed Study of Quercetin
Initial Evaluation (Single Sections) Renal Tubule: Hyperplasia Overall ratesa Logistic regressionb
Renal Tubule: Adenoma Overall rates Adjusted rates' Terminal ratesd First incidence ( a s dy) Logistic regression Renal Tubule: Adenocarcinoma Overall rates Renal Tubule: Adenoma or Adenocarcinoma' Overall rates Adjusted rates Terminal rates First incidence (days) Logistic regression
1/50 (2%) Pe0.079
0/50 (0%) 0.0%
2/50 (4%)
P-0.752N
3/50 (6%)
P =0.492
4/50 (8%) P=O.182 3/50 (6%) 11.1%
(9%)
oem (0%)
0/50 (0%) 0.0%
o m (0%)
P=O.o42
0/50 (0%)
0/50 (0%) 0.0% o m (0%)
0/50 (0%) 0.0% o (0%) m
= 676
P=O.122
1/50 (2%)
0/50 (0%)
0/50 (0%)
0/50 (0%)
O b 0 (0%) 0.0% o m (0%)
0.0%
4/50 (8%)
15.3%
o m (0%)
P=O.o02
3L23 (13%) 676 P -0.064
Evaluation of Step Sections Renal Tubule: Hyperplasia Overall rates
Renal Tubule: Adenoma Overall rates
2/50 (4%)
2/50 (4%) 2/50 (4%)
6/50 (12%)
7/50 (14%) (12%) 6/50
8/50 (16%)
1/50 (2%)
Single and Step Sections Combined Renal Tubule: Hyperplasia Overall rates Logistic regression
Renal Tubule: Adenoma Overall rates Logistic regression Renal Tubule: Adenoma or Adenocarcinoma Overall rates Logistic regression
a
3/50 (6%)
P=O.o06
3/50 (6%)
P=0.655N PE.O.022
2/50 (4%)
8/50 (16%)
P=O.o99
11/50 (22%)
1/50 (2%) P=O.O12
1/50 (2%) P=O.o05
P=O.526
7/50 (14%) P~O.032 7/50 (14%) P=O.O32
8/50 (16%)
P1.0.018
2/50 (4%)
P=O.526
9/50 (18%) P-0.010
'
e
Number of lesion-bearing animals/number of animals examined at site Beneath the control incidence are the P values associated with the trend test. Beneath the dosed group incidence are the P values correspondingto pairwise comparisons betweenthe controls and that dosed group. The logistic regression tests regard these lesions as nonfatal. A lower incidence in a dose group is indicated by N. Kaplan-Meier estimated neoplasm incidence at the end of the study after adjustment for intercurrent mortality Observed incidence at terminal kill Not applicable; no neoplasms in animal group Historical incidence for 2-year NTP feed studies with untreated control group (mean 2 standard deviation): 4/499 (0.8% f 1.1%, range 0%-4%)
Results
31
TABLE7 Selected Kidney Lesions in Female Rats in the 2-YearFeed Study of Quercetin'
0 PP"
Initial Evaluation (Single Sections) Renal Tubule: Hyperplasia Overall rates
Renal Tubule: Adenomab Overall rates
1/49 (2%) 04 ( % 19 0 )
1/49(2%) 04 ( % 19 0 )
3/50(6%) (2%) 1/50
1/50 ( % 2)
0150 ( % 0)
Evaluation of Step Sections Renal Tubule: Hyperplasia Overall rates
Renal Tubule: Adenoma Overall rates
14 /9
(% 2)
3/50 (6%)
1/49(2%)
05 ( % 10 0 )
Single and Step Sections Combined Renal Tubule: Hyperplasia Overall rates
Renal Tubule: Adenoma Overall rates
2/49
(4%)
4/50 (8%)
0/50 (0%)
1/49(2%)
'
Step sections were not evaluated in the 1,OOO ppm and 1 , O ppm dose groups. 0OO Historical incidence for 2-year NTP feed studies o f untreated control groups (mean f standard deviation): 1 4 9 19 range 0%-2%
( . % 0.6%), 02
TABLE 8 Incidences and Severity of Nephropathy in Male and Female Rats in the of Quercetin'
0 Male
Minimal (grade 1) Mild (grade 2) Moderate (grade 3) ) Marked (grade 4 Average severity grade
2-YearFeed Studies
10,Ooo
PPm
1,080 PPm
PP"
40,OOO p p m
1/48 18/48 19/48 118 04 2.7 A 0.14
2/50 19/50 m/50 9/50 2.7 f 0 1 .1
1/50 13/50 23/50 13/50 30 f 0 1 . .1
24 /9 7/49 16/49 24/49 3 2 f 01. . .4.
Female
Minimal (grade 1) Mild (grade 2 ) Moderate (grade 3 ) Marked (grade 4 ) Average severity grade
12/48 m/48 13/48 3/48 .3 21 f 0 1 .
7/48 25/48 12/48 4/48 2.2 f 0 1 .2
110 05 1/50 .0 21 f 0 1 .
30150
9/50
8/48 21/48 14/48 5/48 22 2 0 1 . .4
'
* * Statistically significant (PsO.01)from the control group by the Mann-Whitney U test
Number of animals with severity grade/number of animals with nephropathy.Severity grade wasbased onthepercentage o f parenchyma involved: Minimal - usually l e s s than 25% o f cortex; mild - 2% t o 50% of cortsr; moderate - 50% to 75% of corteq 5 marked -greater than 75% of cortex. Average seventy grade given as the mean -C standard error.
32
Quercetin,
NTP TR 409
Parathyroid g l a d
Hyperplasia o f the parathyroid glands, characterized by bilateral diffuse enlargement o f the glands, occurred with a dose-related increased incidence in malerats (0 ppm, 1/43; 1 O ppm, OO , 6/43; 40,OOO ppm, 17/43) 6/45; l0,OOO ppm, (Table A5). 'Qkally, the hyperplasias occurred with greater frequency and severity in animals with marked nephropathy. This is characteristic o f renal secondary hyperparathyroidism.
Tongue: A single squamous cell papilloma o f the tongue was present in a high-dose male (Table Al). Two squamous cell carcinomas werepresent in highdose females (Table Bl). The historical control incidence o f oral cavity neoplasms for female rats is 4/500 (OS%, range 0%-2%) for all NTP studies (Table B4b). Mummay g l a d Fibroadenomas o f the mammary
Uterus: Uterine stromal polyps O C C U K ~ ~more frequently in mid-dose female rats than in controls (7/50,9/50, 16/50, 11/50) (Table Bl). The incidence of stromal polyps in the high-dose group, however, was similar to controls. A uterine stromal sarcoma occurred in one female rat in the mid-dose group as well. Due to the marginal increased incidence in stromal polyps, the lack o f a dose response, and because only the incidence in the mid-dose group exceeded the range in untreated N T P historical were controls (Table B4d), these neoplasms not considered related to quercetin administration. Gastrointestinal tract: There was a significant dose-
gland occurred with a highly significant,dose-related, negative trend in femalerats (Table 9). The incidences o f fibroadenoma in the mid- and high-dose female groups were significantly lower than that in the controls. Fibroadenoma is the most common neoplasm o f the mammaryglandin female rats, occurring in 178/500 (35.6%, range 8%-56%) o f NTP untreated historical controls (Table B4c). Although the incidence o f fibroadenoma in the controls o f this study (58%) slightlyexceeds the range for historical controls, the incidence in the high-dose group is about one-half o f the mean rate of historical controls. The lower number o f female rats with fibroadenomas in the high-dose group is considered chemically related, and may be associated with the lower body weights in this group.
related accumulation o f a fine granular yellow to light brown pigment in the epithelial cells lining the glandular stomach, jejunum, ileum, and, to a lesser extent, the duodenum and colon (Table 10). Special stains to further characterize the pigment were not used, but the pigment was believed to be quercetin or one o f its metabolites.
Other organs/tissues: Other nonneoplastic lesions occurred with statistically significant increased incidence, but the biological significance o f their occurfence is uncertain. Highdose female rats had a marginally increased incidence o f chronic inflammation involving the liver (Table B5). However, all malegroupshad higher incidence than the highdose females. High-dosemaleratshadareduced incidence o f bile duct hyperplasia, an increased incidence o f lymphatic ectasia within mesenteric lymph nodes, and a marginal dose-related increased incidence in testicular interstitial cell hyperplasia (Table A5).
Results
33
TABLE 9
Mammary Gland Neoplasms in Female Rats in the
2-Year Feed Study of Quercetin
Fibroadenomaa overall ratesb Adjusted rates' Terminal ratesd First incidence (days) Logistic regression testse
a
29/50 (58%) 66.4% 16/30 (53%) 597 P<0.001N
27/50 (54%) 72.3%
18/28 (64%) 597 P=O.553N
16/50 (32%) 3.4% 1OB5 (29%) 605 P=0.008N
9/50 (18%) 30.1% w f i (2%) 549 P<0.001N
Historical incidence for 2-year NTP feed studies of untreated control groups (mean f standard deviation): 178/500 15.0%), range 8%-56% (35.6% Number of neoplasm-bearing animalshumber of animals n m p s i e d Kaplan-Meier estimated neoplasm incidence at the end of the study after adjustment for intercurrent mortality Observed incidence at terminal kill Beneath the control incidence are the P values associated with the trend test. Beneath the dosed group incidence are the P values corresponding to pairwise comparisons between the controls and that dosed group. The logistic regression tests regard these lesions as nonfatal. For all tests, a negative trend or a lower incidence in a dose group is indicated by N.
TABLE 10
Gastrointestinal Pigmentation in Rats in the 2-Year Feed Studies of Quercetin'
Male
Glandular Stomach Epithelium Large Intestine Colon, epithelium Small Intestine Duodenum, epithelium Ilium, epithelium Jejunum, epithelium
0/50
0150
3/49
34/48.
0/49
0148
1/47 0147 1/47 0/44
0/47
0148
Female
Glandular Stomach Epithelium Small Intestine Duodenum, epithelium Ileum, epithelium Jejunum, epithelium
0/50
0/49
0/50 0149
0150
0/48 0/48 0147
0150
19/49.. 3/49
1/49 32/49. U)/49.
. .
*. Significantly different (PsO.01) from the control group by the logistic regression test
a
Number of lesion-bearing animalslnumber of tissues examined
34
Quercetin, NTP TR 409
Exposure to quercetin (0.3-1,OOO &plate) produced a strong, dose-related increase in gene mutations in Salmonella typhimurium strains TAlOO and TA98 both in the presence and in the absence of Aroclor 1254-induced male Sprague-Dawley rat or Syrian hamster liver S9 (Table Cl). Incytogenetic tests with Chinese hamster ovary cells, quercetin induced marked increases in both sister chromatid exchanges and chromosomal aberrations, with and without 3. metabolic activation (Tables C 2 and C ) In the sister chromatid exchange test without S9, positive
GENETIC TOXICOLOGY
of responses were observed over range a dose 0 6 to 20 pg/mL quercetin; with S9, effective doses .7 rangedfrom 2 to 45 pg/mL. In the chromosomal aberration test, the trials conducted in the absence of S9 activation employed a delayed harvest protocol to offset quercetin toxicity; positive responses occurred with 10 to 50 pg/mL quercetin. With S9, standard harvest times were employed andstrong increases in aberrations were observed with 25 to 75 pg/mL quercetin. the At highest dose (75 pg/mL), all cells scored contained aberrations.
PLATE
Renaltubulehyperplasiawithhyperplasticcellsfilling the lumen in t h e kidney o f a male F344/N rat administered 40,000 ppmquercetin in feed for 2 years. H&E, 5OX.
PLATE 2
Highermagnification o f Plate 1 demonstratingcellularmorphologytypical o f hyperplasia. H&E, 1OOX.
m 3 T E
PLATE 4 i n t h e kidney of a male Higher magnification o f Plate 3 demonstrating cellular morphology typical Renal tubule adenoma with microtubular pattern F344/N ratadministered 40,000 ppmquercetininfeedfor 2 years. H&E, o f the adenomas. H&E, 1OOX. 5OX.
PLATE 5 Ekpansilerenal tubule adenomawithcompression of an adjacent tubule in the kidney o f a male F344/N ratadministered 40,000 ppm quercetin i n feed for 2 years. H&E, 1OOX.
PLATE 6 Renal tubule adenocarcinomawithvariablysizedtubular structures in the kidney of a male F344/N rat administered 40,OOO ppmquercetinin feed for 2 years. Tubular structures often had necrotic Centers. H&E, 1 O X .
Higher magnification o f Plate 6 demonstrating cellularanaplasiaand atypicalmitoticfigures. H&E, 1OOX.
PLATE 7
35
DISCUSSION AND CONCLUSIONS
Quercetin, a flavonoid, is found in many food plants In the present NTP studies, quercetin administered 4O O , including citrus fruits, berries, leafy vegetables,roots, at 4% (0 O ppm) in the diet also caused a herbs and spices,legumes, cereal grains, tea, and gradual reduction in body weightgain; final mean cocoa (Brown, 1980). The flavonoids as a group are body weights of male and female rats were 87% o f reported to have a wide range o f possible uses in those of the controls. The depressed weightgain in the high-dose rats in these studies medicine. To date, though, there have been no observed reported controlled clinical trials or toxicity testing demonstrates that the doses were sufficient to elicit o f these compounds to demonstrate efficacy as general toxicity. The high dose (4%) also antiviral or anticancer agents. Thus, flavonoids are approached the maximumlevel of quercetin that notapproved for drug use in the United States could be givenin the diet (5%) without adverse 2-year rodent (Havsteen, 1983; Cody, 1988, Gilman et al., 1990). nutritional effects. Experience with Interest in the toxicity and carcinogenicity o f the studies has shown that control and treated animals within approxithe mid-lVO's when it was must have body weight differences flavonoids began in shown that some o f these naturally occurring mately 10% to 15% to maintain similar rates of chemicals were mutagenic in the Salmonella background tumors. Thus, these 2-year studies of ryphimurium assaysystem. Quercetin is one o f the quercetin were considered adequate for assessing most common flavonoidsin plants and is also a toxicity and carcinogenic activity because the difcomponent o f bracken fern, a plant shown to cause ferences in body weights between the high-dose and toxicity and death in cattle. Quercetin was control groups were within this limit. nominated by the Food and DrugAdministration for toxicity and carcinogenicity studies in the rat The principal lesions associated with the adminisbecause it is widely distributed in natural foods and tration o f quercetin occurred in the kidney of dosed because of conflicting information in the literature male rats and included severe chronic nephropathy, of quercetin in renal tubule hyperplasia, and renal tubule adenomas. regarding the carcinogenicity Chronic nephropathy, a common condition in aging previous animal studies. rats, showed a treatment-related increased severity. Previous studies have shown that quercetin Hyperplasia o f the renal pelvic epithelium (tranadministered in feed to rodents at levels up to 5% sitional epithelium overlying the renal papilla) is a (the maximum dose usually in used feed studies component of severe nephropathy and occurred in without adverse effects on nutrition) caused little males witha similar treatment-related positive trend. organ toxicity and had no effect on mortality. This transitional hyperplasia is characteristic of Rutin, a glycoside conjugate of quercetin, has also chemical-related toxicity and is not considered to be been tested and found to be negative for carcino- a preneoplastic lesion. The dose-related increased genicity when fed in the diet to rats for up to incidence o f parathyroid hyperplasia in male rats is 850 days (Hirono et at!, 1981). However, long-term an indication that nephropathywas severe enough to administration of quercetin in feed has resulted in compromise renal function. Hyperparathyroidism gradually decreased body weights o f dosed animals. frequently accompanies severe nephropathy in rats loss of renal function Hirono et aL (1981)observed thatmale rats fed because the progressive 5% quercetin in the diet for morethan 100 days disrupts calcium and phosphorus homeostasis, which had an approximate 15% decrease in body weight leads to prolonged parathyroid gland stimulation. from that o f the control animals. It0 et at! (1989) This results in hyperplasia and elevated levels o f reported that F344DuCrj rats fed 1.25% or 5.0% parathyroid hormone. An associated mineralization was present in vascularwalls and several organs. quercetin in CRF-1 diet for 104weekshadfinal body weights that were 91% and 93% o f those of Severe nephropathy induced by chemical exposure may be life-threatening and has been the cause o f the controls for males and females.
36
Quercetin,
NTP TR 409
reduced survival among chemical-exposed rats in the survival several NTP 2-yearstudies.However, rates of exposed rats in the quercetin studies were similar to that o f the controls. The treatment-related increase in the severity o f nephropathy was Seen onlyinmales. This greater sensitivity to quercetin toxicity is apparently due to a greater susceptibility o f male rats to spontaneous nephropathy during aging and the exacerbation of this disease by chemical administration. Changes in glomerular permeability, resulting in proteinuria, progressive glomerular sclerosis, tubule damage, inflammation, and interstitial fibrosis, are associated with the process o f aging in rats.
related increase in the incidence of renal tubule hyperplasia. Although the incidence o f renal neoplasms in the high-dose group was not significantly greater than in the controls by pairwise comparisons, the trend test wassignificant.Even though renal neoplasms are relatively uncommon in NTP untreated historical control male rats (8/499, mean 1.6%, range 0%-6%, Table A&), the low number of neoplasms was difficult to interpret.
The NTP and Kurokawa ef al. (1983) have found that multiple sectioning o f the kidney may enable a more precise evaluation of the potential chemicalneoplasms related induction of renal tubule compared with observations from single-section sampling. The majority o f renal neoplasms in these One factor that may contribute in part to the studies are microscopic (i.e., not observed by greater severity of tubule damage in male rats than macroscopic examination at necropsy), thus, multiple , in female rats is their production of more asections might be expected to increase the number globulin, a low molecular weight protein. Vandoren of neoplasms observed and allow for more a et al. (1983) showed thatfemale rats excrete less rigorous statistical evaluation. The residual halves than 1% o f the amount of ah-globulin excreted by of the formalin-fmed kidneys from all the rats were male rats. Further, in males, approximately 60% o f step sectioned to provide approximately eight the ah-globulin is reabsorbed by epithelial cells o f additional tissue sections for microscopic examinathe proximal convoluted tubule, primarily in the Pz tion. Renal tubule focal hyperplasia wasobserved segment, whereit is slowly or poorly hydrolyzed and in eight high-dose males (one o f these animals had accumulates in lysosomes (Charbonneau et aL, 1988). been identified in the initial evaluation), and renal in six highdose Short et aL (1987) showed that cells containing the tubule adenomaswereobserved males (one of these animals had been identified in a,-globulin undergo degeneration, necrosis, and a , higher rate o f cell turnover in the P segment com- the initial evaluation). Focal renal tubule hyperplapared with other segments of the proximal con- sia was seen in two additional control males and a renal tubule adenoma was observed in one control voluted tubule. male. Histopathologically, the cell turnover is normally cellular regeneration, but under conditions not fully The renal tubule hyperplasia observed in these understood, hyperplasia results. Several authors studies was distinguished from backgroundregenerasuggest that this hyperplastic response o f renal tive hyperplasia, which commonly accompanies the tubules in spontaneous nephropathy may be similar degenerative tubule changeso f age-related or chemical-induced nephropathy, on the basis o f cellular to those o f tubules responding to chemical toxins. atypia and prominent stratification o f the epiincreased Konishi and Ward (1989) observed 'H-thymidine labeling indices in the tubule epi- thelium. These cytological features suggest a loss o f thelium with corresponding increased severity o f cell growth regulation and failure o f cellular differnephropathy.Short et al. (1987) also showed in- entiation. This lesion is similar to those induced by creased cell necrosis and regeneration associated potent renal carcinogens and appears to represent with an accumulation of ah-globulin induced by the early stages of renal tubule adenoma and adenocarcinomadevelopment (Hard, 1986, Tsuda et aL, chemical administration. 1986). Although focal hyperplasia, adenoma,and constitute morphological In the initial evaluation of single sections from each adenocarcinoma a left and right kidney, three renal tubule adenomas continuum, the rates o f possible progression or and one adenocarcinomawere seen in the high- regression o f hyperplasia or adenoma are not known vary the inducing agent andthe dose rats male with a concomitant slight dose- and likely with
Discussion and Conclusions
37
mechanism of induction. It has been postulated that increases in cellular proliferation secondary to chemical-related cytotoxicity may create the appropriate environment for the development of neoplasia, perhaps by increasing the frequency of spontaneous mutations through clonal expansion of initiated cells or by other means (Farber, 1980; Pitot and Sirica, 1980; Stott et aL, 1981; Butteworth, 1989; a h e n and Ellwein, 1990). An increase in chemical-related accumulation o f a,-globulin in the P, segment of the nephron has been associated with the development of renal neoplasms (Goldsworthy andPopp, 1987; Goldsworthy et aL, 1988). This syndrome, also known as hyaline dropletnephropathy or a,-globulin nephropathy, is best identified in 13-week studies; such studies were not conducted by the N l T with quercetin. However, the linear tubule mineralization within the papilla,whichis commonly seen in the 2-year studies of chemicals producing this syndrome, was not seen in the quercetin studies. Previous rodent toxicity and carcinogenicity studies of quercetin did not identify the kidney as a target organ (Saito et aL, 1980, Hirono et aL, 1981; Takanashi et aL, 1983; Ito et ai., 1989). However, in the N l T 2-year studies of quercetin, the renal tubule cell neoplasms observed inmalerats were judged to show some evidence for carcinogenicity due to supportive evidence for this neoplastic response by an increase in renal tubule hyperplasia. Step-sectioned kidneysshowed an increase in the incidence o f kidney neoplasms, which supported the original findings of only a few neoplasms at this site. Since most o f the neoplasms were adenomas, this effect was judged to be some evidence rather than clear evidence for carcinogenic activity. In a series of NCI/NlT long-term rodent carcinogenicity studies o f chemicals, treatment-related kidney neoplasms were found more frequently in malerats (23) than in female rats (8), male mice (3), or female mice (1) (Table 11). Based on this information, the kidneys of male rats appear to be more sensitive to chemical-induced formation than are the kidneys of female rats or mice of either sex The reasons for the susceptibility of the male rat kidney to chemicaltoxicity or carcinogenicity may vary from chemical to chemical. There is no one particular chemical structure that is associated with the induction of kidney neoplasms in male rats and
some of the chemicals causing kidney tumors demonstrate genetic toxicity in in vizro tests while others do not. An increase in chemical concentration in the kidney o f male F344/N rats, an animal with a significant age-related background o f kidney disease, may make this animal particularly susceptible to the induction of renal tubule cell neoplasms. The two squamous cell carcinomas o f the tongue observed in the high-dose female rats were not considered to be related to treatment. Squamous cell papillomas or carcinomas have been observed sporadically in N T P study animals in both treated and control groups, occurring with a historical mean incidence o f 0.8% in untreated controls (4/500, range 0%-2%; Table B4b). Due to the Occurrence of these oral cavityneoplasms, a complete histopathologic examination was performed on the tongue. There was no supportive evidence for hyperplasia or other neoplasms at this site. Chemicalswhichhave been shown to causeoral cavityneoplasms, such as benzidine congeners or dyes, are generally characterized as potent genotoxic agents and cause neoplasms at a variety of other sites. summary, In the oral cavity neoplasms observed in the high-dose female rats were not considered to be related to chemical administration because of the lowincidence,lack o f supportive nonneoplastic lesions at this site, and lack of supportive evidence of neoplasms in related tissues o f epidermal origin. There was a dose-related decrease in mammary gland fibroadenomas in female rats. Previous studies showed that decreases in some naturally occurring benign neoplasms, especially neoplasms of the mammarygland and reproductive organs, are associated with decreased bodyweight relative to controls (Rao et aL, 1987). Some investigators have reportedthat quercetin mayhave some undefined antitumor activity (Hirose et aL, 1983; Kat0 et aL, 1983; Nishino et a!, 1984b). While most rat studies o f quercetin have shown no evidence of carcinogenicity,Pamukcu et at! (1980) reportedthat 0.1% quercetin administered in the diet to Norwegian rats for 58 weeks caused an 80% increase in the incidence o f intestinal neoplasms and a 20% increase in the incidence of urinary bladder neoplasms. The mechanism for this increase could not be explained based on the information given but
38
Quercetin, NTP TR 409
may be dueto different experimental conditions, study animals, or chemical preparations. The N T P quercetin studies showed no evidence o f chemicalinduced neoplasms of the urinary bladder or intestine. Quercetin has a marked ability to cause mutations in various genetic toxicity tests including the Salmonella lyphimurium assay systems. Due to these results, the chemical might be expected to cause neoplasms at a variety of sites in male rats besides the kidney. The mutagenic flavonoids generally contain a free hydroxy group at the 3 position. Brown and Griffiths (1983) have shown that rats are capable of metabolizing quercetin and other 3-hydroxyl flavonoids to the 3 s-methyl ethers. The 3 s-methyl ether o f quercetin is considerably
less mutagenic in the Salmonella assay thanthe parent compound (MacGregor and Jurd, 1978). This ability of rats to form 3 +methyl ethers may be important in protecting against the carcinogenic action of quercetin at various sites in the body.
Under the conditions of these 2-year feed studies there was some evidence of carcinogenic activ@+ of quercetin in male W rats based on an increased / N incidence of renal tubule cell adenomas. There was no evidence of carcinogenicactivity of quercetin i n / N rats receiving l O , 10,OOO or OO , female W 4 O ppm. Incidence of renal tubule hyperplasia O 0 , O and the severity of nephropathy were increased in exposed male rats.
CONCLUSIONS
Explanation of Lmels of Evidence o f Carcinogenic Activity is on page 8. A summary of peer review comments and the public discussion on this Technical Report appear on page 10.
Discussion and Conclusions
39
TABLE11 Evidence of Kidney Neoplasms and scrlmonelh Mutagenicity in Rats and Mice for Selected Chemicals Tested by the National Toxicology Program
Chemical Name and Structure Technical Report Number 111 Kidney NeoDlasmsa
@ R a t s
Rats
#Mice
Mice
SuZmone2.h Results
NTP
1-Amino-2-Methylanthraquinone
2-Amino-4-Nitrophenol W
339
0-Aniiidine
m .
89
+b
Benzofuran
370
Bromodichloromethane
CI
321
Chlorinated Paraffins
CH,(CH2CHCICH2CHCICH;),CH,CI (appmximation)
308
Chlorothalonil
41
40
Quercetin, NTP TR 409
11 TABLE Evidence of Kidney Neoplasms and ScrImoneua Mutagenicity in Rats and Mice for Selected Chemicals Tested by the National Toxicology Program (continued)
Chemical Name and Structure Technical Report Number 335
eRats
Kidney NeoDlasms 0 Rats "ice
0 Mice
NTP S h n e h Results
GI. Acid Orange 3
+b
Cinnamyl Anthranilate
1%
319
Dimethyl Methylphosphonate
ii
323
+c
Hexachloroethane
CI--c----c-U
PC" b b
361
NT
NT
Hydroquinone
on I
366
Isophorone
291
Discussion and Conclusions
41
TABU 11 Evidence of Kidney Neoplasms and Scrlmonellrr Mutagenicity in Rats and Mice for Selected Chemicals Tested by the National Todcology Program (continued)
Chemical Name and Structure &Limonene Technical Report Number
~
#Rats
Kidnev Neodasms 0 Rats #Mice
0 Mice
Salmonelh Results
NTP
31 4
a-Methylbenzyl Alcohol
9"
369
359
NT
NT
Mirex
a
313
+b
Monuron
266
Nitrilotriacetic Acid
CH2COOH
Cti&OOH-N-CH&OOH
42
Quercetin, NTP TR 409
TABLE11 Evidence of Kidney Neoplasms and salmonellrr Mutagenicity in Rats and Mice for Selected Chemicals Tested by the National Toxicology Program (continued)
Chemical Name and Structure Nitrofurantoin Technical Report Number
.
Rats
Kidney NeoJasms Q Rats d Mice
Q Mice
h h o m h Results
NTP
341
Ochratoxin
358
NT
NT
Phenylbutazone
367
Tetrachloroethylene
C I
CI
311
\c = c
I C '
'
' a
391
+b
Tris(2-Chloroethyl) Phosphate
0
CICH&H,kP-OCHzCH,CI
II
Tris(2,3-Dibromopropyl) Phosphate
76
Primarilyrenaltubule cell neoplasms observed unlessotherwisenoted; Transitional cell neoplasms Transitional cell neoplasms and renal tubule cell neoplasms
+ = present,
NT = species nottested.
43
REFERENCES
Aeschbacher, H.U. (1982). The significance o f mutagens in food. In Mutagens in Our Environment, pp. 349-362. A R . Liss,NewYork. Ambrose, AM., Robbins, D.J., and DeEds, E (1952). Comparative toxicities o f quercetin and quercitrin. J. A m Pharm. Assoc. 41, 119-122. Aravindakshan, M., Chauhan, P.S., and Sundaram, K (1985). Studies on germinal effects o f quercetin, a naturally occurring flavonoid. Mutat. Res. 144, 99-106. Armitage, P. (1971). Statistical M e t h d in Medical Research,pp. 362-365. John Wiley and Sons,New York. Bartholomew, R.M., and Ryan, D.S. (1980). Lack of mutagenicity o f some phytoestrogens in the Salmonella/mammalian microsome Mutat. assay. Res. 78, 317-321. Bjeldanes, L.F., and Chang, G.W. (1977). Mutagenic activity of quercetin and related compounds.Science 197, 577-578. Bokkenheuser, V.D., Shackleton, C.H.L., and Winter, J. (1987). Hydrolysis of dietary flavonoid glycosides by strains o f intestinal Bacteroides from humans.Biochem. J. 248, 953-956. Boorman, G.A, Montgomery, C . k , Jr., Eustis, S.L., Wolfe, M.J., McConnell, E.E., and Hardisty, J.F. (1985). Quality assurance in pathology forrodent carcinogenicity studies. In Handbook of Carcinogen Testing ( H A Milman and E.K. Weisburger, Eds.), pp. 345-357. Noyes Publications, Park Ridge, NJ. Booth, AN., Murray, C.W., Jones, ET., and DeEds, F. (1956). The metabolic rate o f rutin and quercetinin the animal body. J. Biol.Chem. 223,
251-257.
Borghoff, S.J., Short, B.G., and Swenberg, J.A (1990). Biochemicalmechanisms and pathobiology of a,-globulin nephropathy. Anna Rev. PhamacoL
TOX~COL 30, 349-367.
Brams, A, Buchet, J.P., Crutzen-Fayt, M.C., De Meester, C., Lauwerys, R., and Leonard, A (1987). A comparative study, with 40 chemicals, of the efficiency o f the Salmonella assay and the SOS chromotest (kit procedure). Lett. Toxkol. 38,
123-133.
Brown, J.P. (1980). A review o f the genetic effects o f naturally occurring flavonoids, anthraquinones and related compounds.Mutat. Res. 75, 243-277. Brown, J.P., and Dietrich, P.S. (1979). Mutagenicity o f plant flavonols in the SaZmoneZZa/mammalian microsome test. Activation o f flavonol glycosides by mixed glycosidases from rat cecal bacteria and other sources.Mutat. Res. 66, 223-240. . Brown, S , and Griffiths, L A (1983). New metabolites of the naturally-occurring mutagen, quercetin, the pro-mutagen, rutin and o f taxifolin. Experientia 39,198-200. Busch,D.B., Hatcher, J.F., and Bryan, G.T (1986). Urine recovery experiments with quercetin and other mutagens using the Ames test. Environ. Mutagen. 8,
393-399.
Butterworth, B.E. (1989). Nongenotoxic carcinogens in the regulatory environment. Re& ToxicoL Pharmacal. 9, 244-256. Carver,J.H., Carrano, AV., and MacGregor, J.T. (1983). Genetic effects o f the flavonols quercetin,
kaempferol and galangin on Chinese hamster ovary cells i vitro. Mutat.Res. 113,45-60. n
44 Castillo, M.H., Perkins, E., Campbell, J.H., Doerr, R., Hassett, J.M., Kandaswami, C., and Middleton, E., Jr. (1989). The effects o f the bioflavonoid quercetin on squamous cell carcinoma of head and neck origin. Am. J. Suq. 158, 351-355.
Quercetin,
NTP TR 409
Farber, E. (1980). The sequential analysis o f liver cancer induction. Biochem Bwphys. Acta 605, 149-166. Galloway, S.M., Bloom, AD., Resnick, M., Margolin, B.H., Nakamura, F., Archer, P., and Zeiger, E. (1985). Development of astandard protocol for in vitro cytogenetic testing withChinesehamster ovary cells: Comparison o f results for 22 compounds in two laboratories. Environ Mutagen 7, 1-51. Galloway, S.M., Armstrong, M.J., Reuben, C., Colman, S , Brown, B., Cannon, C., Bloom, AD., . . Nakamura, E , Ahmed, M., Duk, S , Rimpo, J., Margolin, B.H., Resnick, M.A., Anderson, B., and Zeiger, E. (1987). Chromosome aberrations and sister chromatid exchanges in Chinese hamsterovary cells: Evaluations of 108 chemicals. Environ MOL Mutagen 10 (Suppl. lo), 1-175. Gart, J.J., Chu, and KC., Tarone, R.E. (1979). Statistical issues ininterpretation o f chronic bioassay tests for carcinogenicity. J. NatL Cancer Znst. 62, 957-974. Gilman, A.G., Rall, T.W., Nies, AS., and Taylor, P.
(Eds.) (1990). Therapeutics, 8th
Charbonneau, M., Stasser, J., Borghoff, S.J., and Swenberg, J.A. (1988). In vitro hydrolysis of ['4C]-ah-globulin isolated from male rat kidney. Toxicologist 8, 135. (Abstr).
Code o Federal Regulations (CFR) 21, part 58. f
of flavonoids. In PlantFlavonoids in Biologyand Medicine Z : Biochemical, Z Cellular and Medicinal Properties. (V. Cody, E. Middleton, Jr., J.B. Harborne, and A Beretz, Eds.). pp. 29-44.
Cody,V.(1988).Crystal
andmolecular structures
A.R. Liss, N Y .
Cohen, S.M., and Ellwein, L.B. (1990). Cell proliferation in carcinogenesis. Science 249, 1007-1011. Cox, (1972). D.R. Regression models and life tables. J. R Stat. Soc. B34, 187-220. Crebelli, R., Aquilina, G., Falcone, E., and Carere, A. (1987). Urinary and faecalmutagenicity Sprague-Dawley in rats dosed with the food mutagens quercetin and rutin. Food Chem. Toxicol.
25, 9-15.
ed. Pergamon, Elmsford, NY.
The Pharmacological
Bask o f
Haseman, J.K. (1986). Logistic Dinse, G.E., and regression analysis o f incidental-tumor datafrom animal carcinogenicityexperiments. Fundum.AppL T~xicoL 44-52. 6, Dinse, G.E., and Lagakos, S.W. (1983). Regression analysis of tumor prevalence data. Appl. Statist. 32, 236-248. Dunn, O.J. (1964). Multiple comparisons using rank sums. Technomem'cs 6, 241-252. Dunnett, W. (1955). A multiple comparison procedure for comparing several treatments with a control. J. Am. Stat. Assoc. 50, 1095-1121. Evans, I.A. (1984). Bracken carcinogenicity. Chem. Soc. 18, 1171-1204.
Am.
Goldsworthy, T.L., and Popp, J.A. (1987). Chlorinated hydrocarbon-induced peroxisomal enzyme activity in relation to species andorgan ToxicoL AppL PharmacoL 88, carcinogenicity. 225-233. Goldsworthy, T.L., Lyght, O., Burnett, V.L., and Popp, J.A.(1988). Potential role of a-;?u-globulin, protein droplet accumulation, and cell replication in the renal carcinogenicity o f rats exposed to trichloroethylene, perchloroethylene, and AppL 9, 6 pentachloroethane. ToxicoL PharmacoL 367-379. Graziani, Y., and Chayoth, R. (1979). Regulation o f cyclic AMP level and synthesis o f DNA, RNA and protein by quercetin in Ehrlich ascites tumor cells. Biochem. PharmacoL 28, 397-403. Griffith, J.Q., Kreivson, C.F., and Naghski, J. (1955). Rutin and Related Flavonoids, pp. 234-242. Mack, Easton, PA.
References
45
. Gugler, R., k c h i k , M., and Dengler, H.J. (1975). Hirono, I., Ueno, I., Hosaka, S , Takanashi, H., . Disposition o f quercetin in man after single oral and Matsushima, T., Sugimura, T., and Natori, S (1981). Carcinogenicity examination o f quercetin and rutin intravenous doses. Eur. J. Clin. PhamacoL 9, in ACI rats. Cancer Lett. 13, 15-21. 229-234.
Hard, G.C. (1986). Experimental models for the sequential analysis of chemically-induced renal carcinogenesis. ToxicoL Pathol. 14, 112-122. Hardigree, k k , and Epler, J.L. (1978). Comparative mutagenesis of plant flavonoids in microbialsystems. Mutat. Res. 58, 231-239. Haseman, J.K. (1984). Statistical issues in the design, analysis and interpretation o f animal carcinogenicity studies. Environ. Health Perspect. 58,
385-392.
Lett. 21, 23-27.
Hirose, M., Fukushima, S , Sakata, T., Inui, M., and . Ito, N. (1983). Effect o f quercetin on two-stage carcinogenesis o f the rat urinary bladder. Cancer
Hollander, M., and Wolfe, D . k (1973). Nonparametric StatisticalMethods, pp. 120-123. John Wiley and Sons, New York.
. Hosaka, S , andHirono, I. (1981). Carcinogenicity test o f quercetin by pulmonary-adenoma bioassay in strain A mice. Jpn. J. Cancer Res. 72, 327-328.
International Agency for Research on Cancer (IARC) (1983). Quercetin. IARCMonographs on Risk of the Evaluation o f the Carcinogenic Chemicals to Humans, Vol. 31. IARC, Lyon, France. International Agency for Research on Cancer some o f its (IARC) (1986). Bracken fern and constituents. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 40. IARC, Lyon, France. International Agency for Research on Cancer (IARC) (1987). Overall evaluations o f carcinogenicity. IARC Monographs on the Evaluation of the Carcinogenic Risk Chemicals to of Humans, Vol. 7. IARC, Lyon, France.
Ito, N., Tsuda, H., Tatematsu, M., Inoue, T., Tagawa, Y., Aoki, T., Uwagawa, S , Kagawa, . M., Ogiso, T., Masui, T., Imaida, IC, Fukushima, S ,and . Asamoto, M. (1988). Enhancing effect o f various hepatocarcinogens on induction o f preneoplastic glutathione S-transferase placental form positive foci in rats - an approach for a new medium-term bioassaysystem. Carcinogenesis 9, 387-394.
Haseman, J.K., Huff, J., and Boorman, G.A. (1984). Use o f historical control data in carcinogenicity studies in rodents. ToxicoL PathoL 12, 126-135. Haseman, J.K, Huff, J.E., Rao, G.N., Arnold, J.E., Boorman, G.A., and McConnell, E.E. (1985). oil Neoplasms observed in untreated and corn gavage control groups o f E344/N rats and (C57B46N x C3H/HeN)F, (B6C3Fl) mice. JNCI
75, 975-984.
Assoc. Cancer Res. Am. Soc. Clin. OncoL 22. 114.
Hatcher, J.F., Pamukcu, A.M., and Bryan, G.T. (1981). Quercetin ( a ) and kaempferol (K) content o f bracken fern (BF) and mutagenic activity in urine of rats ingesting Q,rutin (R), or B E Roc. Am. (Abstr.)
Phamacol. 32, 1141-1148.
Havsteen, B. (1983). Flavonoids, a classof natural products of high pharmacological potency. Biochem.
. Haworth, S , Lawlor, T., Mortelmans, K., Speck, W., and Zeiger, E. (1983). SaZmonella mutagenicity test results for 250 chemicals. Environ. Mutagen. 5 (SUPPI. l), 3-142.
Herrmann, K. (1976). Flavonols and flavones in food plants: A review. J. Food TechnoL 11,433-448. Hirono, I. (1986). Carcinogenic principles isolated from bracken fern. CRC Cnt. Rev. ToxicoL 17, 1-22.
Ito, N., Hagiwara, A, Tamano, S , Kagawa, . M., Shibata, M - k , Kurata, Y., and Fukushima, S. (1989). Lack o f carcinogenicity o f quercetin in F344DuCrj rats. Jpn. J. CancerRes. 8 0 , 317-325.
46
Quercetin, NTP TR 409
Jonckheere, A. (1954). A distribution-free k-sample test against ordered alternatives. Bwmefriku 41, 133-145. Kaplan, E.L., and Meier, P.(1958). Nonparametric estimation o f incomplete observations. J. Am. Stat. ASS@. 53, 457-481. Kato, R., Nakadate, T., Yamamoto, S , and . Inhibition of 12-0Sugimura, T. (1983). tetradecanoylphorbol-13-acetate-induced tumor promotionandornithine decarboxylaseactivity by quercetin: Possible involvement of lipoxygenase inhibition. Carcinogenesis 4, 1301-1305. Kato, IC, Mori, H., Tanaka, T., Fujii, M., Kawai, T., Hirono, I. Nishikawa, A., Takahashi, M., and (1985). Absence o f initiating activity by quercetin in the rat liver. EcotoxicoLEnviron.Safety 10, 63-69. Konishi, N., and Ward, J.M. Increased (1989). levels of DNA synthesis in hyperplastic renal tubules o f aging nephropathy in female F344/NCr rats. Vet. Kuhnau, J. (1976). The flavonoids. A class of semi-essential food components: role Their in World Rev. Nun: Diet. 24, human nutrition. 117-191. Kuroda, IC, Yoo, Y.S., Yoshikura, T., and Ishibashi, T. (1985).Mutagenicity of natural food
additives. A O I S D 47, 24-30.
MacGregor, J.T., Wehr, C.M., Manners, G.D., Jurd, L, Minkler, J.L, andCarrano, A.V. (1983). In vivo exposure to plant flavonols: Influence on frequencies of micronuclei in mouse erythrocytes and sister chromatid exchange in rabbit lymphocytes. Mutat. Res. 124, 255-270 MacGregor, J.T., and Wilson, R.E. (1988). Flavone mutagenicity in Salmonella typhimurium: Dependence on the pKMlOl plasmid and excision-repair deficiency. Environ. MOL Mutagen. 11, 315-322. Maronpot, R.R., and Boorman, G.A. (1982). Interpretation o f rodent hepatocellular proliferative alterations and hepatocellular tumors in chemical ParhoL 10, 71-80. safetyassessment. ToxicoL Marzin, D., Phi, H.V., Olivier, P., and Sauzieres, J. (1987). Study o f mutagenic activity o f troxerutin, a flavonoidderivative. ToxicoL Lett. 35, 297-305. McConnell, E.E. (1983a). Pathologyrequirements for rodent two-year studies. I. A review o f current procedures. ToxicoL PathoL 11, 60-64. McConnell, E.E. (1983b). Pathologyrequirements 11. Alternative for rodent two-year studies. approaches. ToxicoL PathoL 11, 65-76. McConnell, E.E., Solleveld, H.A., Swenberg,J.A., and Boorman, G.A. (1986). Guidelines for combining neoplasms for evaluation o f rodent carcinogenesis studies. JNCZ 76, 283-289. McCoy, E.C., Anders, M., andRosenkranz, H.S. (1983). The basis o f the insensitivity o f Salmonella lyphimurium strain TA98/1,8-DNP6 to the mutagenic action o f nitroarenes. Mutar.Res. 121, 17-23. b i g h t , B., and Crowley, J. (1984). Tests for differences in tumor incidence based on animal carcinogenesis experiments. J. Am. Stat. Assoc. 79, 639-648.
The Muck Index. (1983). 10th ed. (M.Windholz, Ed.), p. 1160. Merck and Company,Rahway,NJ.
PathoL 26, 6-10.
Kuroda, M., Yoshida, D., and Mizusaki, S . (1985). Mutagenicity o f pyrolyzates o f natural substances toward Salmonella typhimurium TA97. A M . BioL Chem. 49, 1893-1895. Kurokawa, Y., Hayashi, Y., Maekawa, A, Odashima, S. Takahashi, M., Kokubo, T., and (1983). Carcinogenicity of potassium bromate administered orally to F344 rats. JNCI 71, %5-971. Ltifroth, G.,Lazaridis, G., and Rudling, L. (1986). Mutagenicity assay o f emission extracts from wood stoves: Comparison with other emission parameters. Sci TotalEnviron. 58, 199-208. MacGregor, J.T., and Jurd, L. (1978). Mutagenicity of plant flavonoids: Structural requirements for mutagenic activity in Salmonella typhimurium. Mutat. Res. 54, 297-309.
Morino, IC, Matsukura, N., Kawachi, T., Ohgaki, H., Sugimura, T., and Hirono, (1982). I. Carcinogenicity test o f quercetin and rutin in golden hamsters by oral administration. Carcinogenesis 3,
93-97.
References
47
Nagao, M., Morita, N., Yahagi, T., Shimizu, M., Kuroyanagi, M., Fukuoka, M., Yoshihira, IC, . Natori, S , Fujino, T., and Sugimura, T. (1981). Mutagenicities of 61 flavonoids and 11 related compounds. Environ. Mutagen 3, 401-419. National Cancer Institute (NCI) (1976). Guidelines for Carcinogen Bioassay in Small Rodents. Technical Report Series No. 1. NIH Publication No. 76-801. U.S. Department of Health, Education, and Welfare; Public Health SeMce; National Institutes o f Health, Bethesda, MD. NationalInstitutes o f Health (NIH) (1978). Open Formula Ratand Mouse Ration (NIH-07). NIH Publication No. 11-1335. National Insitutes o f Health, Bethesda, MD.
Rao, G.N., Piegorsch, W.W., and Haseman, J.K. (1987). Influence ofbodyweight on the incidence o f spontaneoustumorsinrats and mice of longterm studies. Am. J. Clin. Nun: 45, 252-260. Rueff, J., Laires, A, Borba, H., Chaveca, T., Gomes, M.I., and Halpern, M.(1986). Genetic toxicology of flavonoids: The role of metabolic conditions in the induction o f reverse mutation, S O S functions and sister chromatid exchanges. Mutagenesk 1, 179-183.
Sadtler Standard Specna. IR Nos. 594 and 1009, U V Sadtler Laboratories, Research No. 18272.
Philadelphia.
Neuhaus, O.W., Flory, W., N., Biswas, and Teratogenesh Carchog. Mutagen. 1, 213-221. Hollerman, C.E. (1981). Urinary excretion of ahglobulin and albumin by adult male rats following Shakhova, M.K., Samokhvalov, G.I., and treatment with nephrotoxic agents. Nephron 28, Preobrazhenskii, N.A. (1%2). Synthetic 133-140. investigations in the field o f flavonoids. 111. total synthesis o f quercetin-3-,9-rutinoside, rutin. Z . h Nishino, H., Iwashima, A., Fujiki, H., and Obshch. Khim. (USSR) 32, 390-3%. Sugimura, T. (1984a). Inhibition by quercetin of the promoting effectof teleocidin on skin papilloma Shirley, E. (1977). A non-parametric equivalent o f formation in mice initiated with 7,12- Williams test for contrasting increasing dose levels dimethylbenz[a]anthracene. Jpn. J. CancerRes. 75, o f a treatment. Biometrics 33, 386-389. Short, B.G., Burnett, V.L., Cox, M.G., Bus, J.S., and Nishino, H., Naito, E.,Iwashima, A., Tanaka, IC, Swenberg, J.A. (1987). Site-specific renal Matsuura, T., Fujiki, H., and Sugimura, T. (1984b). cytotoxicity and cell proliferation in rats male Interaction between quercetin and Ca*+-calmodulin exposed to petroleum hydrocarbons. Lab. Invest. 57, complex: Possible mechanism for anti-tumor- 564-577. promotingaction of the flavonoid. Jpn. J. Cancer Res. 75, 311-316. Stich, H.F., Rosin, M.P., Wu, C.H., and Powrie, W.D. (1981). The action o f transition metals on the . Pamukcu, A.M., Yalciner, S , Hatcher, J.F., and genotoxicity of simple phenols, phenolic acids and Bryan, G.T. (1980). Quercetin, a rat intestinal and cinnamic acids. Cancer Lett. 14, 251-260. bladder carcinogen present in bracken fern (Pteridium aquilinum). CancerRes. 40, 3468-3472. Stoewsand, G.S., Anderson, J.L., Boyd, J.N., Hrazdina, G., Babish, J.G., Walsh, KM., and Petrakis, P.L., Kallianos,A.G.,Wender, S.H., and Losco, P. (1984). Quercetin: A mutagen, not a Shetlar, M.R. (1959). Metabolic studies of quercetin carcinogen, in Fischer rats. J. ToxicoL Environ. labeled with C14. Arch. Biochem. Biophys. 85, Health 14, 105-114.
264-271. 113-116.
Saito, D., Shirai, A, Matsushima, T., Sugimura, T., andHirono, I. (1980). Test of carcinogenicityof quercetin, a widely distributed mutagen in food.
Biochem. Biophys. Acta 605, 191-215.
Pitot, H.C., and Sirica, A.E. (1980). The stages of initiationandpromotionin hepatocarcinogenesis.
Stott, W.T., Reitz, R.H., Schumann,
A.M., and Watanabe, P.G. (1981). Genetic nongenetic and events in neoplasia. Food Cosmet. ToxicoL 19,
567-576.
48
Quercetin, NTP TR 409
Vandoren, G., Mertens, B., Heyns, W., Van Baelen, H., Rombauts, W., and Verhoeven, G. (1983). Different forms o f azll-globulin in male and female Sullivan, M., Follis, R.H.,Jr., and Hilgartner, M. rat urine. Eur. J. Biochem. 134, 175-181. (1951). Toxicology of podophyllin. Proc. S c Exp. o. BioL Med 77, 269-272. Van Duuren, B.L, and Goldschmidt, B.M. (1976). Cocarcinogenic tumor-promoting and agents in . Swenberg, J.A., Short, B., Borghoff, S , Strasser, J., tobacco carcinogenesis. J. NatL Cancer Znst. 56, and Charbonneau, M. (1989). The Comparative 1237-1242. Pathobiology o f a,-globulin nephropathy. ToxicoL Watson, W A F . (1982). The mutagenic activity o f AppLPharmacoL 97, 35-46. quercetin kaempferol and in Drosophila Takanashi, H., Aiso, S , Hirono, I., Matsushima, T., . and Sugimura, T. (1983). Carcinogenicity test o f quercetin and kaempferol in rats by oral administration. J. Food Safety 5, 55-60. Tamura, G., Gold, C., Ferro-Luzzi, A., and Ames, B.N. (1980). Fecalase: A model for activation o f dietary glycosides to mutagens by intestinal flora. Tanaka, IC, Ono, T., and Umeda, M. (1987). Pleiotropic effects o f quercetin the on transformation o f BALB 3T3 cells. Jpn. J. Cancer
mehogaster. Mutat.Res. 103, 145-147.
Sugimura, T., Nagao, M., Matsushima, T., Yahagi, T., Seino, Y., Shirai, A, Sawamura, M., Natori, S , Yoshihira, IC, Fukuoka, M., and . Kuroyanagi, M. (1977). Mutagenicity o f flavone derivatives. Roc. Jpn. Acad B53, 194-197.
Ueno, I., Nakano, N., and Hirono, I. (1983). Metabolic fateo f [14C]quercetinin the ACI rat. Jpn. J. Exp. Med 53, 41-50.
Weast, R.C. ( d ) (1979). Handbook o Chemisoy E.. f and Physics, 60th ed. CRC, Boca Raton, FL. Willhite, C.C. (1982). Teratogenic potential of quercetin in the rat. Food Chem. ToxicoL 20,75-79. Williams, D . k (1971). A test for differences between treatment means when several dose levels are compared with a zero dose control Biomeziics
27, 103-117.
Roc. NatL Acad Sci USA 77, 4%1-4%5.
Res. 78, 819-825.
Williams,D.A. (1972). The comparison o f several dose levels with a zero dose control. Biometrics 28,
519-531.
Tarone, R.E. (1975). Tests for trend in life table analysis. Biome& 62,679-682.
Yoshida, M A , Sasaki, M., Sugimura, IC, and Kawachi, T. (1980). Cytogenetic effects o f quercetin on cultured mammalian cells. Roc. Jpn. Acad
Ser. B 56, 443-447.
Tsuda, H., Hacker, H.J., Katayama,H.,Masui, T., . Ito, N., and Bannasch, P. (1986). Correlative Zeiger, E., Anderson, B., Haworth, S , Lawlor, T., Mortelmans, and K (1988). Salmonella histochemical studies on preneoplastic and neoplastic lesions in the kidney of rats treated with mutagenicity tests. IV. Results from the testing of Envwon. MOL Mutagen 11 nitrosamines. Vichows Arch. [Cell PathoL] 51, 300 chemicals. (Suppl. 12),1-158. 385-404.
49
APPENDIX A SUMMARY OF LESIONS IN MALE RATS IN THE 2-YEAR FEED STUDY OF QUERCETIN
TABLE 1 A TABLE A2 TABLEA 3 Summary of the Incidence of Neoplasms in Male Rats in the 2-Year Feed Study of Quercetin Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin Statistical Analysis of Primary Neoplasms in Male Rats i the 2-Year Feed Study of Quercetin n Historical Incidence of Renal Tubule Neoplasms in Untreated Male Summary of the Incidence of Nonneoplastic Lesions in Male Rats in the 2-Year Feed Study of Quercetin
A4 TABLE TABLEA5
................................. ................................. .................................
F W / N Rats
50
54
.................................
...
78 83 84
50
Quercetin, NTP TR 409
TABLE AI
Summary o f the Incidence o f Neoplasms in Male Rats in the
2-Year Feed Study of Quercetin
Disposition Summary Animals initially in study 6-Month interim e a u t o vlain IS-Montb interim ~aluall~n Early deaths Natural deaths Moribund SuMvors Moribund Terminal sacrifice Died last week o f study
Animals examined microscopically
70
10 10
70
10 10
70 10
10
70 10
10
6 2 1
1 19
3 2 1
25 1
50
7 15
27 1
50
3 22
1 22 2
50
50
Alimentary System Intestine large, cecum Intestine large, colon Intestine small, duodenum Intestine small, ileum Adenoma Intestine small, jejunum Liver Cholangiocarcinoma Hemangiosarcoma Hepatocellular carcinoma Hepatocellular adenoma Hepatocellular adenoma, multiple Neoplastic nodule Neoplastic nodule, multiple Sarcoma, metastatic, uncertain primary site Mesentery Sarcoma, poorly differentiated Pancreas Adenoma Salivary glands Stomach Stomach, forestomach Stomach, glandular Tongue Papilloma squamous Cardiovascular System Heart Schwannoma benign Schwannoma malignant
(49) (49) (47)
(8 4)
(8 4)
(50)
3 (6%)
1 (2%)
1 (% 2)
(7) (50) (16)
(50)
(49) (50) (45)
( 8(50) 1)
1 (% 2)
(8 1)
(50)
1 (6%)
Lesions in Male Rats
51
TABLE 1 A
Summary of the Incidence of Neoplasms in Male Rats in the 2-Year Feed Study of Quercetin (continued)
Endocrine System Adrenal gland, cortex Adrenal gland, medulla Pheochromocytoma malignant Pheochromocytoma benign Pheochromocytoma benign, multiple Islets, pancreatic Adenoma Carcinoma Parathyroid gland Adenoma Pituitary gland Pars distalis, adenoma Pars distalis, adenoma, multiple Pars distalis, pars intermedia, pars nervosa, leukemia mononuclear Thyroid gland C-cell, adenoma Ccell, carcinoma Follicular cell, adenocarcinoma Follicular cell, adenoma
General Body System Tissue NOS Basosquamous tumor benign Genital System Epididymis Preputial gland Adenoma Carcinoma Squamous cell carcinoma Prostate Seminal vesicle Testes Interstitial cell. adenoma Hematopoietic System Bone m a w Lymph node Carcinoma, metastatic, thyroid gland Mediastinal, sarcoma, metastatic, uncertain primary site Lymph node, mandibular Carcinoma, metastatic, thyroid gland Lymph node, mesenteric Spleen Hemangioma Hemangiosarcoma Sarcoma Thymus Mediastinum, basosquamous tumor NOS
(50)
4 (8%) 1 (2%)
1 (2%)
(49) 1 (2%)
1 (2%) 1 (2%)
(1) 1 (100%)
(14)
1 (7%)
52
Quercetin,
NTP TR 409
TABLE 1 A
Summary of the Incidence of Neoplasms in Male Rats in the 2-Year Feed Study of Quercetin (continued)
Integumentary System Mammary gland Fibmadenoma Skin Basal cell carcinoma Basosquamous tumor benign Keratoacanthoma Papilloma squamous Subcutaneous tissue, fibroma Subcutaneous tissue, fibrosarcoma Subcutaneous tissue, lipoma Subcutaneous tissue, myxoma Subcutaneous tissue, sarcoma Musculoskeletal System Bone Osteoma Skeletal muscle Sarcoma Nervous System Brain Meningioma benign Choroid plexus, sarcoma, metastatic, uncertain primary site
Respiratory System Lung Ahreolar/bronchiolar adenoma Alveolar/bronchiolar carcinoma, multiple Carcinoma, metastatic, thyroid gland Cholangiocarcinoma, metastatic, liver Osteosarcoma, metastatic, bone Pheochromocytoma malignant, metastatic, adrenal gland Sarcoma, metastatic, uncertain primary site Squamous cell carcinoma Mediastinum, schwannoma malignant, metastatic, heart Nose Papilloma squamous Glands, carcinoma Trachea
(50)
(15)
1 (7%)
(12)
(50)
1 (2%)
(49) 1 (2%)
Special Senses System Eye
Lesions in Male Rats
53
TABLE 1 A
Summary of the Incidence of Neoplasms in Male Rats in the 2-YearFeed Study of Quercetin (continued)
Urinary System Kidney Sarcoma, metastatic, uncertain primary site Proximal convoluted renal tubule, adenoma Renal tubule, adenocarcinoma Renal tubule, adenoma Urinary bladder (49) Systemic Lesions Multiple organsa Leukemia mononuclear Lymphoma malignant histiocytic Lymphoma malignant lymphocytic Lymphoma malignant mixed Lymphoma malignant undifferentiated cell Mesothelioma benign Mesothelioma malignant
(50) 1 (2%)
(50)
(50)
(50)
(50)
(49)
(8 4)
2 (4%)
1 (2%) 1 (2%)
(50) 16 18 (32%)
(50)
(36%)
(50)
3 (6%)
22 (44%) 1 (2%)
(50)
13 (26%)
4 (8%)
(6%) 3
1 (2%) 1 (2%) 1 (2%)
1 (2%)
(4%)
1 (2%)
2
1 (2%)
Total animals with primary neoplasmsb
Tumor Summary
Total primary neoplasms Total animals with benign neoplasms Total benign neoplasms Total animals with malignant neoplasms Total malignant neoplasms Total animals with metastatic neoplasms Total metastatic neoplasms Total animals with malignant neoplasms of uncertain primary site Total animals with neoplasms uncertainbenign or malignant Total uncertain neoplasms
a
50
125 49 95 27
29
50 111 48 82 27
29
4 8
1 2
50 139 50 102 32 37 3 4
48 113 21 25
48 88
1 1
1 1
Number of animals with any tissue examined microscopically Primary neoplasms: all neoplasms except metastatic neoplasms
54
Quercetin, NTP TR 409
TABLE 2 A
Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 0 ppm
Number of Days on Study
2 4 5 5 5 5 5 6 6 6 6 6 6 6 6 6 6 6 6 7 7 7 7 7 7 2 5 1 6 7 7 9 2 3 3 4 5 5 5 6 7 7 8 8 0 1 1 2 2 2 6 8 9 2 3 5 0 0 6 6 0 4 4 6 4 4 9 2 9 4 7 7 0 1 4
0 0 1 4 0 0 3 5 0 1 3 5 0 0 2 3 0 0 1 5 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Carcass ID Number
1 0 1 0 1 1 0 1 0 1 1 0 0 1 0 0 1 1 0 0 2 6 4 1 2 1 9 1 8 0 0 3 5 1 3 6 1 3 5 4 4 4 4 2 3 4 5 3 2 4 3 4 3 2 3 3 1 4 2 3
Alimentary S s e ytm Esophagus Intestine large Intestine large, cecum Intestine large, colon Intestine large, rectum Intestine small Intestine small, duodenum Intestine small, ileum Intestine small, jejunum Liver Hepatocellular adenoma Sarcoma, metastatic, uncertain primary site Mesentery Pancreas Salivary glands Stomach Stomach, forestomach Stomach, glandular Tongue Cardiovascular S s e ytm B l o o d vessel Heart Schwannoma benign
+ + + + + + + + + + + + + + + + + A + + + + + + +
. . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . .
+ + + + + + + + + + + + + + + + + A + + + + + + + + + + + + + + + + + + + + + + + + A + + + + + + +
. + + +
. . . . . . . . . . . . . . . . . . . . . . . . . . + . . . . + . . . . + . . .
. M + +
. + + +
. + + +
. + + A
. + + +
. + + +
. + + +
. + + +
. + + +
. + + +
. + + +
. . . . . . . . . . . . . + + + + + A + + + + + + + + + M + + A + + + + + + M + + + + + A + + + + + + +
+ + + M + + + + + + + + + + + + + +
+ . . . . + + + . . . . . . . . . . . .
+ + . . . . . + + + + . . . . . . . . . . . . . . .
. . . .
. . . .
. . . .
. . . .
. . + . . . . . .
. . . .
. . + . . . . . .
. . . . +
. . . . +
. . . . +
. M . . . ++
. . . . . . . . . . . . . . . . . . . . . . . . .
X
ytm Endocrine S s e Adrenal gland Adrenal gland, cortex Adrenal gland, medulla Pheochromocytoma malignant Pheochromocytoma benign Pheochromocytoma benign, multiple Islets, pancreatic Parathyroid gland Adenoma Pituitary gland Pars distalis, adenoma
+: Tissue examined microscopically
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . X xx x x X X + + + + + + + + + + + + + + + + + + ++ + + +
+ M M + + + + + + + + + + + + + + M M + + + M + + A + + + M + + + + + + + + + M + + + + + + + + + + X X X xx x M: Missing tissue X Lesion present
A: Autolysis precludes examination
I: Insufficient tissue
Blank Not examined
Lesions in Male Rats
TABLE 2 A
Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 0 ppm (continued)
Number of Days on Study 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 4 4 4 4 4 4 7 7 7 7 7 8 9 9 9 9 9 9 9 9 9 9 9 9 9
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Carcass ID Number
0 0 0 0 0 1 0 0 0 0 1 0 0 0 0 0 0 0 0 0 1 1 1 1 1 4 5 7 9 9 4 5 6 6 7 0 3 1 2 3 4 4 8 9 9 2 2 3 3 3 4 1 3 3 4 3 5 1 2 2 2 2 3 1 1 1 2 1 1 2 1 2 1 2 3
Total
Tissues/
Tumors
Alimentary System Esophagus Intestine large Intestine large, cecum Intestine large, colon Intestine large, rectum Intestine small Intestine small, duodenum Intestine small, ileum Intestine small, jejunum Liver Hepatocellular adenoma Sarcoma, metastatic, uncertain primary site Mesentery Pancreas Salivary glands Stomach Stomach, forestomach Stomach, glandular Tongue Cardiovascular System Blood vessel Heart Schwannoma benign Endocrine System Adrenal gland Adrenal gland, cortex A d r e n a l gland, medulla Pheochromocytoma malignant Pheochromocytoma benign Pheochromocytoma benign, multiple Islets, pancreatic Parathyroid gland Adenoma Pituitary gland Pars distalis, adenoma
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
X
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . xx
50 49 49 49 49 49 48 47 48
50
+ . . . . . . . . . . . . . . . . . . . . . . . . . + M + + + . . . . . . . . . . . . . . . . . . . . . . . . . + + + + + + + + + + + + + + + M + + + + + + + + . . . . . . . . . . . . . . . . . . . . . . . . .
+ M + + + + + + + + + + + + + + + + M + + + + + +
1 7 50 1 6 50 49 50 45
. . . . . . . . . . . . . . . . . . . . . . . . .
50 1
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
X
. . . . . . . . . . . . . . . . . . . . . .
+ + + + + + + M + + + + + + + + + + + + M + + + + X + + + + + + + + + + + + + + + + + + + + + + + M + xxx x x x x X
++
50 50 50 1 8 4 47 43 1 46 14
56
Quercetin, NTP
TR 409
TABLE A2
Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 0 ppm (continued)
Number of Days on Study
2 4 5 5 5 5 5 6 6 6 6 6 6 6 6 6 6 6 6 7 7 7 7 7 7 2 5 1 6 7 7 9 2 3 3 4 5 5 5 6 7 7 8 8 0 1 1 2 2 2 6 8 9 2 3 5 0 0 6 6 0 4 4 6 4 4 9 2 9 4 7 7 0 1 4
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Carcass ID Number
0 0 1 0 0 1 0 1 0 1 1 0 1 0 1 1 0 0 1 0 0 1 1 0 0 1 3 3 2 1 2 6 4 1 2 1 9 1 8 0 0 3 5 1 3 6 1 3 5 4 4 5 5 3 5 4 4 4 2 3 4 5 3 2 4 3 4 3 2 3 3 1 4 2 3
Endocrine System (continued) Thyroid gland C e l l , adenoma C e l l , carcinoma Follicular cell, adenoma General Body System None Genital System Coagulating gland Epididymis Preputial gland Adenoma Carcinoma Prostate Seminal vesicle Testes Interstitial cell, adenoma Hematopoietic System Bone marrow Lymph node Mediastinal, sarcoma, metastatic, uncertain primary site Lymph node, mandibular Lymph node, mesenteric Spleen
l-hymus
. . . . . . . . . . . . . . . . . . . . . . . . .
X
X
+ + . . . . . . . . . . . . . . . . . . . . . . . . . I d + + + + + + + + + + + +
X X
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . xxxxxxx xxx xx
. . . x
. . . x
. . . x
. . . x
. . . . . . . . . . . . x
+ + + + + + + + + + + . . . . . . . . . . . . . . . . . . . . . . . . .
+ + + + M + + + + + + + + + + + + + + + + + + + M + + + + + + ++ + + + + + M + M + + + M +
Mediastinum, basosquamous tumor NOS
. . . . . . . . . . . . . . . . . . . . . . . . . + + + + + M+++ + + + +
Integumentary System Mammary gland Fibroadenoma Skin Basal cell carcinoma Papilloma squamous Subcutaneous tissue, fibroma Subcutaneous tissue, fibrosarcoma Subcutaneous tissue, lipoma
M M + + + M + + M + + +
+++++++++++
X
+
X
Lesions in Male Rats
57
TABLE2 A Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 0 ppm (continued)
~ ~ ~
Number of Days on Study
7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 4 4 4 4 4 4 7 7 7 7 7 8 9 9 9 9 9 9 9 9 9 9 9 9 9
0 0 4 4 0 0 5 1 0 0 7 3 0 0 9 3 0 0 9 4 0 1 4 3 0 0 5 5 0 0 6 1 0 0 6 2 0 0 7 2 0 1 0 2 0 0 3 2 0 0 1 3 0 0 2 1 0 0 3 1 0 0 4 1 0 0 4 2 0 0 0 0 0 0 0 0 0 0 0 1 1 1 1 1 8 9 9 2 2 3 3 3 1 1 2 1 2 1 2 3
Total
TissuesJ
Tumom
Endocrine System (continued) Thyroid gland C-cell, adenoma C e l l , carcinoma Follicular cell, adenoma General Body System None Genital System Coagulating gland Epididymis Preputial gland Adenoma Carcinoma Prostate Seminal vesicle Testes Interstitial cell, adenoma Hematopoietic System Bone marrow Lymph node Mediastinal, sarcoma, metastatic, uncertain primary site Lymph node, mandibular Lymph node, mesenteric Spleen nymus Mediastinum, basosquamous tumor NOS Integumentary System Mammary gland Fibroadenoma Skin Basal cell carcinoma Papilloma squamous Subcutaneous tissue, fibroma Subcutaneous tissue, fibrosarcoma Subcutaneous tissue, lipoma
. . . . . . . . . . . . . . . . . . . . . . . . .
X
50
4 1 1
. . . . . . . . . . . . . . . . . . . . . . . . . +
2 50 13 2 49 50
50
1
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . xxxxxxxxxxxxxxxxxxxxxxxxx
+ + + + + + + + + + + + + + M + + + + + + + + + +
44
+ + M + + + + + + + + + + + + + + + + + + + + + + X + + M + + + + + + + + + M + + + + + + + + + + + + + M + + M +
11 49
1 46 22 50 14 1
. . . . . . . . . . . . . . . . . . . . . . . . . + M M + M
X
+ +
+ +
X
+
X
+
+
X
+ +
X
13 5 20 2 2 1 1
58
Quercetin,
NTP TR 409
TABLE 2 A Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 0 ppm (continued)
Number of Days on Study
2 4 5 5 5 5 5 6 6 6 6 6 6 6 6 6 6 6 6 7 7 7 7 7 7 2 5 1 6 7 7 9 2 3 3 4 5 5 5 6 7 7 8 8 0 1 1 2 2 2 6 8 9 2 3 5 0 0 6 6 0 4 4 6 4 4 9 2 9 4 7 7 0 1 4 0 0 1 4 0 0 3 5 0 1 3 5 0 0 2 3 0 0 1 5 0 1 2 4 0 0 6 4 0 1 4 4 0 0 1 2 0 1 2 3 0 1 1 4 0 0 9 5 0 1 1 3 0 0 8 2 0 1 0 4 0 1 0 3 0 0 3 4 0 0 5 3 0 1 1 2 0 0 3 3 0 0 6 3 0 1 1 1 0 1 3 4 0 0 5 2 0 0 4 3
Carcass ID Number
Musculoskeletal System Bone osteoma Skeletal muscle Sarcoma Nervous System Brain Choroid plexus, sarcoma, metastatic, uncertain primary site Respiratory System Lung Alveolar/bronchiolar adenoma Carcinoma, metastatic, thyroid gland Osteosarcoma, metastatic, bone Pheochromocytoma malignant, metastatic, adrenal gland Sarcoma, metastatic, uncertain primary site
Trachea
+ + +
+ + + +
+++
X
. . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . .
X
X
NOW
+ + + + + + + + + + + + + + + + + + + + + + . . . . . . . . . . . . . . . . . . . . . . . . .
I
Special Senses System Eye Harderian gland Urinary System Kidney Sarcoma, metastatic, uncertain primary site Urinary bladder
. . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . x x x x x x x x x x x X xx
X X X
Systemic Lesions
Multiple organs Leukemia mononuclear Lymphoma malignant lymphocytic Mesothelioma malignant
Lesions in Male Rats
59
TABLE A2
Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 0 ppm (continued)
Number of Days on Study 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 1 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 4 4 4 4 4 4 7 7 7 7 7 8 9 9 9 9 9 9 9 9 9 9 9 9 9
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Carcass ID Number
0 0 0 0 0 1 0 0 0 0 1 0 0 0 0 0 0 0 0 0 1 1 1 1 1 4 5 7 9 9 4 5 6 6 7 0 3 1 2 3 4 4 8 9 9 2 2 3 3 3 4 1 3 3 4 3 5 1 2 2 2 2 3 1 1 1 2 1 1 2 1 2 1 2 3
Total Tissue4 Tumors
Musculoskeletal System Bone Osteoma Skeletal muscle Sarcoma Nervous System Brain Choroid plexus, sarcoma, metastatic, uncertain primary site Respiratory System Lung Alveolaribronchiolar adenoma Carcinoma, metastatic, thyroid gland Osteosarcoma, metastatic,bone Pheochromocytoma malignant, metastatic, adrenal gland Sarcoma, metastatic, uncertain primaly site
Trachea
10 1 1 1
. . . . . . . . . . . . . . . . . . . . . . . . .
X
50 1
. . . . . . . . . . . . . . . . . . . . . . . . .
X
50 2 1
1
N W
+ + + + + + + + + + + + + + + + + + + + + + . . . . . . . . . . . . . . . . . . . . . . . . .
M
1 44 50
Special Senses System Eye Harderian gland Urinary System Kidney Sarcoma, metastatic, uncertain primary site Urinaly bladder Systemic Lesions Multiple organs Leukemia mononuclear Lymphoma malignant lymphocytic Mesothelioma malignant
2 1
. . . . . . . . . . . . . . . . . . . . . . . . .
50
. . . . . . . . . . . . . . . . . . . . . . . . .
X
1 50
. . . . . . . . . . . . . . . . . . . . . . . . . X xx X
X
50
16 3 4
60
Quercetin, NTP
TR 409
TABLE 2 A
Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 1 O ppm OO ,
4 5 5 5 5 5 5 5 6 6 6 6 6 6 6 6 6 7 7 7 7 7 7 7 7 5 0 4 5 6 7 8 9 1 1 2 4 5 7 7 8 9 0 1 2 2 2 2 2 2 8 9 6 6 2 2 4 9 8 8 0 3 4 1 8 5 5 4 6 0 1 3 4 4 7
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Number of Days on Study
Carcass ID Number
2 2 2 1 1 2 2 1 1 1 2 1 1 2 2 1 1 2 2 2 2 2 2 2 1 2 3 2 8 9 5 4 8 8 9 2 7 6 7 7 7 7 2 8 5 6 3 1 6 5 5 4 4 3 3 4 4 2 1 2 3 4 4 4 3 3 2 2 4 3 1 2 5 5 1
Alimentary System Esophagus Intestine large Intestine large, cecum Intestine large, colon Intestine large, rectum Intestine small Intestine small, duodenum Intestine small, ileum Intestine small, jejunum Liver Hepatocellular carcinoma Hepatocellular adenoma Neoplastic nodule Neoplastic nodule, multiple Mesentery Pancreas Adenoma Salivary glands Stomach Stomach, forestomach Tongue
Stomach, glandular
++++++++++++
+ A + + A + + + + + + +
+ A + + + + + + + + + + + A + + A + + + + + + + + A + + + + + + + + + +
+ + + M A + + + + + + + A + + + + + + + + + + + + + A + + + + + + + M + + A + + + + + + + + + + + + + A + M A + + + + + + + A + A + + + + + + + + + +
. . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . .
. + . .
. . . . . . . . . . . . . . . . . . . . . . . . .
. + . .
. + . .
. + . .
+ + + . . . . . . . . . . . . . . . . . . . . . ++++++++ + + . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . .
Cardiovascular System Heart Endocrine System Adrenal gland Adrenal gland, cortex Adrenal gland, medulla Pheochromocytoma benign Islets, pancreatic Adenoma Parathyroid gland Pituitary gland Pars distalis, adenoma Pars distalis, adenoma, multiple Thyroid gland C-cell, adenoma C-cell, carcinoma
+++++ + + + + + + + + + + + + + + + + + + + + + + + +
++++++
. . . . . . . . . . . . . . . . . . . . . . . . . X X x xx X X xx +++++++++++++
X + M + + + + + + M + + + + + + + + + + + + + + + +
+ + + +
+ + + +
+ + + +
++ ++ + + X ++
+ + + +
++
+
X
+ + + +
+ +
Lesions in Male Rats
61
TABLE 2 A Individual Animal Tumor Pathology of Male Rats in the 2-Year
(continued)
Feed Study of Quercetin: 1 O ppm OO ,
Number of Days on Study
7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 3 3 3 3 3 3 3 3 3 3 7 7 7 7 7 8 9 9 9 9 9 9 9 9 9 0 0 0 0 0 0 0 0 0 0
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Carcass ID Number
1 2 2 2 2 2 1 1 2 2 2 2 2 2 2 1 1 2 2 2 2 2 2 2 2 6 1 1 6 7 2 6 9 0 1 1 5 7 8 8 6 7 0 0 3 4 4 5 6 8 3 3 4 4 2 1 2 1 4 1 2 2 1 2 3 1 1 1 3 1 1 2 1 3 1
Total
Tissue4
Tumors
Alimentary System Esophagus Intestine large Intestine large, cecum Intestine large, colon Intestine large, rectum Intestine small Intestine small, duodenum Intestine small, ileum Intestine small, jejunum Liver Hepatocellular carcinoma Hepatocellular adenoma Neoplastic nodule Neoplastic nodule, multiple Mesentery Pancreas Adenoma Salivary glands Stomach Stomach, forestomach Stomach, glandular Tongue Cardiovascular System Hart Endocrine System Adrenal gland Adrenal gland, cortex Adrenal gland, medulla Pheochromocytoma benign Islets, pancreatic Adenoma Parathyroid gland Pituitary gland Pars distalis, adenoma Pars distalis, adenoma, multiple Thyroid gland C-cell, adenoma C-cell, carcinoma
+ . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
X X
+ + +
. . . . . . . . . . . . . . . . . . . . . . . . . + + + + + A + + + + + + + + + + + + + + + + + + + . . . . . . . . . . . . . . . . . . . . . . . . .
X
X
+ + . . . . . . . . . . . . . . . . . . . . . . . . .
X
. . . . . . . . . . . . . . . . . . . . . . . . . + + + + + + + + + M + + + + + + + + + + + + + + . . . . . . . . . . . . . . . . . . . . . . . . .
+ + + + + + + M + + + + + + + + + + + + + + + + +
12 12 11 12 11 50 47 47 44 50 1 1 1 1 6 50 2 14 50 49 50
48
+ ++
X
+ + +
+
+ +
X
18
+ + + +
+ + + + + + + + + + + + + + + + + + I + + + + + + X X X xx xx X
+ + + + + + M + + M + + + + + + + + + + + + M + +
18 18 18 4 15 2 45 49 14 3 17 1 2
62
Quercetin, NTP
TR 409
TABLE 2 A
(continued)
Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 1 O ppm OO ,
4 5 5 5 5 5 5 5 6 6 6 6 6 6 6 6 6 7 7 7 7 7 7 7 7 5 0 4 5 6 7 8 9 1 1 2 4 5 7 7 8 9 0 1 2 2 2 2 2 2 8 9 6 6 2 2 4 9 8 8 0 3 4 1 8 5 5 4 6 0 1 3 4 4 7
Number of Days on Study
Carcass ID Number
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
2 2 2 1 1 2 2 1 1 1 2 1 1 2 2 1 1 2 2 2 2 2 2 2 1 2 3 2 8 9 5 4 8 8 9 2 7 6 7 7 7 7 2 8 5 6 3 1 6 5 5 4 4 3 3 4 4 2 1 2 3 4 4 4 3 3 2 2 4 3 1 2 5 5 1
General Body System Tissue NOS B a q u a m o u s tumor benign Genital System Coagulating gland Epididymis Preputial gland Adenoma Squamous cell carcinoma Prostate Seminal vesicle Testes Interstitial cell, adenoma Hematopoietic System Bone marrow Lymph node Lymph node, mandibular Carcinoma, metastatic, thyroid gland Lymph node, mesenteric Spleen
Thymus
M + + + + + + + + + + + +
++++++++++++
X
++++++++++++ ++++++++++++ ++++++++++++ xx xxxx xxx
+ + + + + + + + + + + + +++++++++++++ +++++++++++++
+ + + + + + + + + + + M + + + + + + + + M + M + +
X X
+ + + + x x
++ + + + xxx
+ + + + + + + + xxxxxxxx
+ +
++
+ +
+ + + + + + + + +
+ +
+
+ + + + + + + + + + + + + +
+ + + +
Integumentary System Mammary gland Fibroadenoma Skin Subcutaneous tissue, fibroma Subcutaneous tissue, Sarcoma Musculoskeletal System Bone Skeletal muscle Nervous System Brain Meningioma benign
+ M + + + + M M + + M +
+ + + + + + + + + + +
+
X
+ + + + + + + + + + + + + +
+++++++++++++
Lesions in Male Rats
63
A TABLE 2 Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 1 O ppm OO ,
(continued)
Number of Days on Study
7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 3 3 3 3 3 3 3 3 3 3 7 7 7 7 7 8 9 9 9 9 9 9 9 9 9 0 0 0 0 0 0 0 0 0 0
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Carcass ID Number
1 2 2 2 2 2 1 1 2 2 2 2 2 2 2 1 1 2 2 2 2 2 2 2 2 6 1 1 6 7 2 6 9 0 1 1 5 7 8 8 6 7 0 0 3 4 4 5 6 8 3 3 4 4 2 1 2 1 4 1 2 2 1 2 3 1 1 1 3 1 1 2 1 3 1
Total Tiissued Tumors
General Body System Tissue NOS Basosquamous tumor benign Genital System Coagulating gland Epididymis Preputial gland Adenoma Squamous cell carcinoma Prostate Seminal vesicle Testes Interstitial cell, adenoma Hematopoietic System Bone marrow Lymph node Lymph node, mandibular Carcinoma, metastatic, thyroid gland Lymph node, mesenteric Spleen
Thymus
1 1
+ + +
+ +
+ + + + + ++++++++++ ++++++ +++++++ X X x x x x x x x xx x x x x xx x x x x x x + + + + + + + + + +
22
1 13 22 5 1 14
46 43
+ +
+
+ + +
12 29 18 1 14 25 11
Integumentary System Mammary gland Fibroadenoma Skin Subcutaneous tissue, fibroma Subcutaneous tissue, sarcoma Musculoskeletal System Bone Skeletal muscle Nervous System Brain Meningioma benign
10 1 18 1 1
12 2
++
X
15 1
64
Quercetin,
NTP TR 409
TABLE 2 A Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 1 O ppm OO ,
(continued)
4 5 5 5 5 5 5 5 6 6 6 6 6 6 6 6 6 7 7 7 7 7 7 7 7 5 0 4 5 6 7 8 9 1 1 2 4 5 7 7 8 9 0 1 2 2 2 2 2 2 8 9 6 6 2 2 4 9 8 8 0 3 4 1 8 5 5 4 6 0 1 3 4 4 7 0 2 2 5 0 2 3 4 0 2 2 4 0 1 8 3 0 1 9 3 0 2 5 4 0 2 4 4 0 1 8 2 0 0 0 0 0 0 1 1 2 1 1 2 8 9 2 7 6 7 ' 1 2 3 4 4 4 0 2 7 3 0 1 7 3 0 1 7 2 0 2 2 2 0 2 8 4 0 2 5 3 0 2 6 1 0 2 3 2 0 2 1 5 0 2 6 5 0 1 5 1
Number of Days on Study
Carcass ID Number
Respiratory System Lung Carcinoma, metastatic, thyroid gland NOS2 Glands, carcinoma Trachea Special Senses System Eye Urinary System Kidney Urinaxy bladder Systemic Lesions Multiple organs Leukemia mononuclear Lymphomamalignantmixed Lymphoma malignant undifferentiated cell type Mesothelioma benign Mesothelioma malignant
++++++++++++++++ ++ + + X . . . . . . . . . . . . . . . . . . . . . . . . . X + + + + + + + + + + + +
+ +
+ I
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . xxxxxxxx xxx x xx xx x
X X
Lesions in Male Rats
65
TABLE 2 A
(continued)
Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 1 O ppm OO ,
7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 3 3 3 3 3 3 3 3 3 3 7 7 7 7 7 8 9 9 9 9 9 9 9 9 9 0 0 0 0 0 0 0 0 0 0
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Number of Days on Study
Carcass ID Number
1 2 2 2 2 2 1 1 2 2 2 2 2 2 2 1 1 2 2 2 2 2 2 2 2 6 1 1 6 7 2 6 9 0 1 1 5 7 8 8 6 7 0 0 3 4 4 5 6 8 3 3 4 4 2 1 2 1 4 1 2 2 1 2 3 1 1 1 3 1 1 2 1 3 1
Total
Tissues/
Tumom
Respiratory System Lung Carcinoma, metastatic, thyroid gland NOSe Glands, carcinoma Trachea Special Senses System Eye
+ ++++ + +++++++++++++++++++
+ + ++++
28 1 48
1 12
Urinary System Kidney UrinaIy bladder
Systemic Lesions Multiple organs Leukemia mononuclear Lymphoma malignant mixed Lymphoma malignant undifferentiated cell type Mesothelioma benign Mesothelioma malignant
. . . . . . . . . . . . . . . . . . . . . . . . .
+ + + + + + + + + M + + + + + + + + + + + + + + +
50 49
. . . . . . . . . . . . . . . . . . . . . . . . .
X X
50 18 1
1 1 3
66
Quercetin, NTP TR 409
TABLEA 2 Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 10,OOO ppm
4 4 5 5 5 5 5 6 . - 6 6 6 6 6 6 6 6 6 6 6 6 6 6 7 7 7
Number of Days on Study
2 2 4 5 5 7 9 0 1 2 4 4 7 7 7 7 8 8 8 9 9 9 0 0 0 0 7 9 5 5 4 2 8 7 4 3 3 3 3 4 4 0 4 7 0 5 9 3 4 4 0 4 0 5 0 3 9 5 0 4 2 3 0 2 9 3 0 3 0 1 0 3 7 5 0 4 2 2 0 3 6 5 0 4 1 4 0 3 8 4 0 3 6 4 0 4 1 5 0 3 7 2 0 4 2 1 0 3 2 4 0 3 3 4 0 3 1 3 0 3 9 3 0 2 9 2 0 3 6 3 0 4 1 2 0 3 8 3 0 3 7 4 0 3 6 1 0 3 6 2
Carcass ID Number
Alimentary System Esophagus Intestine large Intestine large, cecum Intestine large, colon Intestine large, rectum Intestine small Intestine small, duodenum Intestine small, ileum Intestine small, jejunum Liver Cholangiocarcinoma Hemangiosarcoma Hepatocellular adenoma, multiple Neoplastic nodule Neoplastic nodule, multiple Mesentery Pancreas Adenoma Salivary glands Stomach Stomach, forestomach Stomach, glandular Tongue Cardiovascular System Blood vessel Hart Schwannoma malignant Endocrine System Adrenal gland Adrenal gland, cortex Adrenal gland, medulla Pheochromocytoma malignant Pheochromocytoma benign Pheochromocytoma benign, multiple Islets, pancreatic Carcinoma Parathyroid gland Pituitary gland Pars distalis, adenoma Pars distalis, adenoma, multiple
+ + + + M + + + + + + +
+ + + + + + + A + + + +
+ + + + + + + A + + + +
+ + M + + + + A + + + +
+ + + + + + + A + + + +
+ + + + + + + A + + + + + + + + + + + + + + + + +
+ + + + + + + A + + + + + M + + + + + + + + + + + + + + + + + + A + + + + + + + + + M + + + + + M +
+ + + + + + + A + + + + + + + + + + + + + + + + +
. . . . . . . . . . . . . . . . . . . . . . . . .
+ + . . . . . . . . . . . . . . X + + + + + + + + + + + + . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
+ . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
+ + + + + + + + M + + + + + + + + + + + + + + + +
++++++++++++
+ + + + ++++ + + + + x + + + +
MI
+ + + + ++++ + + + + x + + + +
+ + + + ++++ + + + + xx + + + +
+ +
X
++ ++
+
. . . . . . . . . . . . . . . . . . . . . . . . . X x xx x x x x X
+ " + + + + I
X + + + + + + + + + + + + + M +
Lesions in Male Rats
67
TABLE A2
(continued)
Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: l , O ppm 0O O
7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 4 4 4 4 4 7 7 7 7 1 8 8 8 8 8 9 9 9 9 9 9 9 9 9 9
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Number of Days on Study
Carcass ID Number
3 3 3 3 4 2 3 3 3 4 3 3 3 3 4 3 3 3 3 3 3 3 3 4 4 2 2 4 5 1 9 1 5 9 0 1 3 3 4 0 2 4 5 7 7 8 8 9 0 0 2 3 4 3 1 1 2 2 2 4 1 2 5 3 3 1 2 1 1 3 1 2 1 1 2
Total Tissues/ Tumors
Alimentary System Esophagus Intestine large Intestine large, cecum Intestine large, colon Intestine large, rectum Intestine small Intestine small, duodenum Intestine small, ileum Intestine small, jejunum Liver Cholangiocarcinoma Hemangiosarcoma Hepatocellular adenoma,multiple Neoplastic nodule Neoplastic nodule, multiple Mesentery Pancreas Adenoma Salivary glands Stomach Stomach, forestomach Stomach, glandular Tongue Cardiovascular System B l o o d vessel Hl3Ut Schwannoma malignant Endocrine System Adrenal gland Adrenal gland, cortex Adrenal gland, medulla Pheochromocytoma malignant Pheochromocytoma benign Pheochromocytoma benign, multiple Islets, pancreatic Carcinoma Parathyroid gland Pituitary gland Pars distalis, adenoma Pars distalis, adenoma, multiple
+ +
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . .
X X X X
+ + + + + M + + + + + + + + + + + + + + + + + + + + + + + + M + + + + + + + + + + M M M + + + M + +
. . . . . . . . . . . . . . . . . . . . . . . . .
X
11 13 13 11 10 49 48 48 42 50 1 1 1 2 1 5
50
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
+ + + + M + + + + + + + + M + + + + + + + + + + +
2 12 50 49 49 41
+ +
1 18 1
+ + +
X
+ + +
+ +
+ + + x
+ + + x
+ + + x
+ + +
+ + + + + + + + + + + + + + + + M + + + + + + + +
. . . . . . . . . . . . . . . . . . . . . . . . . x xX X xx x x
X
2 1 2 1 2 1 1 9 1 13 1 43 50 18 1
68
Quercetin, NTP TR 409
TABLEA 2 Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 10,OOO ppm
(continued)
4 4 5 5 5 5 5 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 7 7 7 2 2 4 5 5 7 9 0 1 2 4 4 7 7 7 7 8 8 8 9 9 9 0 0 0 0 7 9 5 5 4 2 8 7 4 3 3 3 3 4 4 0 4 7 0 5 9 3 4 4 0 4 0 5 0 3 9 5 0 4 2 3 0 2 9 3 0 3 0 1 0 3 7 5 0 4 2 2 0 3 6 5 0 4 1 4 0 3 8 4 0 3 6 4 0 4 1 5 0 3 7 2 0 4 2 1 0 3 2 4 0 3 3 4 0 3 1 3 0 3 9 3 0 2 9 2 0 3 6 3 0 4 1 2 0 3 8 3 0 3 7 4 0 3 6 1 0 3 6 2
Number of Days on Study
Carcass ID Number
Endocrine System (continued) Thyroid gland C c e l l , adenoma Ccell, carcinoma General Body System None Genital System Coagulating gland Epididymis Preputial gland Adenoma Carcinoma Prostate Seminal vesicle Testes Interstitial cell, adenoma Hematopoietic System Bone marrow Lymph node Carcinoma, metastatic, thyroid gland Lymph node, mandibular Lymph node, mesenteric Spleen Hemangioma Hemangiosarcoma Sarcoma Thymus Integumentary System Mammary gland Fibroadenoma Skin Basosquamous tumor benign Keratoacanthoma Papilloma squamous Subcutaneous tissue, fibroma Subcutaneous tissue, myxoma Subcutaneous tissue, sarcoma
+ + + + + + + + + + + +
X
+ + + +
X X
+ ++++++++++++ + + + + + + + + + + + +
+ +
+ + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + x xxxxxxxx x + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + +
++++++++++++ xxxxx xxxxxx + + + + ++
+ + ++ ++ + + + + + + + +
X
++++++++++++
M + M + + + M + + + + +
+ I " + + + + + + + + +
+
X
Lesions in Male Rats
69
TABLE 2 A Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 1 , O ppm 0O O
(continued)
Number of Days on Study
7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 4 4 4 4 4 7 7 7 7 7 8 8 8 8 8 9 9 9 9 9 9 9 9 9 9
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Carcass ID Number
3 3 3 3 4 2 3 3 3 4 3 3 3 3 4 3 3 3 3 3 3 3 3 4 4 2 2 4 5 1 9 1 5 9 0 1 3 3 4 0 2 4 5 7 7 8 8 9 0 0 2 3 4 3 1 1 2 2 2 4 1 2 5 3 3 1 2 1 1 3 1 2 1 1 2
Total
Tissud
Tumors
Endocrine System (continued) Thyroid gland Ccell, adenoma C-cell, carcinoma General Body System None Genital System Coagulating gland Epididymis Preputial gland Adenoma Carcinoma Prostate Seminal vesicle
Testes
+ +
22 4 1
+ x
+ x
++
X
Interstitial cell, adenoma
+ + + + + + + + . . . . . . . . . . . . . . . . . . . . . . . . xxxxxxxxxxxxxxxxxxxxxxxx
2 13 19 3 1 12 23 48 45
Hematopoietic System Bone marrow Lymph node Carcinoma, metastatic, thyroid gland Lymph node, mandibular Lymph node, mesenteric Spleen Hemangioma Hemangiosarcoma Samma Thymus Integumentary System Mammary gland Fibroadenoma Skin Basosquamous tumor benign Keratoacanthoma Papilloma squamous Subcutaneous tissue, fibroma Subcutaneous tissue, myxoma Subcutaneous tissue, sarcoma
+ + + + + + + + + +
X
+ + +
+ +
+ + + +
+ + + + +++++ + +
X
12 2 6 1 15 17 38 1 1 1 13
+
X
+
X
+
X
+ + + xxx
X
14
+
X
19 1 1 3 1 1 1
70
Quercetin, NTP TR 409
TABLE A2
(continued)
Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 10,OOO ppm
4 4 5 5 5 5 5 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 7 7 7 2 2 4 5 5 7 9 0 1 2 4 4 7 7 7 7 8 8 8 9 9 9 0 0 0 0 7 9 5 5 4 2 8 7 4 3 3 3 3 4 4 0 4 7 0 5 9 3 4 4 0 4 0 5 0 3 9 5 0 4 2 3 0 2 9 3 0 3 0 1 0 3 7 5 0 4 2 2 0 3 6 5 0 4 1 4 0 3 8 4 0 3 6 4 0 4 1 5 0 3 7 2 0 4 2 1 0 3 2 4 0 3 3 4 0 3 1 3 0 3 9 3 0 2 9 2 0 3 6 3 0 4 1 2 0 3 8 3 0 3 7 4 0 3 6 1 0 3 6 2
Number of Days on Study
Carcass ID Number
Musculoskeletal System Bone Nervous System Brain Peripheral nerve Spinal cord Respiratory System Lung Alveolarbronchiolar adenoma Alveolarbronchiolar carcinoma, multiple Carcinoma, metastatic, thyroid gland Cholangiocarcinoma, metastatic, liver Mediastinum, schwannoma malignant, metastatic, heart Trachea Special Senses System
++++++++++++
++++++++++++
+ +
++++++++++++
X
X
+++
+ +
X
+++++
X
N W
++++++++++++
++ +
. . . . . . . . . . . . . . . . . . . . . . . . .
Ear
Eye Harderian gland Urinary System Kidney Urinary bladder Systemic Lesions Multiple organs Leukemia mononuclear Lymphoma malignant histiocytic Lymphomamalignantmixed Mesothelioma benign Mesothelioma malignant
++
. . . . . . . . . . . . . . . . . . . . . . . . .
+ + + + + + + + + + + + + M + + + + I + + + + + + +
. . . . . . . . . . . . . . . . . . . . . . . . . x x xx x xxx X x x
X
X X
Lesions in Male Rats
71
A TABLE 2 Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: l0,OOO ppm
(continued) 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 4 4 4 4 4 7 7 7 7 7 8 8 8 8 8 9 9 9 9 9 9 9 9 9 9
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 Carcass ID Number
Number of Days on Study
3 3 3 3 4 2 3 3 3 4 3 3 3 3 4 3 3 3 3 3 3 3 3 4 4 2 2 4 5 1 9 1 5 9 0 1 3 3 4 0 2 4 5 7 7 8 8 9 0 0 2 3 4 3 1 1 2 2 2 4 1 2 5 3 3 1 2 1 1 3 1 2 1 1 2
Total Tissues/ Tumors
Musculoskeletal System Bone Nervous System Brain Peripheral newe Spinal cord Respiratory System Lung Alveolarbmnchiolar adenoma Alveolarbmnchiolar carcinoma, multiple Carcinoma, metastatic, thyroid gland Cholangiocarcinoma, metastatic, liver Mediastinum, schwannoma malignant, metastatic, heart
15
12 1 2
+ +
+++
31 1
1 1
1
NOS
Trachea Special Senses System
++++++++++++
+ + + + + + + + + + + +
1 49 12
Ear
Eye Harderian gland Urinary System Kidney Urinary bladder Systemic Lesions Multiple organs Leukemia mononuclear Lymphomamalignanthistiocytic Lymphomamalignantmixed Mesothelioma benign Mesothelioma malignant
2 6 1
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
50 49
xx
xx
xx
50
22 1 2
1 1
72
Quercetin,
NTP TR 409
TABLE 2 A
Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 40,OOO ppm
3 3 4 5 5 5 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 7 7 7 7 1 2 2 5 7 8 1 2 6 6 7 7 7 7 8 9 9 9 9 9 9 0 0 1 1 5 6 2 5 4 1 7 6 2 7 3 4 6 6 6 0 2 3 4 7 8 0 4 3 5
0 4 3 2 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Number of Days on Study
Carcass ID Number
4 5 5 5 5 4 4 5 4 4 5 4 5 4 4 5 5 5 4 5 4 4 5 5 7 3 2 3 0 9 7 3 9 4 2 8 2 5 3 6 1 0 8 4 3 7 4 4 4 2 4 5 2 2 2 4 1 3 2 5 1 4 4 4 3 1 4 5 3 1 4 3
Alimentary System Esophagus Intestine large Intestine large, cecum Intestine large, colon Intestine large, rectum Intestine small Intestine small, duodenum Intestine small, ileum Adenoma Intestine small, jejunum Liver Hepatocellular carcinoma Mesentery Sarcoma, poorly differentiated Pancreas Salivary glands Stomach Stomach, forestomach Stomach, glandular Tongue Papilloma squamous Cardiovascular System B l o o d vessel Hart Endocrine System Adrenal gland Adrenal gland, cortex Adrenal gland, medulla Pheochromocytoma malignant Pheochromocytoma benign Pheochromocytoma benign, multiple Islets, pancreatic Adenoma Parathyroid gland Pituitary gland Pars distalis, adenoma Pars distalis, adenoma, multiple
M + + + + + + + + + + + + + + + I + + + + + + + +
+ M + A A A A
+ + + + + + A
+ + + + + + +
+ + + + + + +
+ + + + + + +
+ + + + + + +
+ + + + + + +
+ + + +
+ + + + + + + A A + + + A + + + + + + + + + + + + A A + + + A + + + + + + + + + + + + A A + + + A + + + + +
+ + + +
+ + + +
+ + + +
+ + + +
+ + + +
+ + + +
A M A A
A A A A
+ + + +
+ + + +
+ + + +
A A A A
+ + + +
+ + + +
+ + + +
+ + + +
+ + + +
A A + + + + + + M + + + + + A A + + + A + + + + +
X M A + + + + + + + + + + + + A + + + + + + + + + +
. . . . . . . . . . . . . . . . . . . . . . . . . + +
+ ++++++++ + + + + + + + + + + + + + + + + + + + A + + + + + + + + + + + + + + + + + + + + + + A + + + + + + + + + + + + + + + + + + + + + + + + A I + + + + . . . . . . . . . . . . . . . . . . . . . . . .
X
+ + . . . . . . . . . . . . . . . . . . . . . . . . .
+ + + + + + + + + M + + + + + + + + + + + + + + + + + + + + + + + + M + + + + + + + + + + + + + + + + + + + + + + + + M + + + + + + + + + + + + + + + X x x x X X X X + + + + + + + + + + + M + + + + X
M A + + + + + + + + + + + + +
M M I M + + + + I
+-I-+++
+ + +
A + + + + + + + + + + + M + + + + + + + + + + + + X X x x X X
Lesions in Male Rats
73
TABLE A2
(continued)
Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 4, O ppm 0 O O
7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 3 3 4 4 4 4 7 7 7 7 7 8 8 8 8 8 8 8 8 9 9 9 9 9 9
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Number of Days on Study
Carcass ID Number
5 5 4 5 5 5 4 4 4 4 5 4 4 4 4 5 5 5 5 4 4 4 4 5 5 1 4 8 3 4 5 3 4 5 5 1 6 6 6 8 3 5 5 6 4 5 6 8 5 5 1 2 3 3 1 5 1 1 2 3 2 1 2 3 1 1 1 2 1 2 1 4 2 3 4
Total Tissues/ Tumors
Alimentary System b P h u s Intestine large Intestine large, cecum Intestine large, colon Intestine large, rectum Intestine small Intestine small, duodenum Intestine small, ileum Adenoma Intestine small, jejunum Liver Hepatocellular carcinoma Mesentery Sarcoma, poorly differentiated Pancreas Salivary glands Stomach Stomach, forestomach Stomach, glandular Tongue Papilloma squamous Cardiovascular System Blood vessel Heart
~~~~~~~~~~
+ + + + + + + + + + + + + + + + I + + + + + + + +
. . . . . . . . . + . . + .
. . . . . . . . .
. . . . . . . . .
+ + + + + + M + + M M + + + + + + + + + + + + + M
. . + .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . X . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . X + + . . . . . . . . . . . . . . . . . . . . + M . . . . . . . . . . . . . . . . . . . . ++++++ + + + + + + + + + + + + . . . . . . . . . . . . . . . . . . . .
. . . . . . . . .
. . . . . . . . .
. . . . . . . . .
47 47
46
47
46 46 46
45 1 44 50 1 5
1
. . + .
. . + .
. . + + .
47 10 49
44 1
46 48
. . . . . . . . . . . . . . . . . . . . . . . . .
2
50
Endocrine System
Adrenal gland Adrenal gland, cortex Adrenal gland, medulla Pheochromocytoma malignant Pheochromocytoma benign Pheochromocytoma benign, multiple Islets, pancreatic Adenoma Parathyroid gland Pituitary gland Pars distalis, adenoma Pars distalis, adenoma, multiple
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . X X xx xx X + + + + + + + + + + + + M + + ++ + + + +
+ + + + + + + + I + + + + + + + + + + + + + + + +
xx
. . . . . . . . . . . . . . . . . . . . . . . . . x x X
49 49 49 2 9 2 41 3 43
48
11 1
I4
Quercetin, NTP TR 409
TABLEA 2 Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 4, O ppm 0 O O
(continued)
-.3 3 4 5 5 5 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 7 7 7 7 1 2 2 5 7 8 1 2 6 6 7 7 7 7 8 9 9 9 9 9 9 0 0 1 1 5 6 2 5 4 1 7 6 2 7 3 4 6 6 6 0 2 3 4 7 8 0 4 3 5
Number of Days on Study
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Carcass ID Number
4 4 5 5 5 5 4 4 5 4 4 5 4 5 4 4 5 5 5 4 5 4 4 5 5 3 7 3 2 3 0 9 7 3 9 4 2 8 2 5 3 6 1 0 8 4 3 7 4 4 2 4 2 4 5 2 2 2 4 1 3 2 5 1 4 4 4 3 1 4 5 3 1 4 3
Endocrine System (continued) Thyroid gland C e l l , adenoma Follicular cell, adenocarcinoma Follicular cell, adenoma General Body System None Genital System Epididymis Preputial gland Adenoma Carcinoma Prostate Seminal vesicle Testes Interstitial cell, adenoma Hematopoietic System Bone marrow Lymph node Lymphnode,mandibular Lymph node, mesenteric Spleen Thymus Integumentary System Mammary gland Fibroadenoma Skin Papilloma squamous Subcutaneous tissue, fibroma Subcutaneous tissue, sarcoma Musculoskeletal System Bone
. . . . . . . . . . . . . . . . . . . . . . . .
X
X
. . . . . . . . . . . . . . . . . . . . . . . . + + + + + + + + + + +
+ + + + + + + + + + M + + + M + + + + + + + + + + + + + + + + + + + + + + + + M + + + + + + + + + +
X
X
. . . . . . . . . . . . . . . . . . . . . . . . .
x x x x x x x x x x x x x x x x x x x x
++++++++ + . . . . . . . . . . . . . . . . . . . . . . . . . + + M M + + + + + + + + + + + M + + + + + + + + M + + + + + + + + +++ + + + + + . . . . . . . . . . . . . . . . . . . . . . . . . M M M + + + + +
M M + +
+ + +
++++++++
X
X
+ +
X
+ + + + + + + + + +
+ +
+ + + +
Lesions in Male Rats
75
TABLE 2 A
(continued)
Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 4O O ppm 0O ,
7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 3 3 4 4 4 4 7 7 7 7 7 8 8 8 8 8 8 8 8 9 9 9 9 9 9
0 5 1 1 0 5 4 2 0 4 8 3 0 5 3 3 0 5 4 1 0 5 5 5 0 4 3 1 0 4 4 1 0 4 5 2 0 4 5 3 0 5 1 2 0 4 6 1 0 4 6 2 0 4 6 3 0 4 8 1 0 5 3 1 0 5 5 1 0 5 5 2 0 5 6 1 0 4 4 2 0 4 5 1 0 4 6 4 0 4 8 2 0 5 5 3 0 5 5 4
Number of Days on Study
Carcass ID Number
Total
Tissues/
Tumors
Endocrine System (continued) Thyroid gland C e l l , adenoma Follicular cell, adenocarcinoma Follicular cell, adenoma General Body System None Genital System Epididymis Preputial gland Adenoma Carcinoma Prostate Seminal vesicle Testes Interstitial cell, adenoma Hematopoietic System Bone marrow Lymphnode Lymphnode,mandibular Lymph node, mesenteric Spleen Thymus Integumentary System Mammary gland Fibroadenoma Skin Papilloma squamous Subcutaneous tissue, fibroma Subcutaneous tissue, Sarcoma Musculoskeletal System Bone
. . . . . . . . . . . . . . . . . . . . . . . . .
49 1 1 1
. . . . . . . . . . . . . . . . . . . . . . . . . + + + +
. . . x
. . . x
. . . x
. . . x
. . . x
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . xxxxxxxxxxxxxxxxxxxx
48
49 50 45
49 15 1 3
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . + + + . . . . . . . . . . . . . . . . . . . . . . . . .
9 50 47 19 50 5
+
+ +
X
+
+ +
9 3 18 1 3 1
23
76
Quercetin, NTP TR 409
TABLE A2
(continued)
Individual Animal Tumor Pathology of Male Rats in the 2-Year Feed Study of Quercetin: 4 O ppm 0 O O ,
3 3 4 5 5 5 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 7 7 7 7 1 2 2 5 7 8 1 2 6 6 7 7 7 7 8 9 9 9 9 9 9 0 0 1 1 5 6 2 5 4 1 7 6 2 7 3 4 6 6 6 0 2 3 4 7 8 0 4 3 s 0 4 3 2 0 4 7 4 0 5 3 2 0 5 2 4 0 5 3 5 0 5 0 2 0 4 9 2 0 4 7 2 0 5 3 4 0 4 9 1 0 4 4 3 0 5 2 2 0 4 8 5 0 5 2 1 0 4 5 4 0 4 3 4 0 5 6 4 0 5 1 3 0 5 0 1 0 4 8 4 0 5 4 5 0 4 3 3 0 4 7 1 0 5 4 4 0 5 4 3
Number of Days on Study
Carcass ID Number
Nervous System Brain Spinal cord Respiratory System Lung Alveolar/bronchiolar adenoma Pheochromocytoma malignant, metastatic, adrenal gland Squamous cell carcinoma Nose Papilloma squamous Trachea Special Senses System Ear Eye Harderian gland Urinary System Kidney Proximal convoluted renal tubule, adenoma Renal tubule, adenocarcinoma Renal tubule, adenoma Urethra Urinary bladder Systemic Lesions Multiple organs Leukemia mononuclear Mesothelioma malignant
. . . . . . . . . . . . . . . . . . . . . . . . . +
. . . . . . . . . . . . . . . . . . . . . . . . .
A
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
+
+ +
. . . . . . . . . . . . . . . . . . . . . . . . .
+
X
+ M + + + + + + + + + + + + A + + + + + + + + + +
. . . . . . . . . . . . . . . . . . . . . . . . .
X
xxxx
x
X
Lesions in Male Rats
77
TABLE 2 A Individual Animal Tumor Pathology of Male Rats in the 2-YearFeed Study of Quercetin: 40,OOO ppm
(continued)
7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 3 3 4 4 4 4 7 7 7 7 7 8 8 8 8 8 8 8 8 9 9 9 9 9 9
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 5 5 4 5 5 5 4 4 4 4 5 4 4 4 4 5 5 5 5 4 4 4 4 5 5
Number of Days on Study
Carcass ID Number
Total
TissUed
1 4 8 3 4 5 3 4 5 5 1 6 6 6 8 3 5 5 6 4 5 6 8 5 5 1 2 3 3 1 5 1 1 2 3 2 1 2 3 1 1 1 2 1 2 1 4 2 3 4
Tumors
Nervous System Brain Spinal cord
. . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . .
X
50 1
Respiratory System Lung Alveolar/bronchiolar adenoma Pheochromocytoma malignant, metastatic, adrenal gland Squamous c e l l carcinoma
Nose
50 1
1
1
Papilloma squamous Trachea Special Senses System Ear Eye Harderian gland Urinary System Kidney Proximal convoluted renal tubule, adenoma Renal tubule, adenocarcinoma Renal tubule, adenoma Urethra Urinary bladder Systemic Lesions Multiple organs Leukemia mononuclear Mesothelioma malignant
+++
. . . . . . . . . . . . . . . . . . . . .
X
. . . . . . . . . . . . . . . . . . . . . . . . .
49 1
50
1 3 1
. . . . . . . . . . . . . . . . . . . . . . . . .
X X X
50 1 1 2 1
. . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . x x x X x xx
48
50 13
78
Quercetin, NTP TR 409
TABLE A3
Statistical Analysis of Primary Neoplasms in Male Rats in the 2-Year Feed Study of Quercetin
Adrenal Medulla: Pheochromocytoma Benign Overall ratesa Adjusted ratesb Terminal rates' First incidence days Life table tests Logistic regression testsd Fisher exact testd
d )
m o (24%) 37.6% 7126 (27%) 575
4/18
(=%le
lo/21(48%)e
11/49 (22%) 6/23 (26%) 662 P=0.568N P=O.503N P=O.522N
36.0%
Adrenal Medulla: Pheochromocytoma (Benign or Malignant) 13/50 (26%) Overall rates 39.7% Adjusted rates Terminal rates 7/M (27%) 575 First incidence (days) Life table tests Logistic regression tests Fisher exact test Kidney (Renal Tubule): Adenoma (Single Sections) Overall rates Adjusted rates Terminal rates dy) First incidence ( a s Life table tests Logistic regression tests Cochran-Armitage testd Fisher exact test
0/50 (0%) 0.0%
4/18 (22%)=
11/21(52%)e
12/49 (24%) 38.5% 6/23 (26%) 662 P=0.574N P=0.501N P=O.523N
3/50 (6%)
0/50 (0%)
0.0%
o p (ow P=O.O07 P=O.o09 P=O.o08
o m (0%)
0150 (0%) 0.0%
of25
(0%)
11.1% 2JB (9%) 676 P=O.114 P=O.122 P=O.121
Kidney (Renal Tubule): Adenoma (Single and Step Sections) Overall rates 1/50 (2%) 3.8% Adjusted rates Terminal rates 1 m (4%) 724 0 First incidence (days) P =o.o08 Life table tests P=O.526 P-0.012 Logistic regression tests P=O.O12 Cochran-Armitage test Fisher exact test
2/50 (4%)
7.1% 2. (7%) Q8 724 0 P=O.526 P=O.5O0
7/50 (14%) 22.5% 4/25 (16%) 617 P=O.O32 P~ 0 . 0 3 2 P=O.O30
0/50 (0%) 0.0% 0 2 (0%) 15
8/50 (16%) 275% 5/23 (22%) 667 P=O.O16 P=O.O18
P=O.O15 4/50 (8%) 15.3% 3/23 (13%) 676 P=O.O56 P=O.o64 P=O.O59
Kidney (Renal Tubule): Adenoma or Adenocarcinoma (Single Sections) Overall rates O b 0 (0%) 0/50 (0%) 0.0% 0.0% Adjusted rates Terminal rates o m (0%) 0 2 (0%) 16 First incidence (days) P=O.O01 Life table tests P=O.O02 Logistic regression tests P=O.o02 Cochran-Armitage test Fisher exact test
Lesions in Male Rats
79
TABLE A3
(continued)
S a i t c l Analysis of Primary Neoplasms in Male Rats in the 2-Year Feed Study of Quercetin ttsia
Kidney (Renal Tubule): Adenoma or Adenocarcinoma (Single and Step s c i n ) etos Overall rates 1/50 (2%) 2/50 (4%) 7.1% Adjusted rates 3.8% Terminal rates 1/26 (4%) 2f2a (7%) F i t incidence ( a s dy) 724 0 724 0 Life table tests P-0.526 P=O.003 Logistic regression tests P=O.526 P= o m 5 P=O.005 Cochmn-Armitage test P=O.500 Fisher exact test Liver: Neoplastic Nodule or Hepatocellular Adenoma Overall rates 3150 (6%) 11.5% Adjusted rates Terminal rates 3/26 (12%) dy) First incidence ( a s 724 0 P=0.631N P=0.105N Life table tests P=0.631N P=0.105N Logistic regression tests P=O.O82N Cochran-Armitage test Fisher exact test
3/50 (6%) 10.7% 3/28 (11%) 724 0
7/50 (14%) 25% 4125 (16%) 617 P =0.032 P=O.O32 P=O.O30 4/50 (8%) 16.0% 4/25 (16%) 724 0 P=O.478 P=O.478 P-0.500 4/50 (8%) 16.0% 4/25 (16%) 724 ( T ) P=O.418 P=O.478 P=O.500
5/50 (10%)
9/50 (18%)
31.5% 6/23 (26%) 667 P=O.o08 P=O.OlO P=O.o08
0150 (0%) 0.0% 0/23 (0%)
P=O.l42N P=O.l42N P=0.121N 1/50 (2%) 4.3% 1/23 (4%) 724 0 P=O.348N P =0.348N P=O.309N
3/50 (6%) 13.0% 3/23 (13%) 724 0 P=0.401N P=O.35ON
P=0.661N
Liver: Neoplastic Nodule, Hepatocellular Adenoma, or Hepatocellular Carcinoma Overall rates 4/50 (8%) 3/50 (6%) Adjusted rates 11.5% 14.3% Terminal rates 4/28 (14%) 3/26 (12%) First incidence ( a s dy) 724 ( T ) 724 0 P =O.u)8N P=O.541 Life table tests Logistic regression tests P=0.208N P=O.541 Cochran-Armitage test P=0.161N P=O.500 Fisher exact test Mammary Gland Fibroadenoma Overall rates Adjusted rates Terminal rates dy) First incidence ( a s Life table tests Logistic regression tests Cochran-Armitage test Fisher exact test Pancreatic Islets: Adenoma or Carcinoma Overall rates Adjusted rates Terminal rates dy) First incidence ( a s Life table tests Logistic regression tests Fisher exact test
5/50 (10%)
16.3% 3/24 (12%) 590 P=O.590 P=O.lOON P=O.546N P=OJSlN
1/50 (2%) 3.6% Ins (4%) 724 0 P=0.094N P=O.l02N
2/15 (13%)e
17.3% 3/25 (12%) 673 P=O.612 P=O.624 P=0.630N
1/13
P=0.357N 3/41 (7%) 11.9% 694 P=O.101 P=O.100 P=O.o97
0147 (0%)
0.0% 0/25 (0%)
1/18 (6%)
80
Quercetin, N T P TR 409
TABLE A3
(continued)
Statistical Analysis of Primary Neoplasms in Male Rats in the 2-Year Feed Study of Quercetin
Pituitary Gland (Pars Distalis): Adenoma Overall rates Adjusted rates Terminal rates First incidence ( a s dy) Life table tests Logistic regression tests Cochran-Armitage test Fisher exact test Skin: Squamous Papilloma Overall rates Adjusted rates Terminal rates First incidence ( a s dy) Life table tests Logistic regression tests Cochran-Armitage test Fisher exact test Skin (Subcutaneous Tissue): Fibroma Overall rates Adjusted rates Terminal rates dy) First incidence ( a s Life table tests Logistic regression tests Cochran-Armitage test Fisher exact test
14/46 (30%) 42.9% 8/25 (32%) 519 P=O.278N P=0.191N P=O.l92N
17/49 (35%) 45.4% 9/27 ( 3 ) 3% 546 P-0.401 P-0.413 P~O.412
0/50 (0%) 0.0% 0 2 (0%) 18
19/50 ( 8 ) 3% 54.0% 1 o m (40%) 592 P~O.219 P -0.245 P10.287 3/50 (6%) 3/25 (12%) 724 0 P50.482 P-0.482 P=O.500
1/50 (2%)
3/23 (13%) 422 P =0.444N P=0.360N
P=OMN
1/50 (2%)
12/48 (25%) 3.% 45
2/50 (4%)
7.7%
2226 (8%)
724 0 P =0.632N P=O.589N P=O.586N
120%
P=0.221N P-0.221N P-0.247N 1/50 (2%) 3.0% 0 2 (0%) 18 704 P=O.WN P=O.489N P=0.500N 1/50 (2%) 3.0%
676 P-0.516N P-0.495N
0/23 (0%)
2.6%
P=0.500N 3/50 (6%) 8.8% 1/23 (4%) 574 P10.476 P=O.500 P-0.500 3/50 (6%) 8.8% 1/23 (4%) 574 P-0.635 P=0.664N P=0.661N
4/50 (8%)
7.1% 1126 (4%) 717 P=O.241 P=O.265 P=O.263
2/50 (4%)
2.1%
0/25 (0%) 555
P=0.52.5N P=O.502N P=0.500N
1/50 (2%)
Skin (Subcutaneous Tissue): Fibroma or Fibrosarcoma 3/50 (6%) Overall rates 8.9% Adjusted rates Terminal rates 1 2 (4%) 16 458 First incidence (days) Life table tests P=O.351 P =0.392 Logistic regression tests P=O.378 Cochran-Armitage test Fisher exact test
o m (0%)
2.1%
704 P=O.292N P=0.320N P=0.309N
om (0%)
555 P=O.33ON P=O.328N P =0.309N
2/50 (4%)
Skin (Subcutaneous Tissue): Fibroma, Fibrosarcoma, or Sarcoma 3/50 (6%) 2/50 (4%) Overall rates 8.9??1 6.2% Adjusted rates Terminal rates 0 2 (0%) 18 12 (4%) 16 704 458 First incidence ( a s dy) .P=0.474N P=O.282 Life table tests P=0.509N P=O.318 Logistic regression tests P=O.304 Cochran-Armitage test P=0.500N Fisher exact test
o m (0%)
5.0%
555 P=0.519N P=O.523N
10.7% 1/23 (4%) 555 P=O.476 P=O.501 P =os00
P=O.500N
Lesions in Male Rats
81
TABLE A3
(continued)
Statistical Analysis of Primary Neoplasms in Male Rats in the 2-Year Feed Study of Quercetin
Testes: Adenoma
Overall rates Adjusted rates Terminal rates First incidence (days) Life table tests Logistic regression tests Cochran-Armitage test Fiiher exact test
100.0% (100%) 573 P=O.252 P=O.617 Pz0.521N
44/50 (88%)
43/46 (B%) 100.0% 26/26 (100%) 509 P=0.477N Pe0.226 P=O.287 3/l7 (18%)e
45/48 (94%)
100.0% 2 2 (100%) /4 420 P=O.341 P=O.218 P=O.264 4/22 (18%)e
45/50 (90%) 100.0% 23/23 (100%) 555 P10.335 P.cO.521 P=O500 1/49 (2%) 2.6%
Thyroid Gland (Call): Adenoma or Carcinoma Overall rates Adjusted rates Terminal rates First incidence ( a s dy) Life table tests Logistic regression tests Fisher exact test All Organs: Mononuclear Leukemia Overall rates Adjusted rates Terminal rates First incidence (days) Life table tests Logistic regression tests Cochran-Annitage test Fisher exact test
5/50 (10%)
15.6% 2 f z (8%) 654
676 P=0.116N P=O.l03N P=0.107N
(0%)
16/50 (32%) 42.4% 6/26 (23%) 590 P=0.257N P=0.164N P=0.164N
3% 18/50 ( 6 ) 39.2% 2/28 (7%) 546 P =0.470 P=O.394
P=O.417
22/50 ( 4 ) 4%
58.8%
11/25 ( 4 ) 4% 549 P=O.168 P=O.151
13/50 (26%) 42.5% 7/23 (30%) 662 P=0.393N P=O.322N P=O.330N
P=O.151
All Organs: Malignant Lymphoma (Histiocytic, Lymphocytic, Mixed, or Undifferentiated Cell Type) Overall rates 3/50 (6%) 2/50 (4%) 2/50 (4%) O b 0 (0%) Adjusted rates 8.3% 5.5% 6.2% 0.0% Terminal rates 0 2 (0%) 16 1128 (4%) 1125 (4%) 0/23 (0%) First incidence ( a s dy) 458 654 592 Life table tests P=O.l18N P=0.117N P=0.514N P=O.488N Logistic regression tests P=0.121N P=O.l02N P=O.S02N P=0.533N Cochran-Armitage test P=0.107N Fisher exact test P=0.121N P=0.500N P=0.500N
All Organs: Mesothelioma (Benign or Malignant)
Overall rates Adjusted rates Terminal rates dy) First incidence ( a s Life table tests Logistic regression tests Cochran-Annitage test Fiiher exact test 4/50 (8%) 4/50 (8%) 10.8% 11.8% 3.6% 2/28 (7%) 1/26 (4%) 573 599 P=O.l92N P=0.501N P=0.626N P=O.l33N P=O.lllN P=O.S34N P=O.l79N P=O.635 P=O.lU)N P =0.643N 3/50 (6%) 6.9% 0 2 (0%) 15 420
1/50 (2%)
o m (0%)
704
P=O.5OON
P=0.181N
82
Quercetin, N T P TR 409
TABLE A3
(continued)
Statistical Analysis of Primary Neoplasms in Male Rats in the 2-YearFeed Study of Quercetin
All Organs. Benign Tumors
Overall rates Adjusted rates Terminal rates First incidence (days) Life table tests Logistic regression tests Cochran-Armitage test Fisher sract test Overall rates Adjusted rates Terminal rates First incidence (days) Life table tests Logistic regression tests Cochran-Armitagetest Fisher exact test Overall rates Adjusted rates Terminal rates dy) First incidence ( a s Life table tests Logistic regression tests Cochran-hitage test Fisher exact test
49/50 (98%) 100.0% 26/26 (100%) 226 PEO.340 P=O.643 P-0.464N
48/50 (96%)
100.0% 28/28 (100%) 509 P-0.359N P=0.307N P=0.500N
27/50 (54%)
100.0% 25125 (100%) 420 P=O.413 P=O.627 P=O.500 32/50 (64%) 73.5% 14E5(56%) 420 P=O.339 P=O.340 P=O.341
50/50 (100%)
50/50 (100%)
48/50 (%%)
100.0%
422 P=O.467 P=O.612N P-O.500N
23/23 (100%)
All Organs: Malignant Tumors
29/50 (58%)
11/26 (42%) 458 P=O.l7ON P=0.047N P=0.050N
6.% 49
54.7% 6/28 (21%) 458 P=O.392N P =0.438N P=0.420N
21/50 (42%) 57.0% 9/23 (39%) 422 P-0.177N P=0.081N P=O.08lN
48/50 (%%)
23/23 (100%)
All Organs: Benign or Malignant Tumors
50/50 (100%)
100.0% 26/26 (100%) 226 P=O.427 Px0.058N P=0.042N
50/50 (100%)
100.0% 28/28 (100%) 458 P=0.419N
2
100.0% 25/25 (100%) 420 P=O.470
2
100.0%
422 P=O.523 P=O.l62N
P=l.OOON
P-1.OOON
Pz0.247N
Terminal sacrifice 'Number of tumor-bearing animalshumber o f animals examined. Denominator is number o f animals examined microscopically for adrenal gland, bone marrow, brain, clitoral gland, epididymis, gallbladder (mouse), heart, kidney, larynx, liver, lung, nose, ovary, pancreas, parathyroid gland, pituitary gland, preputial gland, prostate gland, salivary gland, spleen, testes, thyroid gland, and urinary bladder, for other tissues, denominator is number o f animals necropsied. Kaplan-Meier estimated tumor incidence at the end o f the study after adjustment for intercurrent mortality Observed incidence at terminal kill Beneath the control incidence are the P values associated with the trend test. Beneath the dosed group incidence are the P values corresponding to paitwise comparisons between the controls and that dosed group. The life table analysis regards tumors in animals dying prior to terminal kill as being (directly or indirectly) the cause o death. The logistic regression tests f regard these lesions as nonfatal. The Cochran-Armitage and Fiiher a c t tests compare directly the overall incidence rates. For all tests, a negative trend or a lower incidence in a dose group is indicated by N. e Tissue was examined microscopically only when it was observed to be abnormal at necropsy; thus statistical comparisons with the controls are not appropriate. Not applicable; no tumors in animal group. g Value of statistic cannot be computed.
Lesions in Male Rats
TABLE A4
Historical Incidence of Renal Tubule Neoplasms In Untreated Male F344/N Rats.
Incidence in Controls Adenoma Adenocarcinoma or Carcinoma Adenocarcimona Adenoma,
or Carcinoma
Historical Incidence at EG&C Mason Research Institute
4-Hydmxyacetanilide Pentaerythritol tetranitrate Total Overall Historical Incidence Total Standard deviation Range
~~
3/50
0149
0150
0149
3/50 0149
3/99 (3.0%)
3/99 (3.0%)
41499 (0.8%) 1.9%
41499 (0.8%)
0-% %6
m-42
1.1%
81499 (1.6%) 2.3%
0-% %6
Data as of 17September 1990
84
Quercetin, NTP
TR 409
TABLE AS
Summary of the Incidence of Nonneoplastic Lesions in Male Rats in the 2-Year Feed Study of Quercetin
Disposition Summary Animals initially in study 6-Month Inbrim evaluation 15-Month inbrim evaluation Early deaths Natural deaths Moribund SuMvors Moribund Terminal sacrifice Died last week of study
Animals examined microscopically
70 10 10 3 2 1
70 10 10 7 15
70 10 10
70 10 10 6 2 1
22 1 22 2
50
25
1
27 1
50
1 1 9 3
50
50
Alimentary System Intestine large, cecum Parasite metazoan Intestine large, colon Parasite metazoan Epithelium, pigmentation Intestine large, rectum Parasite metazoan Intestine small Autolysis Intestine small, duodenum Epithelium, pigmentation Intestine small, ileum Necrosis, coagulative Epithelium, pigmentation Peyer's patch, hyperplasia Intestine small, jejunum Epithelium, pigmentation Liver Angiectasis Basophilic focus Clear cell focus Congestion Cyst Cyst multilocular Cytoplasmic alteration Degeneration Degeneration, cystic Eosinophilic focus Fatty change Fibrosis Hemorrhage Hepatodiaphragmatic nodule Hyperplasia, focal Inflammation, chronic Mixed cell focus Mixed cell focus, multiple Necrosis, coagulative Thrombus Bile duct, hyperplasia Centrilobular, necrosis, coagulative
Lesions in Male Rats
TABLE AS
Summary of the Incidence of Nonneoplastic Lesions in Male Rats in the 2-Year Feed Study of Quercetin (continued)
Alimentary System (continued)
Mesentery Fibrosis Inflammation, chronic Inflammation, granulomatous, chronic Mineralization Necrosis, coagulative Pigmentation Pancreas Atrophy cyst Cytoplasmic alteration Ectopic liver Fibrosis Hyperplasia Inflammation, chronic Necrosis, coagulative Pigmentation Thrombus Artery, fibrosis Artery, inflammation, necrotizing, chronic active Artery, mineralization Duct, dilatation Perivascular, inflammation, chronic Serosa, hyperplasia Salivary glands Parotid gland, vacuolization cytoplasmic Stomach, forestomach Acanthosis Edema Fibrosis Hyperkeratosis Hyperplasia, basal cell Hyperplasia, pseudoepitheliomatous Inflammation, acute Inflammation, chronic active Mineralization Ulcer Muscularis, pigmentation Stomach, glandular Edema Hemorrhage Inflammation, chronic active Necrosis, coagulative Epithelium, pigmentation Mucosa, mineralization Muscularis, mineralization Submucosa, fibrosis Tongue Hemorrhage Inflammation, necrotizing, acute Metaplasia, osseous Artery, mineralization Artery, endothelium, hyperplasia
86
Quercetin,
NTP TR 409
TABLE A5
Summary of the Incidence of Nonneoplastic Lesions in Male Rats in the 2-Year Feed Study of Quercetin (continued)
~~~~~~~~
~~
Cardiovascular System Blood vessel Aorta, mineralization
Hart
Cardiomyopathy Cytomegaly Edema Inflammation, chronic Metaplasia, osseous Mineralization Thrombus Artery, mineralization Coronary artery, inflammation, chronic active
2 (11%) 6 (12%)
1 (% 6)
5 (28%)
Endocrine System Adrenal gland, cortex
Angiectasis
Atrophy Congestion Hematopoietic cell proliferation Hemorrhage Hyperplasia Necrosis, coagulative Vacuoliition cytoplasmic Adrenal gland, medulla Atrophy Congestion Hyperplasia Necrosis, coagulative Islets, pancreatic Hyperplasia Parathyroid gland Hyperplasia Pituitary gland Autolysis Hemorrhage Pars distalis, angiectasis Pars distaliis, autolysis Pars distalis, congestion Pars distalis, cyst Pars dsai , hyperplasia i t ls Pars distalii, necrosis Pars distalis, pigmentation Pars intermedia, angiectasis Pars intermedia, crystals Pars intermedia, cyst Pars intermedia, ectopic tissue Pars intermedia, hyperplasia Pars nervosa, cyst Pars nervosa, ectopic tissue Pars nervosa, hyperplasia Rathkes cleft, cyst
Angiectasis
Lesions in Male Rats
87
TABLE A5 Summary of the Incidence of Nonneoplastic Lesions in Male Rats in the 2-Year Feed Study of Quercetin (continued)
Endocrine System (continued) Thyroid gland Congestion Inflammation, acute Ultimobranchial cjst C-cell, hyperplasia Follicle, pigmentation Follicular cell, cyst Follicular cell, hyperplasia General Body System None Genital System Coagulating gland Adventitia, inflammation, chronic active Preputial gland
Abscess
Cyst
(50)
1 2 17 1
(% 2) (% 4) (% 2)
(17) 1 (% 6)
(22)
(49)
(4) 3%
3 (18%)
(33%) 6 16 (27%) 1 (5%)
2 (4%)
1 (5%)
6 (12%) 3 (6%) 1 (2%)
Hyperplasia Inflammation, chronic Duct, dilatation Prostate Fibrosis Hemorrhage Inflammation, acute Inflammation, chronic Inflammation, chronic active Epithelium, hyperplasia Seminal vesicle Atrophy Testes Infarct Necrosis, coagulative Interstitial cell, hyperplasia Seminiferous tubule, atrophy
10 (83%)
(23)
(8 4)
9 (39%)
4 (5) 1 8% 43 ( 0 ) 9%
Hematopoietic System a m Bone m Fibrosis Lymph node Angiectasis Artery, pancreatic, thrombus Inguinal, ectasia Lumbar, ectasia Lumbar, hemorrhage Lumbar, hyperplasia, plasma cell Lumbar, infiltration cellular, histiocyte Lumbar, inflammation, acute Lumbar, pigmentation
(49)
88
Quercetin, NTP TR 409
TABLE A5
of Quercetin (continued)
Summary of the Incidence of Nonneoplastic Lesions in Male Rats in the 2-Year Feed Study
Hematopoietic System (continued) Lymph node (continued) Mediastinal, depletion lymphoid Mediastinal, ectasia b k " , hemorrhage Mediastinal, infiltration cellular, histiocyte Mediastinal, pigmentation Pancreatic, ectasia Pancreatic, hemorrhage Pancreatic, hyperplasia, plasma cell Pancreatic, infiltration cellular, histiocyte Pancreatic, pigmentation Renal, ectasia Renal, fibrosis Renal, hemorrhage Renal, hyperplasia, lymphoid Renal, hyperplasia, plasma cell Renal, infiltration cellular, histiocyte Renal, pigmentation Lymph node, mandibular Angiectasis Congestion Depletion lymphoid Ectasia Hemorrhage Hyperplasia, plasma cell Infiltration cellular, histiocyte Pigmentation Lymph node, mesenteric Ectasia Hemorrhage Hyperplasia, plasma cell Infiltration cellular, histiocyte Pigmentation Spleen Congestion Depletion lymphoid Fibrosis Hematopoietic cell proliferation Hyperplasia, lymphoid Infarct Necrosis, coagulative Pigmentation Thrombus Thymus Congestion Depletion lymphoid Hemorrhage
Lesions in Male Rats
89
TABLE A5
Summary of the Incidence of Nonneoplastic Lesions in Male Rats in the 2-Year Feed Study of Quercetin (continued)
Integumentary System Mammary gland
Abscess Galactocele Hyperplasia Pigmentation Skin Acanthosis Cyst epithelial inclusion Fibrosis Hyperkeratosis Hyperplasia, basal cell Inflammation, necrotizing, acute Subcutaneous tissue, abscess Subcutaneous tissue, edema Subcutaneous tissue, inflammation, chronic active Subcutaneous tissue, inflammation, granulomatous Subcutaneous tissue, necrosis, coagulative
(3 1)
1 7
(20) 1
2
1 1 1
Musculoskeletal System Skeletal muscle Hindlimb, mineralization Nervous System Brain Hemorrhage Cerebellum, infarct Cerebrum, degeneration, focal Respiratory System Lung Congestion Edema Hemorrhage Infiltration cellular, histiocyte Inflammation, chronic active Metaplasia, osseous Necrosis, coagulative Alveolar epithelium, hyperplasia Artery, mineralization Bronchiole, epithelium, hyperplasia
(50)
1 1 1 43
1 1
1 (% 2)
2 3 43 1
34 (68%)
4 (14%) 13 (46%)
2 17 1
90
Quercetin,
NTP TR 409
TABLE A5
Summary of the Incidence of Nonneoplastic Lesions in Male Rats in the %Year Feed Study o f Quercetin (continued)
Respiratory Syatem (continued) NOX Foreign body Metaplasia, squamous Glands, inflammation, acute Lumen, hemorrhage Lumen, inflammation, acute Mucosa, congestion Nasopharyngeal duct, inflammation, acute Nasopharyngeal duct, inflammation, chronic Special Senses System Eye Atrophy Synechia Artery, mineralization Cornea, fibrosis Cornea, inflammation, chronic Posterior chamber, inflammation, chronic Retina, degeneration Sclera, metaplasia, osseous Harderian gland Hyperplasia Inflammation, chronic Urinary System
Kidney
(44)
11 ( 5 ) 2%
2 (5%)
1 (2%)
(2)
() 9
(6)
1 (50%) 1 (50%)
(1) 1 (100%)
1 (11%) 1 (11%)
1 (17%)
(3) 1 (3) 3% 1 (33%)
1 (33%) 1 (3) 3% 1 (33%)
1 (11%)
(1)
(1) 1 (100%)
Autolysis Congestion Cyst Hemorrhage Hydronephrosis Nephropathy Artery, inflammation, necrotizing, chronic active Collecting tubule, mineralization Interstitial tissue, inflammation, acute Interstitial tissue, proximal convoluted renal tubule, inflammation, acute Proximal convoluted renal tubule, mineralization Proximal convoluted renal tubule, necrosis Proximal convoluted renal tubule, epithelium, pigmentation Renal tubule, hyperplasia Renal tubule, hyperplasia, cystic Transitional epithelium, hyperplasia Transitional epithelium, mineralization
2 (4%) 7 (14%) 49 (98%)
4 (8%) 27 (54%)
Lesions in Male Rats
91
TABLE A5
Summary of the Incidence of Nonneoplastic Lesions in Male Rats in the 2-Year Feed Study of Quercetin (continued)
0 PPm
1,m ppm
1 , O ppm 0O O
4 , oPPm 0 f oJ
(1) 1 (100%) 1 (2%) 1 (2%)
Urinary System (continued) Urethra Calculus micro observation only Urinary bladder Calculus micro observation only Inflammation, chronic Artery, mineralization Serosa, mineralization Submucosa, hemorrhage Subserosa, mineralization Transitional epithelium, hyperplasia Wall, mucosa, muscularis, inflammation, necrotizing, acute, diffuse
(49)
(49)
(8 4)
1 (2%) 1 (2%) 1 (2%) 1 (2%)
2 (4%)
1 (2%) 1 (2%)
1 (2%)
93
APPENDIX B SUMMARY OF LESIONS IN FEMALE RATS IN THE 2-YEAR FEED STUDY OF QUERCETIN
TABLE l B the TABLE B2 the TABLE B3 Summary of the Incidence of Neoplasms in Female Rats in 2-Year Feed Study of Quercetin Individual Animal Tumor Pathology of Female Rats in 2-Year Feed Study of Quercetin Statistical Analysis of Primary Neoplasms in Female Rats in the 2-Year Feed Study of Quercetin TABLE B4a Historical Incidence of Renal Tubule Neoplasms in Untreated Female F344/N Rats TABLE B4b Historical Incidence of Oral Cavity Neoplasms in Untreated Female F344/N Rats TABLE B4c Historical Incidence of Mammary Gland Fibroadenomas Female in Untreated F344/N Rats TABLE B4d Historical Incidence of Uterine Stromal Polyps Female in Untreated F344/N Rats TABLE B5 Summary of the Incidence of Nonneoplastic Lesions in Female Rats in the 2-Year Feed Study of Quercetin
................................. ................................. .................................
94
98
.................................... .................................... .................................
. ...
122 127 127 128 128 129
94
Quercetin, N T P TR 409
TABLE B1
Summary of the Incidence of Neoplasms in Female Rats in the 2-YearFeed Study of Quercetin
Disposition Summary Animals initially in study 6-Month Interim evaluation 15-Month interim evaluation Early deaths Natural deaths Moribund sulvivors Terminal sacrifice Moribund Died last week o f study
Animals examined microscopically
70 1 0 10
70 10 10 4 18
70 10 10 2 1 3 3 5
70 10 10 3 1 9 2 7 1
50
1 1 9
29
28
1
50
50
50
Alimentary System Intestine large, CeCum Intestine large, colon Intestine large, rectum Polyp adenomatous Intestine small, duodenum Leiomyoma Intestine small, ileum Intestine small, jejunum Liver Neoplastic nodule Mesentery Pancreas Adenoma Salivary glands Stomach, forestomach Stomach, glandular Tongue Squamous cell carcinoma Cardiovascular System
Alveolarbronchiolar carcinoma, metastatic, lung Fibrosarcoma, metastatic, skin
(50)
Hat er
(3 1)
(7)
1 (4) 1%
(50)
1 (% 8)
Endocrine System Adrenal gland Adrenal gland, cortex Adenoma Fibrosarcoma, metastatic, skin Adrenal gland, medulla Pheochromocytoma malignant Pheochromocytoma benign Islets, pancreatic Adenoma
Lesions in Female Rats
95
TABLE 1 B
(continued)
Summary of the Incidence of Neoplasms in Female Rats in the
2-Year Feed Study of Quercetin
Endocrine System (continued) Parathyroid gland Adenoma Pituitary gland Pars distalis, adenoma Pars distalis, adenoma, multiple Pars distalis, carcinoma Pars distalis, pars intermedia, pars nelvosa, leukemia mononuclear Thyroid gland C-cell, adenoma C e l l , carcinoma Follicular cell, adenoma General Body System None Genital System Clitoral gland Adenoma Carcinoma Sarcoma ovary Granulosa cell tumor benign Granulosa-theca tumor malignant Uterus Adenocarcinoma Leiomyoma Polyp stromal Polyp stromal, multiple Sarcoma stromal Hematopoietic System Bone marrow Lymph node Lumbar, fibrosarcoma,metastatic, skin Lymph node, mandibular Lymph node, mesenteric Spleen Hemangioma Thymus Alveolar/bronchiolar carcinoma, metastatic, lung
(16%) 8 1 (2%) 2 (4%)
(8 4)
(46)
(9) (50) 1 (2%) (8)
(8)
Quercetin, NTP TR 409
TABLE B1
(continued)
Summary of the Incidence of Neoplasms in Female Rats i the 2-Year Feed Study of Quercetin n
Integumentary System Mammary gland Adenocarcinoma Elbmadenoma Fibroadenoma, multiple Skin Subcutaneous tissue, carcinosarcoma, poorly differentiated Subcutaneous tissue, fibroma Subcutaneous tissue, fibrosarcoma Subcutaneous tissue, sarcoma, poorly differentiated Musculoskeletal System Skeletal muscle Sarcoma Nervous System Brain Carcinoma, extension, metastatic, pituitary gland Medulla, carcinoma, metastatic, pituitary gland Spinal cord Respiratory System Lung Alveolarbronchiolar adenoma Alveolarbronchiolar carcinoma Carcinosarcoma, metastatic, skin Granulosa-theca tumor malignant, metastatic, ovary Hepatocellular carcinoma,metastatic, uncertain primary site Pheochromocytoma malignant, metastatic Sarcoma, metastatic, lung Sarcoma, poorly differentiated, metastatic, skin Pleura, ahreolarbronchiolar carcinoma, metastatic, lung NOSe Special Senses System Ear
1 (% 9) 2 (18%)
3 (2Qw
1 (13%) 1 (13%)
(3) 1 (33%)
(50)
(15)
() 8
(50)
'
1 (% 2) (1) 1 (% 7) () 2
(2)
Fibrosarcoma Zymbal's gland Adenoma Squamous cell carcinoma
Lesions in Female Rats
97
TABLE B1
(continued)
Summary of the Incidence of Neoplasms in Female Rats in the 2-Year Feed Study of Quercetin
Urinary System Kidney Renal tubule, adenoma Urinary bladder Papilloma
(49) (49)
Multiple organs* Leukemia monocytic Leukemia mononuclear
Systemic Lesions
Tumor Summary Total animals with primary neoplasmsb Total primary neoplasms Total animals with benign neoplasms Total benign neoplasms Total animals with malignant neoplasms Total malignant neoplasms Total animals with metastatic neoplasms Total metastatic neoplasms Total animals with malignant neoplasms of uncertain primary site
49 118 49 101 15 17 1 1
48 103 44 82 19 21 4 6
43 106 42 82 19 24 1 3
44 81
59 19 22 4 4
1
35
f Number o animals with any tissue examined microscopically Primary neoplasms: all neoplasms except metastatic neoplasms
98
Quercetin, NTP TR 409
TABLE B2
Individual Animal Tumor Pathology of Female Rats in the 2-Year Feed Study of Quercetin: 0 ppm
~
Number of Days on Study
4 5 6 6 6 6 6 6 6 6 6 6 6 6 6 7 7 7 7 7 7 7 7 7 7 2 9 0 1 1 2 3 4 6 6 7 7 8 8 8 0 0 1 1 1 2 2 2 2 2 2 7 6 3 9 5 2 1 0 0 2 2 6 7 7 0 0 8 8 8 3 3 3 3 4 0 6 4 5 0 6 9 5 0 6 0 3 0 5 8 4 0 6 9 4 0 6 4 2 0 6 1 4 0 5 9 5 0 6 5 3 0 6 8 3 0 5 8 5 0 6 3 3 0 6 1 3 0 6 0 2 0 6 7 3 0 6 8 2 0 6 9 3 0 5 7 3 0 6 7 2 0 6 9 2 0 6 0 1 0 6 7 1 0 7 0 3 0 7 0 4 0 5 7 2
Carcass ID Number
Alimentary System Erophaf3us Intestine large Intestine large, cecum Intestine large, colon Intestine large, rectum Intestine small Intestine small, duodenum Intestine small, ileum Intestine small, jejunum Liver Mesentery Pancreas Salivary glands Stomach Stomach, forestomach Stomach, glandular Tongue Cardiovascular System Heart Endocrine System Adrenal gland Adrenal gland, cortex Adrenal gland, medulla Pheochromocytoma malignant Pheochromocytoma benign Islets, pancreatic Adenoma Parathyroid gland Adenoma Pituitary gland Pars distalis, adenoma Pars distalis, adenoma, multiple Thyroid gland Ccell, adenoma Ccell, carcinoma
+: Tissue examined microscopically
. . . . . . . . . . . . . . M + . . . . + . . M + . . . . . .
M +
. . . . . . . + . . . + . . .
. . . . . . . . . . . . . . + + . . . . . . + + . . . . . . ++
. . . . . . . + . . . + . . .
. . . . . . . + . . . + . . .
. . . . . . . + . . . + . . .
. . . . . . . + . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . + + + + + + + + + + . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . +
. . . . . . . + . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . + + + + . . . . . . . . . . . . . . . . . . . . . . . . + + + +
. . . . . . . + . . . . . . +
. . . . . . . . . . . . . + +
. . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . +++++++++
X X
+++++
X
+ + + + + + +
+ + + + + M M + + + + + + + + + + + + + M + + M +
. . . . . . . . . . . . . . . . . . . . . . . . . x x x x x x x x x x x x x x x X X X X . . . . . . . . . . . . . . . . . . . . . . . . .
X
A: Autolysiis precludes examination
M Missing tissue I: Insufficient tissue
X Lesion present Blank Not examined
Lesions in Female Rats
99
TABLE B2 Individual Animal Tumor Pathology of Female Rats in the 2-YearFeed Study of Quercetin: 0 ppm (continued)
Number of Days on Study 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 3 3 3 3 3 3 3 3 3 3 3 3 4 4 4 4 4 5 5 8 9 9 9 9 9 1 1 1 1 1 1 1 2 2 2 2 2
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Carcass
ID Number
5 6 6 6 6 5 6 6 5 6 6 6 7 5 5 5 6 6 6 7 5 6 6 6 6 9 4 5 6 9 8 6 2 9 1 5 6 0 7 9 9 3 5 8 0 8 1 2 3 6 4 1 4 4 1 2 3 1 2 1 2 1 2 1 1 3 1 1 1 1 1 2 2 2 2
Tissues/
Tumors
Total
Alimentary System Esophagus Intestine large Intestine large, cecum Intestine large, colon Intestine large, rectum Intestine small Intestine small, duodenum Intestine small, ileum Intestine small, jejunum Liver Mesentery Pancreas Salivary glands Stomach Stomach, forestomach Stomach, glandular Tongue Cardiovascular System Heart Endocrine System Adrenal gland Adrenal gland, cortex Adrenal gland, medulla Pheochromocytoma malignant Pheochromocytoma benign Islets, pancreatic Adenoma Parathyroid gland Adenoma Pituitary gland Pars distalis, adenoma Pars dsai ,adenoma, multiple i t ls Thyroid gland C-cell, adenoma C-cell, carcinoma
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . + + + + + + + + M + + + + + + + + + + + + M + + . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . + . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ++++++++++++++++ ++++++++ . . . . . . . . . . . . . . . . . . . . . . . . . + + +++ ++++++++++ + + +
~~~~~~~ ~
+ 48
50 50 50 50
50 50 49 50 50 2
50 7 50 49
50 29
. . . . . . . . . . . . . . . . . . . . . . . . .
50
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
++++
++++++++
X X
50 50 50
+++++++++++
X
. . . . . . . . . . . . . . . . . . . . . . . . . x xxxxxx X xx xx X . . . . . . . . . . . . . . . . . . . . . . . . . X X xxx x
X
+ M + I + + + M + M + + + + + + + + + + + M M + + X
1 3 44 2 40
1 50
32
5
50
6 2
100
Quercetin,
NTP TR 409
TABLE B2 Individual Animal Tumor Pathology of Female Rats in the 2-Year Feed Study of Quercetin: 0 ppm (continued)
Number of Days on Study 4 5 6 6 6 6 6 6 6 6 6 6 6 6 6 7 7 7 7 7 7 7 7 7 7 2 9 0 1 1 2 3 4 6 6 7 7 8 8 8 0 0 1 1 1 2 2 2 2 2 2 7 6 3 9 5 2 1 0 0 2 2 6 7 7 0 0 8 8 8 3 3 3 3 4 0 6 4 5 0 6 9 5 0 6 0 3 0 5 8 4 0 6 9 4 0 6 4 2 0 6 1 4 0 5 9 5 0 6 5 3 0 6 8 3 0 5 8 5 0 6 3 3 0 6 1 3 0 6 0 2 0 6 7 3 0 6 8 2 0 6 9 3 0 5 7 3 0 6 7 2 0 6 9 2 0 6 0 1 0 6 7 1 0 7 0 3 0 7 0 4 0 5 7 2
Carcass ID Number
General Body System None Genital System Clitoral gland Adenoma Carcinoma maIy Granulosa cell tumor benign Uterus Leiomyoma Polyp stromal Hematopoietic System Bone marrow Lymph node Lymphnode,mandibular Lymphnode,mesenteric Spleen Hemangioma
Thymus
+ + +
+ +
+ + +
X
. . . . . . . . . . . . . . . . . . . . . . . . . X . . . . . . . . . . . . . . . . . . . . . . . . . X xx X
+ + + + + + + +
+ + + + + + + + + + + + + + + + + + + + + + M + + M + + + + + M + + + + + + + + + + + + + + + M + + X + + + + + + M +
+ + + + + + + + + . . . . . . . . . . . . . . . . . . . . . . . . . .
Integumentary System Mammary gland Adenocarcinoma Fibroadenoma Fibroadenoma, multiple Skin Subcutaneous tissue, carcinosarcoma, poorly differentiated Subcutaneous tissue, fibroma Musculoskeletal System Bone Nervous System Brain Spinal cord
++++++++++++++ X xxx x xx x x X X ++++++++
X
+
x
+
x +
X
+ + + + +
x
X
+ + + + + + + +
. . . . . . . . . . . . . . . . . . . . . . . . . +
Lesions in Female Rats
101
TABLE B2 Individual Animal Tumor Pathology of Female Rats in the 2-YearFeed Study of Quercetin: 0 ppm (continued)
Number of Days on Study 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 3 3 3 3 3 3 3 3 3 3 3 3 4 4 4 4 4 5 5 8 9 9 9 9 9 1 1 1 1 1 1 1 2 2 2 2 2
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Carcass ID Number
5 6 6 6 6 5 6 6 5 6 6 6 7 5 5 5 6 6 6 7 5 6 6 6 6 9 4 5 6 9 8 6 2 9 1 5 6 0 7 9 9 3 5 8 0 8 1 2 3 6 4 1 4 4 1 2 3 1 2 1 2 1 2 1 1 3 1 1 1 1 1 2 2 2 2
Total
Tissues/
Tumors
General Body System None Genital System Clitoral gland Adenoma Carcinoma ovary Granulosa c e l l tumor benign Uterus Leiomyoma Polyp stromal Hematopoietic System Bone marrow Lymph node Lymphnode,mandibular Lymph node, mesenteric Spleen Hemangioma Thymus Integumentary System Mammary gland Adenocarcinoma Fibroadenoma Fibroadenoma, multiple Skin Subcutaneous tissue, carcinosarcoma, poorly differentiated Subcutaneous tissue, fibroma Musculoskeletal System Bone Nervous System Brain Spinal cord
+ + + xx X . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . X X xx
M +
14 4 1 50 1 50 1
7
+ + + + + + + + + + + + + + + + + + + + + + + M + + + + + + + + + + + + + + + + + + + + + + + + M +
48
. . . . . . . . . . . . . . . . . . . . . . . . .
46 9 50 1 8
+ +
X
+ x
+++++ x x xx x x
X
+ +
+ x
+ + + x xx
+ x
+
X
36 1 2 1 8 11
1 2
. . . . . . . . . . . . . . . . . . . . . . . . .
50 1
102
Quercetin, NTP
TR 409
TABLEB2 Individual Animal Tumor Pathology of Female Rats i the 2-Year Feed Study of Quercetin: 0 ppm (continued) n
Number of Days on Study 4 5 6 6 6 6 6 6 6 6 6 6 6 6 6 7 7 7 7 7 7 7 7 7 7 2 9 0 1 1 2 3 4 6 6 7 7 8 8 8 0 0 1 1 1 2 2 2 2 2 2 7 6 3 9 5 2 1 0 0 2 2 6 7 7 0 0 8 8 8 3 3 3 3 4
0 6 4 5 0 6 9 5 0 6 0 3 0 5 8 4 0 6 9 4 0 6 4 2 0 6 1 4 0 5 9 5 0 6 5 3 0 6 8 3 0 5 8 5 0 6 3 3 0 6 1 3 0 6 0 2 0 6 7 3 0 6 8 2 0 6 9 3 0 5 7 3 0 6 7 2 0 6 9 2 0 6 0 1 0 6 7 1 0 7 0 3 0 7 0 4 0 5 7 2
Carcass ID Number
Respiratory System Lung Ah.eolar/bronchiolar adenoma Carcinosarcoma, metastatic, skin Trachea Special Senses System Ear Fibrosarcoma Eye Zymbals gland Adenoma Squamous cell carcinoma
. . . . . . . . . . . . . . . . . . . . . . . . .
NO92
+ + +++++ +++++ +++++++++++++++++++
X X
+
X
Urinary System
Kidney Urinary bladder Papilloma
. . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . .
Systemic Lesions Multiple organs Leukemia mononuclear
. . . . . . . . . . . . . . . . . . . . . . . . . X X x x x
Lesions in Female Rats
103
TABLE B2
Individual Animal Tumor Pathology of Female Rats in the 2-Year Feed Study of Quercetin: 0 ppm (continued)
Number o f Days on Study
7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 3 3 3 3 3 3 3 3 3 3 3 3 4 4 4 4 4 5 5 8 9 9 9 9 9 1 1 1 1 1 1 1 2 2 2 2 2
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Carcass ID Number
5 6 6 6 6 5 6 6 5 6 6 6 7 5 5 5 6 6 6 7 5 6 6 6 6 9 4 5 6 9 8 6 2 9 1 5 6 0 7 9 9 3 5 8 0 8 1 2 3 6 4 1 4 4 1 2 3 1 2 1 2 1 2 1 1 3 1 1 1 1 1 2 2 2 2
Total
TirsUd Tumors
Respiratory System Lung Alveolsrbmnchiolar adenoma Carcinosarcoma, metastatic, skin
NOSe Trachea
. . . . . . . . . . . . . . . . . . . . . . . . .
X X
50
. . . . . . . . . . . . . . . . . . . . . . . . .
49
Special Senses System
Ear
Fibrosarcoma Eye Zymbal's gland Adenoma Squamous cell carcinoma Urinary System Kidney Urinaly bladder Papilloma Systemic Lesions Multiple organs Leukemia mononuclear
+
+ +
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
X
49 50 1
. . . . . . . . . . . . . . . . . . . . . . . . .
X
50 9
104
Quercetin, N T P TR 409
TABLE B2
Individual Animal Tumor Pathology of Female Rats in the 2-Year Feed Study of Quercetin: 1 O ppm OO ,
1 4 5 5 5 5 5 6 6 6 6 6 6 6 6 6 6 6 6 6 7 7 7 7 7 8 9 0 1 4 9 9 0 0 1 1 2 4 6 7 8 8 8 8 9 0 2 2 2 2 3 7 4 5 3 6 7 3 3 2 3 6 5 8 6 0 0 3 7 6 4 1 3 3 3
0 8 0 3 0 7 6 5 0 8 3 4 0 7 3 5 0 7 8 5 0 8 1 4 0 7 2 5 0 7 2 2 0 7 2 3 0 7 3 4 0 7 1 5 0 7 3 3 0 7 5 4 0 7 1 2 0 8 3 3 0 7 1 4 0 7 3 2 0 7 7 4 0 7 8 4 0 8 2 5 0 7 2 1 0 8 0 2 0 7 3 1 0 7 5 3 0 7 6 2
Number of Days on Study
Carcass ID Number
Alimentary System Esophagus Intestine large Intestine large, cecum Intestine large, colon Intestine large, rectum Intestine small Intestine small, duodenum Leiomyoma Intestine small, ileum Intestine small, jejunum Liver Neoplastic nodule Mesentery Pancreas Salivary glands Stomach Stomach, forestomach Stomach, glandular Tongue Cardiovascular System Hart Alveolar/bronchiolar carcinoma, metastatic, lung Endocrine System Adrenal gland Adrenal gland, cortex Adrenal gland, medulla Pheochromocytoma malignant Islets, pancreatic Adenoma Parathyroid gland Pituitary gland Pars distalii, adenoma Pars distalis, adenoma, multiple Pars distalis, carcinoma Thyroid gland C e l l , adenoma C e l l , carcinoma
++++++++++++
+ + + + + + + + + + + + A A A A A A + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + +
+ + + + + + + + + + + + + + + + + + + + + A + + + + + + + + + +
+ + A + + + + + + + + + + + A + + + + + + + + + + + + A + M + + + + + + + + + A + + + + + + + + + +
. . . . . . . . . . . . . . . . . . . . . . . . .
M
+ + A + + + + + + + + + + + + + + + + + + + + + +
+ + + + + + + + + + + + . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
+ + A + + + + + + + + + + + + + + + + + + + + + +
+++++++++
+ + + + + M M M M + + + +
+ + + + + + + + + + + +
X
+ + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + +
X + + A + + + + + + + + +
+
+ +
+ + M + M + + + + + + + + + + M M M + + + + + + + + + M + + + + + + + + + + + + + + + + + + + + + + xx x xx xx xx x xx X X X
+ + + + + + + + + + + + + +
+ + + +
+ + +
Lesions in Female Rats
105
TABLE B2
(continued)
Individual Animal Tumor Pathology of Female Rats in the 2-Year Feed Study of Quercetin: 1 O ppm OO ,
7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 3 3 3 3 3 3 3 3 3 3 4 4 5 5 5 5 5 5 5 5 8 9 9 9 9 1 1 1 1 1
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Number of Days on Study
Carcass ID Number
7 7 7 7 8 7 7 7 7 7 8 8 8 8 8 7 7 7 7 7 7 7 8 8 8 7 7 9 9 4 8 9 1 4 6 2 3 3 4 4 4 1 5 5 7 8 9 1 2 2 1 2 1 2 1 2 4 3 2 4 4 1 2 3 4 1 1 1 2 3 1 3 1 1 2
Total
Tissue4
Tumors
Alimentary System Esophagus Intestine large Intestine large, cecum Intestine large, colon Intestine large, rectum Intestine small Intestine small, duodenum Leiomyoma Intestine small, ileum Intestine small, jejunum Liver Neoplastic nodule Mesentery Pancreas Salivary glands Stomach Stomach, forestomach Stomach, glandular Tongue Cardiovascular System Heart Aiveolar/bronchiolar carcinoma, metastatic, lung
. . . . .
. . . . . . . . . . + + . . . . . . . . . . . . . . . .
. . . . .
. . . . .
. . . . .
. . . . .
. . . . .
. . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . + . . . . . .
. . . . .
. . . . . X + . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . .
. . . . .
. . . . X . . . . . .
. . . . .
. . . . .
. . . . .
. . . . .
. . . . .
. . . . .
. . . . .
. . . . .
+
12 11 11 11 11 49 48 1 48 47 50 1 6 49 12 50 50 49 43
13
1
Endocrine System
Adrenal gland Adrenal gland, cortex Adrenal gland, medulla Pheochromocytoma malignant Islets, pancreatic Adenoma Parathyroid gland Pituitary gland Pars distalis, adenoma Pars distalis, adenoma, multiple Pars distalis, carcinoma Thyroid gland C-cell, adenoma C-cell, carcinoma
X X X + + + + M + + + + M + + + + + M + + M + M + + M +
. . . . . . . . . . . . . . . . . . . . . . . . . x x xx xxxxxxx xx X + + + + + + +++++++++++++++ X X X X xx
14 13 13 1 15 3 39 49 27 4 1 43 3 3
106
Quercetin,
NTP TR 409
B2 TABLE Individual Animal Tumor Pathology of Female Rats in the 2-Year Feed Study of Quercetin: 1O ppm OO ,
(continued)
1 4 5 5 5 5 5 6 6 6 6 6 6 6 6 6 6 6 6 6 7 7 7 7 7 8 9 0 1 4 9 9 0 0 1 1 2 4 6 7 8 8 8 8 9 0 2 2 2 2 3 7 4 5 3 6 7 3 3 2 3 6 5 8 6 0 0 3 7 6 4 1 3 3 3 0 8 0 3 0 7 6 5 0 8 3 4 0 7 3 5 0 7 8 5 0 8 1 4 0 7 2 5 0 7 2 2 0 7 2 3 0 7 3 4 0 7 1 5 0 7 3 3 0 7 5 4 0 7 1 2 0 8 3 3 0 7 1 4 0 7 3 2 0 7 7 4 0 7 8 4 0 8 2 5 0 7 2 1 0 8 0 2 0 7 3 1 0 7 5 3 0 7 6 2
Number of Days on Study
General Body System None Genital System Clitoral gland Adenoma Carcinoma ovary Uterus Polyp stromal Polyp stromal, multiple Sarcoma stromal Hematopoietic System Blood Bone marrow Lymph node Lymphnode,mandibular Lymph node, mesenteric Spleen
Thymus
+++++++++++
X
+ + + + + + + + + + + + M + + . . . . . . . . . . . . . . . . . . . . . . . . .
X
+ + A + + + + + + + + +
+
+++++++ + + + + + + + + + ++ +
+
+ + + + + + + + + + + +
+ + M + + + + + + M M +
+ + + + + + + + + + + + ++++++++++++ + + + + + + + + + + + +
Alveolar/bronchiolar carcinoma, metastatic, lung
Integumentary System Mammarygland Fibroadenoma Fibroadenoma, multiple Skin Subcutaneous tissue, fibroma Musculoskeletal System Bone Nervous System Brain Medulla, carcinoma, metastatic, pituitary gland Spinal cord
++++++++++++ +++ ++ X x x x x x x X + ++++++++++ +
++++++++++++
+
+ + + + + + + + + + + +
X
Lesions in Female Rats
107
TABLEB2 Individual Animal Tumor Pathology of Female Rats in the 2-Year Feed Study of Quercetin: 1 O ppm OO ,
(continued)
7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 3 3 3 3 3 3 3 3 3 3 4 4 5 5 5 5 5 5 . 5 5 8 9 9 9 9 1 1 1 1 1 0 7 7 1 0 7 7 2 0 7 9 1 0 7 9 2 0 8 4 1 0 7 8 2 0 7 9 4 0 7 1 3 0 7 4 2 0 7 6 4 0 8 2 4 0 8 3 1 0 8 3 2 0 8 4 3 0 8 4 4 0 7 4 1 0 7 1 1 0 7 5 1 0 7 5 2 0 7 7 3 0 7 8 1 0 7 9 3 0 8 1 1 0 8 2 1 0 8 2 2
Carcass ID Number
Total Thud Tumors
General Body System None Genital System Clitoral gland Adenoma Carcinoma ovary Uterus Polyp stromal Polyp stromal, multiple Sarcoma stromal Hematopoietic System Blood Bone marrow Lymph node Lymphnode,mandibular Lymph node, mesenteric Spleen
l-hymus
+
X
+++ x x
++
20
+ + + . . . . . . . . . . . . . . . . . . . . . . . . . X xx X xx
4 1 17 50 8 1 2
+
+
+
+ +
+ +
+ +
Alveolar/bronchiolar carcinoma, metastatic, lung
1 11 25 19 14 23 10
Integumentary System Mammary gland Fibroadenoma Fibroadenoma, multiple Skin Subcutaneous tissue, fibroma Musculoskeletal System Bone Nervous System Brain Medulla, carcinoma, metastatic, pituitary gland Spinal cord
+++++ xx x X X +
X
+ + + + + + + xx X xxxx xx
++ + + + xx
36 17 10 15 3
13
15 1 2
108
Quercetin, N T P TR 409
TABLEB2 Individual Animal Tumor Pathology of Female Rats i the 2-Year Feed Study of Quercetin: 1O ppm n OO ,
(continued)
1 4 5 5 5 5 5 6 6 6 6 6 6 6 6 6 6 6 6 6 7 7 7 7 7 8 9 0 1 4 9 9 0 0 1 1 2 4 6 7 8 8 8 8 9 0 2 2 2 2 3 7 4 5 3 6 7 3 3 2 3 6 5 8 6 0 0 3 7 6 4 1 3 3 3
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Number of Days on Study
Carcass ID Number
8 7 8 7 7 8 7 7 7 7 7 7 7 7 8 7 7 7 7 8 7 8 7 7 7 0 6 3 3 8 1 2 2 2 3 1 3 5 1 3 1 3 7 8 2 2 0 3 5 6 3 5 4 5 5 4 5 2 3 4 5 3 4 2 3 4 2 4 4 5 1 2 1 3 2
Respiratory System Lung Alveolarlbronchiolar carcinoma Pheochromocytoma metastatic malignant, Sarcoma, metastatic, lung Pleura, alveolarlbronchiolar carcinoma, metastatic, lung
Nose
X
X
++++++++++++
X
+ +
X
++++
Trachea Special Senses System Ear Eye Harderian gland Zymbals gland Squamous cell carcinoma
Urinary System
+ + + + + + + + + + + + + + + + + + + + + + + +
+
X
I 1 1
X
Kidney Urinary bladder Systemic Lesions Multiple organs Leukemia mononuclear
+ + A + + + + + + + + + + + + + + + + + + + + + +
+ + + + + + + + + + +
+ + + + + + + + + + + + +
. . . . . . . . . . . . . . . . . . . . . . . . . x xxx xx x
Lesions in Female Rats
19 0
TABLE B2
(continued)
Individual Animal Tumor Pathology of Female Rats in the 2-Year Feed Study of Quercetin: 1 O ppm OO ,
7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 3 3 3 3 3 3 3 3 3 3 4 4 5 5 5 5 5 5 5 5 8 9 9 9 9 1 1 1 1 1
~ ~~~
Number of Days on Study
Carcass ID Number
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
7 7 7 7 8 7 7 7 7 7 8 8 8 8 8 7 7 7 7 7 7 7 8 8 8 7 7 9 9 4 8 9 1 4 6 2 3 3 4 4 4 1 5 5 7 8 9 1 2 2 1 2 1 2 1 2 4 3 2 4 4 1 2 3 4 1 1 1 2 3 1 3 1 1 2
Total Tissue4 Tumors
Respiratory System Lung Ahreolar/bronchiolar carcinoma Pheochromocytoma malignant, metastatic Sarcoma, metastatic, lung Pleura, ahreolar/bronchiolar carcinoma, metastatic, lung NOSe Trachea Special Senses System Ear Eye Harderian gland Zymbals gland Squamous cell carcinoma Urinary System Kidney Urinary bladder Systemic Lesions Multiple organs Leukemia mononuclear
20
1 1 1
1 12 12
+ I
+ +
+ +
+ I +
2 8 4 2 2
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
49 49
. . . . . . . . . . . . . . . . . . . . . . . . .
X
X
50 10
110
Quercetin, NTP
TR 409
TABLE B2
Individual Animal Tumor Pathology of Female Rats in the 2-Year Feed Study of Quercetin: l , O ppm 0O O
3 4 5 5 5 6 6 6 6 6 6 6 7 7 7 7 7 7 7 7 7 7 7 7 7 3 4 2 4 4 0 0 6 6 8 9 9 0 0 1 2 2 2 2 2 2 2 2 2 2 7 1 1 8 8 3 5 0 6 5 7 7 7 9 8 3 3 3 3 3 3 4 4 4 4
Number of Days on Study
o o o o o o o o o o o o o o o o o o o o o o a o o
Carcass ID Number
9 9 8 9 9 9 9 8 8 9 9 9 8 8 8 8 8 9 9 9 9 8 8 8 8 6 8 9 6 7 3 4 8 7 8 6 7 5 8 5 7 9 4 5 6 7 5 6 8 8 5 5 4 4 4 4 4 5 3 2 2 3 5 4 4 2 1 1 1 1 1 1 2 1 2
Alimentary System Esophagus Intestine large Intestine large, cecum Intestine large, colon Intestine large, rectum Intestine small Intestine small, duodenum Intestine small, ileum Intestine small, jejunum Liver Mesentery Pancreas Salivary glands Stomach Stomach, forestomach Stomach, glandular Tongue Cardiovascular System Heart Fibrosarcoma, metastatic, skin
+ + + + + . . + . . . + . . . +
++++++ + + + + + + + + + + + + ++++++ ++++++ . . . . . . . . . . . . . . . . . . . . . . + + + + + + + + + + . . . . . . . . . . . . . . . . . . . . . . M . . . . . . . . . . . + + + + + + . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . + + + + + + + + M M
. . + . . . . . .
M M + + + + + M + + + + +
. . . . . . . . . . . . . . . . . . . . + M + + + + + + + . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . + . . . . . .
. . + . . + . . . .
+ +
+++++++
X
Endocrine System
Adrenal gland Adrenal gland, cortex Adenoma Fibrosarcoma, metastatic, skin Adrenal gland, medulla Pheochromocytoma benign Islets, pancreatic Adenoma Parathyroid gland Pituitary gland Pars distalis, adenoma Pars distalii, adenoma, multiple Thyroid gland C e l l , adenoma Ccell, carcinoma
+ + + + + + + +++++++
+++++++ + + + + + + +
X
+ +
+ + ++
++
X M M + + + + + + + + M + + M M + + + M + + + + + +
. . . . . . . . . . . . . . . . . . . . . . . . . X xxx xx x xxx xx x xx X X . . . . . . . . . . . . . . . . . . . . . . . . .
X X
Lesions in Female Rats
111
TABLE B2 Individual Animal Tumor Pathology of Female Rats in the 2-Year Feed Study of Quercetin: l0,OOO ppm
(continued)
7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 3 3 3 3 4 4 4 4 4 4 4 4 4 5 5 5 5 5 5 9 9 9 9 9 9 1 1 1 1 0 8 9 2 0 8 9 3 0 9 0 3 0 9 0 5 0 9 1 1 0 9 1 2 0 9 2 1 0 9 2 2 0 9 3 1 0 8 6 3 0 9 2 3 0 9 3 3 0 9 4 3 0 9 5 4 0 9 7 2 0 8 6 1 0 8 8 3 0 9 0 2 0 9 1 5 0 9 4 2 0 9 5 3 0 8 5 2 0 9 0 1 0 9 1 4 0 9 3 2
Number of Days on Study
Carcass ID Number
Total Tissues/ Tumors
Alimentary System MPhagm Intestine large Intestine large, cecum Intestine large, colon Intestine large, rectum Intestine small Intestine small, duodenum Intestine small, ileum Intestine small, jejunum Liver Mesentery Pancreas Salivary glands Stomach Stomach, forestomach Stomach, glandular Tongue Cardiovascular System Hart Fibrosarcoma, metastatic, skin Endocrine System Adrenal gland Adrenal gland, cortex Adenoma Fibrosarcoma, metastatic, skin Adrenal gland, medulla Pheochromocytoma benign Islets, pancreatic Adenoma Parathyroid gland Pituitary gland Pars distalis, adenoma Pars distalis, adenoma, multiple Thyroid gland C e l l , adenoma C e l l , carcinoma
+ + . . . + . . . . . . . . + . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . + + + + + + + M + + + + . . . . . . . . . . . . . + . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . + . . . . . . . . + . . . . . . . . + . . . . . . . . + . . . . . . . . + . . . . . . . . . . . . . . . . . . . . + + + + + . . . . . . . . . . . . . . . . . . . . . . . . .
+ + + + + + + + + + + M + + + + + + + + + + + + +
7 8 8 7 7 50 50 49 49 50 2 50 7 50 50 50 43
I
1
+ +
X
+ +
X
+ +
X
13 13 1 1 12 3 8
. . . . . . . . . . . . . . . . . . . . . . . . . x xx X x x x x x x X xx x X X xx + + + + + + + + + + + + + + + + + + + + + + X x x
X
+ + + + + + + M M + + + + M M M M + M + + + + M +
36 50 26
9 47 4 2
112
Quercetin, NTP
TR 409
TABLEB2 Individual Animal Tumor Pathology of Female Rats in the 2-Year Feed Study of Quercetin: l , O ppm 0O O
(continued)
3 4 5 5 5 6 6 6 6 6 6 6 7 7 7 7 7 7 7 7 7 7 7 7 7 3 4 2 4 4 0 0 6 6 8 9 9 0 0 1 2 2 2 2 2 2 2 2 2 2 7 1 1 8 8 3 5 0 6 5 7 7 7 9 8 3 3 3 3 3 3 4 4 4 4 0 9 6 5 0 9 8 5 0 8 9 4 0 9 6 4 0 9 7 4 0 9 3 4 0 9 4 4 0 8 8 5 0 8 7 3 0 9 8 2 0 9 6 2 0 9 7 3 0 8 5 5 0 8 8 4 0 8 5 4 0 8 7 2 0 8 9 1 0 9 4 1 0 9 5 1 0 9 6 1 0 9 7 1 0 8 5 1 0 8 6 2 0 8 8 1 0 8 8 2
Number of Days on Study
Carcass ID Number
General Body System None Genital System Clitoral gland Adenoma Carcinoma Sarcoma ovary Uterus Adenocarcinoma Polyp stromal Sarcoma stromal Vagina Hematopoietic System Bone marrow Lymphnode Lumbar, fibrosarcoma, skin metastatic, Lymphnode,mandibular Lymph node, mesenteric Spleen Thymus Integumentary System Mammarygland Adenocarcinoma Fibroadenoma Fibroadenoma, multiple Skin Subcutaneous tissue, fibroma Subcutaneous tissue, fibrosarcoma Musculoskeletal System Bone Nervous System Brain
+ M + + + + +
X
+
X
. . . . . . . . . . . . . . . . . . . . . . . . . X xx X xx +
+ + + + + + + + + + + + + +
X + + + M + + +
+ + + + + + + M M M M M + M M M M + M + M + M + M
X
+
+ + +
+ + xx
+ + +
X
+ + + + + + + + + + + + + + + + +++++++
M + + + + + + + + +
+ +
xxx
+
X
+ + + + + + +
X
X
+ + + + + + +
+ + + + + + +
+
Lesions in Female Rats
113
TABLE B2
(continued)
Individual Animal Tumor Pathology of Female Rats in the 2-YearFeed Study of Quercetin: l0,OOO ppm
7 7 7 7 7 7 7 7 7 7 . 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 3 3 3 3 4 4 4 4 4 4 4 4 4 5 5 5 5 5 5 9 9 9 9 9 9 1 1 1 1
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Number of Days on Study
Carcass ID Number
8 8 9 9 9 9 9 9 9 8 9 9 9 9 9 8 8 9 9 9 9 8 9 9 9 9 9 0 0 1 1 2 2 3 6 2 3 4 5 7 6 8 0 1 4 5 5 0 1 3 2 3 3 5 1 2 1 2 1 3 3 3 3 4 2 1 3 2 5 2 3 2 1 4 2
Total
TissUesl
Tumors
General Body System None Genital System Clitoral gland Adenoma Carcinoma Sarcoma ovary Uterus Adenocarcinoma Polyp stromal Sarcoma stromal Vagina
Hematopoietic System
+
X
14 3 1 15 50 1 16 1 1
. . . . . . . . . . . . . . . . . . . . . . . . . X X xxx x x x x
X
+ M M M M M M + M M M M M M M M M M M M M M + M M X
Bone marrow Lymphnode Lumbar, fibrosarcoma, metastatic, skin Lymphnode,mandibular Lymph node, mesenteric Spleen
Thymus
+ + + + + +
X
+
+ +
+
X
+ + + + + + + + + + +
+
+ + + + xx x + x
7 17 1 10 12 24) 7
Integumentary System Mammary gland Adenocarcinoma Fibroadenoma Fibroadenoma, multiple Skin Subcutaneous tissue, fibroma Subcutaneous tissue, fibrosarcoma Musculoskeletal System Bone Nervous System Brain
+
X
24 3 14 2 8 1 1
114
Quercetin,
NTP TR 409
TABLE B2
(continued)
Individual Animal Tumor Pathology of Female Rats in the 2-Year Feed Study of Quercetin: 10,OOO ppm
3 4 5 5 5 6 6 6 6 6 6 6 7 7 7 7 7 7 7 7 7 7 7 7 7 3 4 2 4 4 0 0 6 6 8 9 9 0 0 1 2 2 2 2 2 2 2 2 2 2 7 1 1 8 8 3 5 0 6 5 7 7 7 9 8 3 3 3 3 3 3 4 4 4 4
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Number of Days on Study
Carcass ID Number
9 9 8 9 9 9 9 8 8 9 9 9 8 8 8 8 8 9 9 9 9 8 8 8 8 6 8 9 6 7 3 4 8 7 8 6 7 5 8 5 7 9 4 5 6 7 5 6 8 8 5 5 4 4 4 4 4 5 3 2 2 3 5 4 4 2 1 1 1 1 1 1 2 1 2
Respiratory System Lung Alveolar/bronchiolar adenoma
Nose
Trachea
+ + + + + + + + +++++++ +++++++
++++
Special Senses System
Ear
Eye Harderian gland
+ +
Urinary System
Kidney Renal tubule, adenoma Urinary bladder
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . X xxx x x xxx
Systemic Lesions Multiple organs Leukemia mononuclear
Lesions in Female Rats
115
TABLE B2
(continued)
Individual Animal Tumor Pathology of Female Rats in the 2-Year Feed Study of Quercetin: 10,OOO ppm
7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 3 3 3 3 4 4 4 4 4 4 4 4 4 5 5 5 5 5 5 9 9 9 9 9 9 1 1 1 1
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
Number of Days on Study
Carcass ID Number
8 8 9 9 9 9 9 9 9 8 9 9 9 9 9 8 8 9 9 9 9 8 9 9 9 9 9 0 0 1 1 2 2 3 6 2 3 4 5 7 6 8 0 1 4 5 5 0 1 3 2 3 3 5 1 2 1 2 1 3 3 3 3 4 2 1 3 2 5 2 3 2 1 4 2
Total Tissud Tumors
Respiratory System Lung Alveddbronchiolar adenoma Nose Trachea Special Senses System Ear Eye Harderian gland Urinary System Kidney Renal tubule, adenoma Urinary bladder Systemic Lesions Multiple organs Leukemia mononuclear
20
1 7 7
+ +
++
1 6 2
. . . . . . . . . . . . . . . . . . . . . . . . . X . . . . . . . . . . . . . . . . . . . . . . . . .
50 1
50
. . . . . . . . . . . . . . . . . . . . . . . . .
X X X
50 13
116
Quercetin, NTP TR 409
TABLEB2 Individual Animal Tumor Pathology of Female Rats in the 2-Year Feed Study of Quercetin: 4O O ppm 0O ,
2 4 4 5 5 5 5 5 6 6 6 6 6 6 6 6 6 6 6 7 7 7 7 7 7 8 5 9 4 5 6 9 9 2 3 3 3 6 6 6 8 8 8 8 1 1 1 2 2 2 4 7 2 9 3 0 4 7 3 0 2 7 0 7 8 6 8 8 8 5 6 6 3 3 3 1 0 5 4 1 0 6 5 0 9 9 3 1 1 1 3 1 0 1 2 1 0 1 3 1 0 7 4 1 0 5 2 1 0 8 4 1 0 6 3 1 1 0 4 1 1 1 2 1 1 1 4 1 0 8 3 1 0 2 5 1 1 0 3 0 9 9 2 1 0 9 4 1 1 1 5 1 0 0 5 0 9 9 5 1 0 7 3 1 0 0 2 1 0 1 1 1 0 1 5
Number of Days on Study
Carcass ID Number
Alimentary System Esophagus Intestine large Intestine large, cecum Intestine large, colon Intestine large, rectum Polyp adenomatous Intestine small Intestine small, duodenum Intestine small, ileum Intestine small, jejunum Liver Neoplastic nodule Mesentery Pancreas Adenoma Salivary glands Stomach Stomach, forestomach Stomach, glandular Tongue Squamous c e l l carcinoma Cardiovascular System Heart Endocrine System Adrenal gland Adrenal gland, cortex Adenoma Adrenal gland, medulla Pheochromocytoma benign Islets, pancreatic Parathyroid gland Pituitary gland Pars distalis, adenoma Pars distalis, adenoma, multiple Pars distalis, carcinoma Thyroid gland C e l l , adenoma C-cell, carcinoma Follicular cell, adenoma
. . . . . . . . . . . . . . . . . . . . . . . . .
+ + + +
+ + + +
+ + + +
+ + + +
. . . . . . . . . . . + . . . . . . . . . . . ++++++++++ . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . + + + + + + + +
X
+ + + +
+ + + +
+ + + +
+ + + +
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
. . . .
A A A A
+ + + +
+ + + +
+ + + +
+ + + +
+ + + +
+ + + +
+ + + +
. . . . . . . . . . . . . .
X
+ + + +
+ + + +
+ + + +
+ + + +
+ + + +
+ + + +
+ + + +
+ + + +
+ + + +
+ + + +
+ + + +
+ + + +
+ + + +
. . + . . . . . .
. . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . + ++ + . . . . . . . . . . . . + + +
. . . . . . . . . . . . . . . . . . . . . . . . .
. . . + . . . + . . . + . . . . . . + . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . X .
+ M + + + + + + + M + M + + M + + + + + + + + + + + + + + M + + + + + + + + + + + + + + + + + + + + X xx X x xxxxx xxx x
. . . . . . . . . . . . . . . . . . . . . . . . .
X
Lesions in Female Rats
117
TABLE B2
(continued)
~~
Individual Animal Tumor Pathology of Female Rats in the 2-Year Feed Study of Quercetin: 4, O ppm 0 O O
7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 3 3 3 3 3 3 3 3 3 3 3 3 3 3 4 4 4 4 4 4 4 4 5 5 9 1 1 1 1 1 1 1 1
Number of Days on Study
Carcass ID Number
1 1 1 1 1 1 0 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 0 0 0 0 1 1 9 0 0 0 0 0 0 0 0 0 1 0 0 0 0 0 0 1 1
7 7 8 9 2 2 9 0 2 3 4 4 6 9 1 2 0 2 2 3 3 4 6 0 2 1 2 2 2 1 2 1 3 4 2 2 4 2 3 4 3 2 1 2 1 3 1 1 1 3
Total Tissues/ Tumors
Alimentary System Esophagus Intestine large Intestine large, cecum Intestine large, colon Intestine large, rectum Polyp adenomatous Intestine small Intestine small, duodenum Intestine small, ileum Intestine small, jejunum Liver Neoplastic nodule Mesentery Pancreas Adenoma Salivary glands Stomach Stomach, forestomach Stomach, glandular Tongue Squamous cell carcinoma Cardiovascular System Halt
+ + + + + + + + + + + + + + + + + M + + + + + +
. + + + . . . . .
. + + + . . . . .
. + + + . . . . .
. . . . + + + +++ +++ . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + +
+ + + + M + + + + + + + + + + + + +
47 49 48 47
1
48
49 49 49 49 50 1 1 50 1 11
50
. . . . .
. . . . .
. . . . .
. . . . .
. . . . .
. . . . .
. . . . .
. . . . .
. . . . .
. . . . .
. . . . .
X
. . . . .
. . . . .
. . . . .
. . . . .
. . . . .
. . . . .
. . . . .
. . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . + + + + + + + + + + + + + + + + + + + + + +
. . . . . . . . . . . . . . . . . . . . . . . . . + + + + + + + + + + + + + + + + ++ + + + + + +
47 50 39 2
. . . . . . . . . . . . . . . . . . . . . . . . .
. . . . M . x . . . . M .
X
50
Endocrine System
Adrenal gland Adrenal gland, cortex Adenoma Adrenal gland, medulla Pheochromocytoma benign Islets, pancreatic Parathyroid gland Pituitary gland Pars distalis, adenoma Pars distalis, adenoma, multiple Pars distalis, carcinoma Thyroid gland C e l l , adenoma C-cell, carcinoma Follicular cell, adenoma
. . . . + . x
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . X . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . + + + + + + + + M + + + + + + + + + + + . . . . . . . . . . . . . . . . . . . . . x x x xx x x xx
X X X
. . . . + .
50
50 50 1 49 43 49 25 2
50
. . . . . . . . . . . . . . . . . . . . . . . . .
X X
1 1 1
118
Quercetin, N T P
TR 409
TABLE B2 Individual Animal Tumor Pathology of Female Rats in the 2-Year Feed Study of Quercetin: 4O O ppm 0O ,
(continued)
~
~-
Number of Days on Study
2 4 4 5 5 5 5 5 6 6 6 6 6 6 6 6 6 6 6 7 7 7 7 7 7 8 5 9 4 5 6 9 9 2 3 3 3 6 6 6 8 8 8 8 1 1 1 2 2 2 4 7 2 9 3 0 4 7 3 0 2 7 0 7 8 6 8 8 8 5 6 6 3 3 3 1 0 5 4 1 0 6 5 0 9 9 3 1 1 1 3 1 0 1 2 1 0 1 3 1 0 7 4 1 0 5 2 1 0 8 4 1 0 6 3 1 1 0 4 1 1 1 2 1 1 1 4 1 0 8 3 1 0 2 5 1 1 0 3 0 9 9 2 1 0 9 4 1 1 1 5 1 0 0 5 0 9 9 5 1 0 7 3 1 0 0 2 1 0 1 1 1 0 1 5
Carcass ID Number
General Body System None Genital System Clitoral gland Adenoma wary Granulosa-theca tumor malignant Uterus Polyp stromal Polyp stromal, multiple Hematopoietic System Bone marrow Lymphnode Lymphnode,mandibular Lymph node, mesenteric Spleen Thymus Integumentary System Mammary gland Adenocarcinoma Fibroadenoma Fibroadenoma, multiple Skin Subcutaneous tissue, sarcoma, poorly differentiated Musculoskeletal System Bone Skeletal muscle Sarcoma Nervous System Brain Carcinoma, extension, metastatic, pituitary gland Spinal cord
+ + + + + + + + + + + X X X . . . . . . . . . . . . . . . . . . . . . . . . . X . . . . . . . . . . . . . . . . . . . . . . . . .
X
X
M + + + + M + + + + + + + + + + + + + + + + + + M
M + + M + + + M + + + +
++++++++++ + . . . . . . . . . . . . . . . . . . . . . . . . .
+ A + + + +
. . . . . . . . . . . . . . . . . . . . . . . . .
M
+ +
++
+ + + + + + + + + + + +
X
++
X
+ + + + + + + + + +
X
++++ +
+++++ +
. . . . . . . . . . . . . . . . . . . . . . . . .
+ +
Lesions in Female Rats
119
TABLE B2 Individual Animal Tumor Pathology of Female Rats in the 2-Year Feed Study of Quercetin: 4, O ppm 0 O O
(continued) 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 3 3 3 3 3 3 3 3 3 3 3 3 3 3 4 4 4 4 4 4 4 4 5 5 9 1 1 1 1 1 1 1 1
1 0 7 1 1 0 7 2 1 0 8 2 1 0 9 2 1 1 2 1 1 1 2 2 0 9 9 1 1 0 0 3 1 0 2 4 1 0 3 2 1 0 4 2 1 0 4 4 1 0 6 2 1 0 9 3 1 0 1 4 1 0 2 3 1 1 0 2 1 0 2 1 1 0 2 2 1 0 3 1 1 0 3 3 1 0 4 1 1 0 6 1 1 1 0 1 1 1 2 3
Number of Days on Study
Carcass ID Number
Total Tissues/ Tumors
General Body System None Genital System Clitoral gland Adenoma ovary Granulosa-theca tumor malignant Uterus Polyp stromal Polyp stromal, multiple Hematopoietic System Bone marrow Lymphnode Lymphnode,mandibular Lymph node, mesenteric Spleen Thymus Integumentary System Mammary gland Adenocarcinoma Fibroadenoma Fibroadenoma, multiple Skin Subcutaneous tissue, sarcoma, poorly differentiated Musculoskeletal System Bone Skeletal muscle Sarcoma Nervous System Brain Carcinoma, extension, metastatic, pituitary gland Spinal cord
+ + + + + M + + + M + + + + + + + + + + + + + + +
. . . . . . . . . . . . . . . . . . . . . . . . . xx X X xxxx
12 4 48 1
50
1 0 1
+ + + + + + + + + + + + + + + + + + + + M + + + +
+ + + + + + + + + + + + + + + + + + + + M + + + +
. . . . . . . . . . . . . . . . . . . . . . . . .
11 49 46 9
50
+
X
+ +
X X
+
X
+
X
+
X
22 2 6 3
11
14 3
. . . . . . . . . . . . . . . . . . . . . . . . .
X
50
1 2
120
Quercetin,
NTP TR 409
TABLE B2
(continued)
Individual Animal Tumor Pathology of Female Rats in the 2-YearFeed Study of Quercetin: 4, O ppm 0 O O
2 4 4 5 5 5 5 5 6 6 6 6 6 6 6 6 6 6 6 7 7 7 7 7 7 8 5 9 4 5 6 9 9 2 3 3 3 6 6 6 8 8 8 8 1 1 1 2 2 2 4 7 2 9 3 0 4 7 3 0 2 7 0 7 8 6 8 8 8 5 6 6 3 3 3 1 0 5 4 1 0 6 5 0 9 9 3 1 1 1 3 1 0 1 2 1 0 1 3 1 0 7 4 1 0 5 2 1 0 8 4 1 0 6 3 1 1 0 4 1 1 1 2 1 1 1 4 1 0 8 3 1 0 2 5 1 1 0 3 0 9 9 2 1 0 9 4 1 1 1 5 1 0 0 5 0 9 9 5 1 0 7 3 1 0 0 2 1 0 1 1 1 0 1 5
Number of Days on Study
Carcass ID Number
Respiratory System Lung Gknulosa-theca tumor malignant, metastatic, ovary Hepatocellular carcinoma, metastatic, uncertain primary site Sarcoma, poorly differentiated, metastatic, skin Nose Trachea Special Senses System Eye Harderiangland Urinary System Kidney Urinary bladder Systemic Lesions Multiple organs Leukemia monocytic Leukemia mononuclear
. . . . . . . . . . . . . . . . . . . . . . . . .
X X
+ . . . . . . . . . . . . . . . . . . . . . . . . .
+ + + + A + + + + +
+ + +
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . X xxx x xx X
Lesions in Female Rats
121
B2 TABLE Individual Animal Tumor Pathology of Female Rats in the 2-Year Feed Study of Quercetin: 4O O ppm 0O ,
(continued) 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 3 3 3 3 3 3 3 3 3 3 3 3 3 3 4 4 4 4 4 4 4 4 5 5 9 1 1 1 1 1 1 1 1 1 0 7 1 1 0 7 2 1 0 8 2 1 0 9 2 1 1 2 1 1 1 2 2 0 9 9 1 1 0 0 3 1 0 2 4 1 0 3 2 1 0 4 2 1 0 4 4 1 0 6 2 1 0 9 3 1 0 1 4 1 0 2 3 1 1 0 2 1 0 2 1 1 0 2 2 1 0 3 1 1 0 3 3 1 0 4 1 1 0 6 1 1 1 0 1 1 1 2 3
Number of Days on Study
Carcass ID Number
Total Tissuesf Tumors
Respiratory System Lung Granulosa-theca tumor malignant, metastatic, ovary Hepatocellular carcinoma, metastatic, uncertain primary site Sarcoma, poorly differentiated, metastatic, skin Nose Trachea Special Senses System Eye Harderian gland Urinary System Kidney Urinary bladder Systemic Lesions Multiple organs Leukemia monocytic Leukemia mononuclear
. . . . . . . . . . . . . . . . . . . . . . . . .
50
1
1
. . . . . . . . . . . . . . . . . . . . . . . . .
1 10 50
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
50 50
. . . . . . . . . . . . . . . . . . . . . . . . . X x x xx
50 1 12
122
Quercetin,
NTP TR 409
TABLE B3
Statistical Analysis of Primary Neoplasms in Female Rats in the 2-Year Feed Study of Quercetin
Adrenal Medulla: Pheochromocytoma (Benign or Malignant) Overall ratesa 3/50 (6%) Adjusted ratesb 7.8% Terminal rates' 1/30 (3%) 625 First incidence days Life table tests Logistic regression testsd Fisher exact testd
1/13
3/12 (25%)"
1/50 (2%) 3.6% 723 0 P=O.34ON P=0305N P=0.309N
d )
1/28 (4%)
Lung: Alveolar/bronchiolar Adenoma or Carcinoma 5/50 (10%) Overall rates Adjusted rates 14.3% Terminal rates 3/30 (10%) First incidence ( a s dy) 641 Life table tests Logistic regression tests Fisher exact test Mammary Gland Fibroadenoma Overall rates Adjusted rates Terminal rates First incidence ( a s dy) Life table tests Logistic regression tests Cochran-Armitage testd Fisher exact test Mammary Gland: Adenocarcinoma Overall rates Adjusted rates Terminal rates dy) First incidence ( a s Life table tests Logistic regression tests Cochran-Armitage test Fisher exact test
29/50 (58%)
1/20 (5%)e
1/20 (5%)e
060 (0%) 0.0% (0%)
op -
P=0.044N P =0.036N P =0.028N
66.4% 16/30 (53%) 597 P<0.001N P<0.001N Pe0.001N
27/50 (54%) 72.3% 18/28 (64%) 597 P~O.531 P=O.553N P-0.4U)N
O b 0 (0%) 0.0% (0%)
16/50 (32%) 38.4% 1OB5 (29%) 605 P =0.O08N P=O.O08N P =0.O08N
3/50 (6%) 7.9% 2/35 (6%) 660 P= . 4 030 P=O.304
9/50 (18%)
30.1% 8/28 (29% 549 P<0.001N P<0.001N
P<0.001N
2/50 (4%) 6.0% 0128 (0%)
1/50 (2%) 2.5%
0/30 (0%)
672 P =0.299 P=O.309 P=O.318
P=0.521N P=O.492N P=O.500N
27/50 (54%)
686
P=O.468 P=O.492
P=O.309
P=O.500
11/50 (22%) 34.3% 8/28 (2%) 549 P=O.o02N P<0.001N P<0.001N
Mammary Gland Fibroadenoma or Menocarcinoma 30/50 (60%) Overall rates 67.2% Adjusted rates 16/30 (53%) Terminal rates 597 dy) First incidence ( a s P<0.001N Life table tests P<0.001N Logistic regression tests P<0.001N Cochran-Armitage test Fisher exact test
72.3% 18/28 (64%) 597 P=0.538N P=0.468N P=O.343N
3% 17/50 ( 4 ) 40.8% 11/35 (31%) 605 P=O.O08N P =0.009N
P=O.O08N
Lesions in Female Rats
13 2
TABLE B3
(continued)
Statistical Analysis of Primary Neoplasms in Female Rats i the 2-Year Feed Study of Quercetin n
Pancreatic Islets: Adenoma Overall rates Adjusted rates Terminal rates First incidence (days) Life table tests Logistic regression tests Fisher exact test Pituitary Gland (Pars Distalis): Adenoma Overall rates Adjusted rates Terminal rates First incidence ( a s dy) Life table tests Logistic regression tests Cochran-Annitage test Fisher exact test
2/44 (5%) 6.3% If27 (4%) 687
18 (13%)e 1
0.0%
0149 (0%)
(0%)
P=0.245N P=0.225N P=0.221N 27/49 (55%) 68.5% 16/28 (57%) 549 P=0.157N Pe0.062N P =0.039N 28/49 (57%) 71.2% 17/28 (61%) 549 P=0.199N P-0.094N P=O.OM)N
0/50 (0%) 0.0%
37/50 (74%) 80.3% 21/30 (70%) 597 P=O.l66N P=0.064N P=0.056N
31/49 (63%) 74.7% 18/28 (64%) 183 P=0.380N P=0.185N P=0.175N 32/49 (65%) 75.3% 18R8 (64%) 183 P=O.446N P=0.239N P=0.235N
3/50 (6%)
35/50 (70%) 77.5% 25/35 (71%) 441 P=0.218N P=0.441N PsO.412N 35/50 (70%) 77.5% 25/35 (71%) 441 Ps0.218N P=0.441N P=0.412N
Pituitary Gland (Pars Distalis): Adenoma or Carcinoma Overall rates 37/50 (74%) Adjusted rates 80.3% Terminal rates 21/30 (70%) First incidence (days) 597 P=0.197N Life table tests Logistic regression tests P=0.083N Cochran-Annitage test P=0.073N Fisher exact test Skin (Subcutaneous Tissue): Fibroma Overall rates Adjusted rates Terminal rates First incidence ( a s dy) Life table tests Logistic regression tests Cochran-Annitage test Fisher exact test
2/50 (4%) 6.3% 1/30 (3%) 718 P=0.114N P=0.108N P=0.107N
10.2% (7%) 704 P=O.461 P=O.449
2.1%
1/50 (2%)
0/35 (0%)
521 Pz0.475N PsO.470N P=0.500N
2/50 (4%)
(0%)
P=O.266N P=O.256N P=0.247N
0150 (0%) 0.0% 0/28 (0%)
P=O.500 3/50 (6%) 10.2% (7%) 704 P=O.461 P=O.449 P=O.500
Skin (Subcutaneous Tissue): Fibroma or Fibrosarcoma Overall rates 2/50 (4%) Adjusted rates 6.3% Terminal rates 1/30 (3%) dy) 718 First incidence ( a s P=O.lU)N Life table tests P=0.105N Logistic regression tests P=O.l12N Cochran-Annitage test Fisher exact test
4.1% on5 (0%) 441 P=O.672N P=O.608N Pz0.691N
P =0.266N
P=O.256N P=0.247N
124
Quercetin, NTP TR 409
TABLE B3
(continued)
Statistical Analysis of Primary Neoplasms in Female Rats in the 2-Year Feed Study of Quercetin
Overall rates Adjusted rates Terminal rates dy) First incidence ( a s Life table tests Logistic r e p i o n tests Cochran-Armitage test Fisher exact test
Skin (Subcutaneous Tissue): Fibroma, Fibrosarcoma, or Sarcoma
2/50 (4%) 6.3% 1/30 (3%) 718 P=O313N P-0.449 P=O.262N P=0.297N
704
3/50 (6%) 10.2% 2/28 (7%)
2/50 (4%)
4.1%
P=O.461 P=O500 3/43 (7%) 12.5% 3/24 (13%) 723 0
441 P=O.672N P-O.608N
OB5 (0%)
2m
1/50 (2%)
284 P=O.527N P=O.339N
o m (0%)
P=0.691N 4/47 (9%) 11.8% 3/32 (9%) 709 P=0.325N P=0.351N P=0.410N 2/47 (4%) 5.7% 1/32 (3%) 707 P=O.666N P=O.669 P=O.668 6/47 (13%) 17.1% 4/32 (13%) 707 P=0.334N P=0.408N P =0.436N
0/50 (0%) 0.0%
O B 5 (0%)
P=O.500N
2/50 (4%)
Thyroid Gland (Cell): Adenoma Overall rates Adjusted rates Terminal rates First incidence (days) Life table tests Logistic regression tests Cochran-Armitage test Fisher exact test Thyroid Gland (Ccell): Carcinoma Overall rates Adjusted rates 12.5% Terminal rates dy) First incidence ( a s Life table tests Logistic regression tests Cochran-Annitage test Fisher exact test
6/50 (12%) 20.0%
6/30 (20%) 723 0 P=O.358N P=0.179N P=0.358N P=0.171N P=O.l62N
6.4% 1/28 (4%) 688 P=0.154N P=0.161N P=0.134N
1/50 (2%) 3.6% 1/23(4%) 723 0 P=O.528N P=0.521N
P=0.324N 3/43 (7%) 3/24 (13%) 723 0
2/50 (4%) 5.6% 1/30 (3%) 660 P=O.408 P=0.294N P=O.371 P=O.282N P=O.UlN
P=O.428 6/43 (14%)
P=OS00N
3/50 (6%)
Thyroid Gland (Ccell): Adenoma or Carcinoma Overall rates 6/50 (16%) Adjusted rates 25.2% Terminal rates 7/30 (23%) dy) First incidence ( a s 660 Life table tests P=0.561N P=0.095N Logistic regression tests P=O.609 P=0.087N Cochran-Armitage test P=O.082N Fisher exact test Tongue: Squamous Cell Carcinoma Overall rates Adjusted rates Terminal rates dy) First incidence ( a s Life table tests Logistic regression tests Cochran-Annitage test Fisher exact test
0/50 (0%)
0.0% o (0%) m
25.0%
6/24 (25%) 723 0
9.9%
2/28 (7%)
688 P=O.l23N P=O.lWN
P=O.508N
0/50 (0%) 0.0% o m (0%)
P=0.100N
2/50 (4%) 4.7% o m (0% 492 P=O.227 P=O.327
P=O.O39 P=O.047 P= o m 2
P=O.247
Lesions in Female Rats
125
TABLE B3
(continued)
Statistical Analysis of Primary Neoplasms in Female Rats in the 2-Year Feed Study of Quercetin
Overall rates Adjusted rates Terminal rates First incidence ( a s dy) Life table tests Logistic regression tests Cochran-Armitage test Fisher exact test
Uterus: Stromal Polyp
7/50 (14%) 18.8% 4/30 (13%) 597 P =0.262 P =0.308 P=O.314
9/50 (18%) 27.7% 6/28 (21%) 515 P-0.333 P=O.387 P=O.393 11/50 (22%) 31.2% 6/28 (21%) 515 P=O.180 P=O.233 P=O.218 10/50 (20%) 26.3% 3/28 (11%) 504 P=O.420 P=O.586 P=O.500 44/50 (88%) 93.5% 25/28 (89%) 183 P=0.503N P=0.069N P=0.056N 19/50 (38%) 44.0% 6/28 (21%) 183 P=O.213 P=O.437 P=O.263
16/50 (32%) 41.4% 13/35 (37%) 521 P=O.O59 P=O.O28 P=O.o28
11/50 (22%) 33.2% 8r28 (2%)
284
P=O.178 P =0.265 P=O.218 11/50 (22%) 33.2% 8/28 (2%)
Overall r a t e s Adjusted rates Terminal rates First incidence (days) Life table tests Logistic regression tests Cochran-Armitage test Fisher exact test
Uterus: Stromal Polyp or Stromal Sarcoma
7/50 (14%) 18.8% 4/30 (13%) 597 P=O.361 P=O.419 P=O.420
3% 17/50 ( 4 ) 44.0% 14/35 (40%) 521 P=O.o40 P=O.O17
P=O.O17 13/50 (26%) 29.6% 5/35 (14%) 548 P10.323 P=O.258 PL.O.235 42/50 (84%) 87.5% 29/35 (83%) 441 P=0.041N P=0.021N P=0.015N 19/50 (38%) 42.5% lO/35 (29%) 441 P=O.3% P=O.284 P=O.263
284
P=O.178 P=O.265 P=O.218
All Organs: Leukemia (Monocytic
Overall rates Adjusted rates Terminal rates dy) First incidence ( a s Life table tests Logistic regression tests Cochran-Armitage test Fisher exact test
or Mononuclear)
9/50 (18%) 22.8% (10%) 422 P=O.W Pe0.336 PtO.322
12/50 (24%) 32.6% 5/28 (18%) 623 P=O.264 P=O.331 P~O.312 35/50 (70%) 84.9% (79%)
Overall rates Adjusted rates Terminal rates First incidence (days) Life table tests Logistic regression tests Cochran-Armitage test Fisher exact test
All Organs: Benign Tumors
49/50 (98%) 98.0% 29/30 (97%) 422 P=O.O52N P<0.001N P<0.001N
= 284
P=O.O6ON P<0.001N P<0.001N B / 5 0 (40%) 46.1% 6/28 (21%)
All Organs: Malignant Tumors
Overall rates Adjusted rates Terminal rates dy) First incidence ( a s Life table tests Logistic regression tests Cochran-Armitage test Fisher exact test
15/50 ( 0 ) 3% 36.4% 6/30 (20%) 422 P=O.255 P=O.341 P=O.283
284
P=O.175 P=O.288 P=O.u)l
126
Quercetin,
NTP TR 409
TABLE B3
(continued)
Statistical Analysis of Primary Neoplasms in Female Rats in the 2-Year Feed Study of Quercetin
All Organs: Benign or Malignant Tumors
Overall rates Adjusted rates Terminal rates 441 d y ) First incidence ( a s Life table tests Logistic regression tests Cochran-Armitage test Fiiher exact test
183
49/50 (98%) 98.0% 29/30 (97%) 422 P=O.O59N P=O.492N P=O.373 P=O.476N P=O.l4ON P=O.138N
48/50 ( 6 ) 9% %o .93.7% % 26/28 (93%)
43/50 (86%)
87.8% 29/35 (83%) P=0.107N P=0.035N
45/50 ( 0 ) 9%
25/28 (89%) 284
P=0.541N
P=O.O3ON P=O.SoON
P=O.l02N
Terminal sacrifice o f tumor-bearing animals/number of animals examined. Denominator is number o f animals examined microscopically 'Number for adrenal gland, bone marrow, brain, clitoral gland, epididymis, gallbladder (mouse), heart, kidney, larynx, liver, lung, nose, ovary, pancreas, parathyroid gland, pituitary gland, preputial gland, prostate gland, salivary gland, spleen, testes, thyroid gland, and urinary bladder; for other tissues, denominator is number o f animals necropsied. Kaplan-Meier estimated tumor incidence at the end of the study after adjustment for intercurrent mortality Obsewed incidence at terminal kill Beneath the control incidence are the P values associated with the trend test. Beneath the dosed group incidence are the P values corresponding to pairwise comparisons between the controls and that dosed group. The life table analysis regards tumors in animals dying prior to terminal kill as being (directly or indirectly) the cause of death. The logistic r e g m i o n tests regard these lesions as nonfatal. The Cochran-Armitage and Fiiher exact tests compare directly the overall incidence rates. For all tests, a negative trend or a lower incidence in a dose group is indicated by N. e Tissue was examined microscopically only when it was observed to be abnormal at n e c r o m thus statistical comparisons with the controls are not appropriate. Not applicable; no tumors in animal group.
Lesions in Female Rats
127
TABLE B4a Historical Incidence o f Renal Tubule N e o p l a s m s in U n t r e a t e d Female F344/N Rt a' s
Study Adenoma Incidence in Controls Adenocarcinoma Adenoma Adenocarcinoma
or
Historical Incidence at EG&C Mason Research Institute 4-Hydroxyacetanilide Pentaerythritol tetranitrate Overall Historical Incidence Total Standard deviation Range
a
0150 0150
0150 0150
0150 0150
11499 (0.2%)
0.6% 0%-2%
01499 (0.0%)
11499 (0.2%) 0.6%
0%-2%
Data as of 17 September 1990
TABLEB4b
Study
Historical Incidence o f Oral Cavity N e o p l a s m s in U n t r e a t e d Female F344/N
Ratsa
Incidence in Controls or Mucosa: Oral Oral Mucosa: Oral Papilloma Squamous Papilloma Cell Squamous Carcinoma Cell Squamous Papilloma, Cell
Mucosa: Papilloma,
or Carcinoma
Historical Incidence at EG&G Mason Research Institute 4-Hydroxyacetanilide Pentaerythritol tetranitrate Total Overall Historical Incidence Total Standard deviation Range
a
0150
0150
0150 0150
0150 1/50
1O (1%) nO
31500 (0.6%) 1.0% 0%-2%
01500
41500 (0.8%)
0%-2%
1.0%
Data as of 17 September 1990; includes oral mucosa, tongue, pharynx (palate), tooth (gingiva), and lip
128
Quercetin, NTP TR 409
TABLE B4c Historical Incidence of Mammary Gland Fibroadenomas
Study Historical Incidence at EG&G Mason Research Institute 4-Hydroxyacetanilide Pentaexythritol tetranitrate Total Overall Historical Incidence Total Standard deviation Range
in Untreated Female F344/N
Incidence in Controls
Ratsa
19/50 27/50
46/loo
(46.0%)
178/500 (35.6%) 15.0%
8%-56%
'
Dataas of 17 September 1990
TABLE B4d Historical Incidence of Uterine Stromal Polyps in Untreated Female F344/N Rats'
Study
~~ ~
Incidence in Controls
Historical Incidence at EG&G Mason Research Institute 4-Hydroxyacetanilide Pentaexythritol tetranitrate Total Overall Historical Incidence Total Standard deviation Range
94/500 (19.6%) 5.4% 15/50
8/50
23/loo (23.0%)
12%-30%
'
Data as of 17 September 1990
Lesions in Female Rats
129
TABLE B5
Summary of the Incidence of Nonneoplastic Lesions in Female Rats in the 2-Year Feed Study of Quercetin
Disposition Summary
Animals initially in study 6-Month interim e v o l ~ U 0 ~ 1CMonth interim evalualions Early deaths Natural deaths Moribund sulvivors Terminal sacrifice Moribund Died last week o f study Animals examined microscopically
70
10
19 10
70 10
10
70
10
70 10
10 19
10
4 18
2 13 35
29
1
50
28
27
50
50
50
Alimentary System Intestine large, cecum Necrosis, coagulative, acute Parasite metazoan Lymphoid tissue, hypoplasia Intestine large, colon Parasite metazoan Intestine large, rectum Parasite metazoan Intestine small, duodenum Autolysis Epithelium, pigmentation Intestine small, ileum Epithelium, pigmentation Serosa, fibrosis Intestine small, jejunum Autolysis Epithelium, pigmentation Liver Angiectasis Basophilic focus Clear cell f o c u s
Cyst multilocular Cytoplasmic alteration Degeneration Developmental malformation Eosinophilic focus Fatty change Fibrosis, focal Granuloma Hematopoietic cell proliferation Hemorrhage Hepatodiaphragmatic nodule
Cyst
130
Quercetin, NTP TR 409
TABLE B5
Summary of the Incidence of Nonneoplastic Lesions in Female Rats in the 2-Year Feed Study of Quercetin (continued)
Alimentary System (continued)
Liver (continued) Infarct Inflammation, acute Inflammation, chronic Inflammation, chronic active Inflammation, granulomatous, chronic M i d cell f o c u s Necrosis, coagulative Pigmentation Bile duct, cyst multilocular Bile duct, hyperplasia Centrilobular, necrosis, coagulative
S r s , hemorrhage eo a Mesentery Fibrosis Inflammation, chronic Inflammation, chronic active Inflammation, granulomatous, chronic Mineralization Necrosis, coagulative Pigmentation Pancreas Atrophy Cytoplasmic alteration Ectopic liver Inflammation, chronic Pigmentation Artery, hyperplasia Artery, perivascular, inflammation, necrotizing, chronic Duct, dilatation Stomach, forestomach Acanthosis Edema Erosion Hyperkeratosis Hyperplasia, basal cell Inflammation, acute Inflammation, chronic active Inflammation, necrotizing, chronic active Necrosis, coagulative Ulcer Artery, mineralization Artery, muscularis, mineralization Muscularis, mineralization Submucosa, mineralition
Serosa, fibrosis
Lesions I n Female Rats
131
TABLE B5
Summary of the Incidence of Nonneoplastic Lesions in Female Rats in the 2-Year Feed Study of Quercetin (continued)
Alimentary System (continued) Stomach, glandular Cyst epithelial inclusion Edema Inflammation, chronic active Ulcer Artery, mineralization Epithelium, pigmentation Mucosa, dilatation Muscularis, developmental malformation Muscularis, mineralization Tongue Hemorrhage Inflammation, chronic Arteriole, necrosis, fibrinoid Cardiovascular System Heart Cardiomyopathy Atrium left, thrombus Coronary artery, inflammation, chronic active Coronary artery, inflammation, necrotizing, chronic active Coronary artery, necrosis, fibrinoid Epicardium, fibrosis Endocrine System Adrenal gland Hyperplasia Adrenal gland, cortex Angiectasis Atrophy Congestion Degeneration, fatty Hyperplasia Necrosis, coagulative Pigmentation Thrombus Vacuolization cytoplasmic Adrenal gland, medulla
Hyperplasia Necrosis, coagulative Islets, pancreatic Ectopic tissue Hyperplasia Parathyroid gland Cyst Hyperplasia
Angiectasis
(49)
1 (2%)
132
Quercetin, NTP
TR 409
TABLE B5
Summary of the Incidence of Nonneoplastic Lesions in Female Rats in the 2-Year Feed Study of Quercetin (continued)
Endocrine System (continued) Pituitary gland
Angiectasis Hyperplasia i t ls Pars ds ai ,angiectasis Pars distalis, cyst Pars ds ai ,cyst, multiple i t ls Pars distalis, hemorrhage Pars distalis, hyperplasia Pars distalis, infiltration cellular, histiocyte Pars distalis, pigmentation Pars intermedia, angiectasis Pars intermedia, cyst Pars intermedia, pigmentation Pars nervosa, angectasis Pars nervosa, cyst Thyroid gland Ultimobranchial cyst C c e l l , hyperplasia Follicle, cyst Follicular cell, hyperplasia
(49)
21 (43%)
11 (22%)
6 (12%) 6 (12%)
1 (2%)
(50) 1 (2%) 38 (76%) 2 (4%)
General Body System None Genital System Clitoral gland
Abscess
Cyst Hyperplasia Inflammation, acute Inflammation, chronic Inflammation, chronic active @ary Congestion Cyst Periovarian tissue, cyst Uterus Cyst Hydrometra Inflammation, acute Inflammation, chronic Inflammation, chronic active Metaplasia, squamous Pigmentation ev ,cyst ra Cervix, inflammation, chronic Endometrium, hyperplasia Lumen, exudate
(14) 1 (7%) 3 (21%) 2 (14%) (43%) 6 3 (21%) (50) 3 (6%) 5 (10%) (50) 3 (18%)
(50)
11 (22%)
9 (18%)
2 (4%)
8 (16%)
(14%) 7
Lesions in Female Rats
133
TABLE B5
Summary of the Incidence of Nonneoplastic Lesions in Female Rats in the 2-Year Feed Study of Quercetin (continued)
Hematopoietic System Lymph node Deep cervical, hyperplasia, plasma cell Lumbar, infiltration cellular, histiocyte Lumbar, pigmentation Mediastinal, depletion lymphoid Mediastinal, hemorrhage Mediastinal, infiltration cellular, histiocyte Mediastinal, necrosis, coagulative Mediastinal, pigmentation Pancreatic, ectasia Pancreatic, hemorrhage Pancreatic, infiltration cellular, histiocyte Pancreatic, pigmentation Renal, ectasia Renal, hemorrhage Renal, infiltration cellular Renal, infiltration cellular, histiocyte Renal, pigmentation Lymph node, mandibular Congestion Ectasia Hemorrhage Hyperplasia, plasma cell Infiltration cellular, histiocyte Necrosis, coagulative Pigmentation Lymph node, mesenteric Angiectasis Ectasia Hemorrhage Infiltration cellular, histiocyte Necrosis, coagulative Pigmentation Thrombus Spleen Depletion lymphoid Fibrosis Hematopoietic cell proliferation Hyperplasia, lymphoid Inflammation, granulomatous, chronic Necrosis Pigmentation Thrombus Capsule, hyperplasia Thymus Depletion lymphoid Hemorrhage Necrosis, coagulative
(17)
3 (18%)
1 (6%) 1 (6%)
(12%) 2 (12%) 2 (12%) 2 (12%) 2 (12%) 2
(10)
(0) 3%
1 (10%) 1 (10%)
(12) 1 (8%) 1 (8%) (17%) 2 10 (83%) (67%) 8 1 (8%)
4
(20)
( 2 w
1 (5%) (15%) 3 (15%) 3
(7) 1 (14%) 1 (14%)
134
Quercetin,
NTP TR 409
TABLE B5
Summary of the Incidence of Nonneoplastic Lesions in Female Rats in the 2-Year Feed Study of Quercetin (continued)
0 PPm
1 o PPm , o O
10,Ooo PPm
W o PPm Oo
Integumentary System Mammary gland Galactocele Hyperplasia Hyperplasia, cystic Skin Inflammation, chronic Inflammation, necrotizing, acute Subcutaneous tissue, edema Musculoskeletal System None Nervous System Brain Hemorrhage Hydrocephalus Meninges, hemorrhage Spinal cord Hemorrhage Respiratory System Lung Crystals Foreign body Hemorrhage Infiltration cellular, histiocyte Inflammation, acute Inflammation, chronic Inflammation, chronic active Leukocytosis Metaplasia, osseous Pigmentation Alveolar epithelium, hyperplasia Arteq, mineralization Nose Congestion Capillary, submucosa, thrombus Glands, inflammation, acute Lumen, inflammation, acute
(2)
1 (50%)
(50)
1 (2%)
(20) 3 (15%) 5 (25%)
(20)
2 (10%)
(50)
4 (8%)
14 (28%)
4 (8%)
1 (5%)
3 (15%)
18 ( 6 ) 3%
1 (2%) 1 (2%)
1 (5%) 1 (5%) 1 (5%)
(12)
1 (2%) 1 (2%) 1 (2%)
1 (2%) 1 (2%)
24 ( 8 ) 4% (7)
2 (4%)
3 (15%)
1 (8%) 1 (% 8)
(7)
2 (10%) 6 (0) 3%
14 (28%) (10)
1 (14%)
1 (10%) 1 (10%)
Lesions in Female Rats
135
TABLE B5
Summary of the Incidence of Nonneoplastic Lesions in Female Rats in the 2-Year Feed Study of Quercetin (continued)
Special Senses System Eye cataract synechia Cornea, fibrosis Retina, degeneration Sclera, metaplasia, osseous Sclera, mineralization Harderian gland Hemorrhage Inflammation, acute Inflammation, chronic
Kidney Autolysis Congestion Inflammation, chronic Nephropathy Artery, fibrosis Artery, thrombus Collecting tubule, mineralization Papilla, necrosis, coagulative Pmximal convoluted renal tubule, degeneration, hyaline Pmximal convoluted renal tubule, inflammation, acute Proximal convoluted renal tubule, pigmentation Renal tubule, hyperplasia Renal tubule, hyperplasia, cystic Transitional epithelium, hyperplasia Urinaty bladder Hemorrhage Inflammation, acute Inflammation, chronic Subserosa, mineralization Transitional epithelium, hyperplasia
Urinary System
1 (% 2)
4 (% 8)
137
APPENDIX C GENETIC TOXICOLOGY
SALMONELLA R ~ ~ O L . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ~ . , . . . . . . . . . . . . . . . . . .138 P . CHINESEHAMSTER OVARY CELL CYTOCENFI~CS ASSAYS 18 3 RESULTS 139 TABLE Mutagenicity of Quercetin in Salmonelha @phimwium C1 , 140 TABLE Induction of Sister Chromatid Exchanges in Chinese Hamster Ovary Cells C2 byQuercetin . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 141 TABLE C3 Induction o f Chromosomal Aberrations in Chinese Hamster Ovary Cells byQuercetin 142
. . . . . ... . . . . . . . . . . . . . . . . . . . . . . . ................................................................ . . . . . . . . . . . . . . . . . . . . .. . .
.....................................................
138
Quercetin,
NTP TR 409
GENETIC TOXICOLOGY
SALMONEUA PROTOCOL
Testing was performed as reported by Haworth et uf (1983) and Zeiger el uf (1988). Quercetin was sent to the laboratory as a coded aliquot from Radian Corporation, Austin, TX. It was incubated with the Salmonella typhimurium tester strains TA98 and TAlOO either in buffer or S9 mix (metabolic activation enzymes and cofactors from Aroclor 1254-induced male Sprague-Dawley rat or Syrian hamster liver) for 20 minutes at 37" C prior to the addition of soft agar supplemented with I-histidine and d-biotin and subsequent plating on minimal glucose agar plates. Incubation was continued for an additional 48 hours. Each test consisted o f triplicate plates o f concurrent positive and negative controls and of at least five doses of quercetin. The high dose was limited by toxicity. Tests were repeated for all negative a s y , sas and all positive assays were retested under the conditions that elicited the positive response. In this assay, a positive response is defined as a reproducible, dose-related increase in histidine-independent (revertant) colonies in any one strain/activation combination. An equivocal response is defined as an increase in revertants which was not dose related, not reproducible, or o f insufficient magnitude to support a determination of mutagenicity. A response was considered negative when no increase in revertant colonies was observed after chemical treatment.
CHINESE HAMSTER OVARY CELLCYTOGENETICS ASSAYS
s Testing was performed as reported by Galloway et u f (1985, 1987) and i briefly described as follows. Quercetin was sent to the laboratory as a coded aliquot from Radian Corporation, Austin, Tx It was tested in cultured Chinese hamster ovary (CHO) cells for induction o f sister chromatid exchanges (SCE) and chromosomal aberrations (Abs) both in the presence and absence o f Aroclor 1254-induced male Sprague-Dawley rat liver S9 and cofactor mix. Cultures were handled under gold lights to prevent photolysis o f bromodmxyuridine-substituted DNA Each test consisted o f concurrent solvent and positive controls and o f at least three doses of quercetin; the high dose was limited by toxicity. In the SCE test without S9, CHO cells were incubated for 26 hours with quercetin in McCoy's 5A medium supplemented with 10% fetal bovine serum, I-glutamine (2mM), and antibiotics. Bromodeoxyuridine (BrdU) was added 2 hours after culture initiation. After 26 hours, the medium containing quercetin was removed and replaced with fresh medium containing BrdU and Colcemid, and quercetin incubation was continued for 2 hours. Cells were then harvested by mitotic shake-off, fixed, and stained with Hoechst 33258 and Giemsa. In the SCE test with S9, cells were incubated with quercetin, serum-free medium, and S9 for 2 hours. The medium was then removed and replaced with medium containing BrdU and no quercetin, and incubation proceeded for an additional 25 to 26 hours, with Colcemid present for the final 2 t o 3 hours. Harvesting and staining procedures were the same as for cells treated without S9. In the Abs test without S9, cells were incubated in McCoy's 5 A medium with quercetin for 18 hours; Colcemid was added and incubation continued for 2 hours. The cells were then harvested by mitotic shake-off, fured, and stained with Giemsa. For the Abs test with S9, cells were treated with quercetin and S9 for 2 hours, after which the treatment medium was removed and the ells were incubated for 10 hours in fresh medium, with Colcemid present for the final 2 hours. Cells were harvested in the same manner as for treatment without S9.
Genetic Toxicology
139
Cell cycle delay was anticipated in the Abs test without S9, based on observance of cell cycle progression
in the SCE test, and the incubation period prior to cell harvest was therefore extended to allow accumulation o f sufficient metaphases for analysis.
Cells were selected for scoring on the basis of good morphology and completeness of karyotype (21 f 2 chromosomes). All slides were scored blind, and those from a single test were read by the same person. For the SCE test, 25 to 50 seconddivision metaphase cells were scored for frequency of SCE per cell from each dose; 1 0 to 200 firstdivision metaphase cells were scored at each dose for the 0 Abs test. Exceptions were made in each test when a culture showed high levels of damage which allowed fewer cells to provide a representative sample o f the whole culture, or which made it difficult to locate a r a b l e cells. Classes o f aberrations included simple (breaks and terminal deletions), complex (rearrangements and translocations), and other (pulverized cells, despiralized chromosomes, and cells containing ten or more aberrations). Statistical analyses were conducted on the slopes of the dose-response curves and on the individual dose points. An SCE frequency 20% above the concurrent solvent control value was chosen as a statistically conservative positive response. The probability o f this level o f difference occurring by chance at one dose point is less than 0.01; the probability for such a chance occurrence at two dose points is less than 0.001. Abs data are presented as percentage o f cells with aberrations. For aberration data, both the dose-response curve and individual dose points were statistically analyzed. A statistically significant (PsO.05) difference for one dose point was considered weak evidence for a positive response (+w); significant differences for two or more doses indicated the trial was positive (+) (Galloway et al., 1987).
RESULTS
OO , Exposure to quercetin (0.3 to 1 O &plate) produced a strong, dose-related increase in gene mutations in Salmonella typhimurium strains TAlOO and TM8 in the presence and in the absence of Aroclor 1254-induced male Sprague-Dawley rat or Syrian hamster liver S9 (Table Cl). In cytogenetic tests with CHO cells, quercetin induced marked increases in both SCE and Abs, with and without Aroclor 1254-induced male Sprague-Dawley rat liver S9 (Tables C2 and C3). In the SCE test without S9, positive responses were observed over a dose range of 0.67 to 20 pg/mL quercetin; with S9, effective doses ranged from 2 to 45 pg/mL. In the Abs test, the trials conducted in the absence of S9 activation employed a delayed harvest protocol to offset quercetin toxicity; positive responses occurred with 10 to 50 pg/mL quercetin. With S9, standard harvest times were employed and strong increases in aberrations were observed with 25 to 75 pg/mL quercetin. At the highest dose (75 pg/mL), 100%of the cells scored contained aberrations.
140
Quercetin,
NTP TR 409
TABLE C1
Mutagenicity of Quercetin in Salmonella typhimuiuma
Revertants/dateb Strain
Dase (&plate)
0 1 3 10 33 66 100 333 666
-s9
Trial 1 134 2 1 . 58 153 f 91 . 253 2 1 . 36
440 f 2 . 67 467 f 1 . 92 512 f 29.1d Positive 402 2 . 37
Trial 2
Til 1 ra
+30%hamster S9
Trial 2
Trial 1
+x&
rat s 9 Trial 2 157 f 164 +. 142 f 306 f 517 f 613 f 67 . 1. 41 58 . 2. 94 1. 98 326
TU00
134 f 3 4 . 156 f 4 4 .
222 f 1 . 36
57 123 f 1 . 106 f 43 . 132 f 7 8 2% f 1 . 78 449 f 7 2 . 828 f 21 2 .
.ooo
0.0
. 303 f 8 2 341 f 2 . 70 426 f 2 . 20
Positive 466 f 1 . 36
1 1 f 88 4 157 f 1 . 45 172 f 10.2 271 f 65 . 566 f 3 . 87 798 f 3 . 17
798 f 3 . 24
Trial summary Positive control'
Positive 654 f 4 . 56
Positive 553 f 5 . 43
Positive 615 f 4 . 10
Positive 498 98 .
TA98
03 . 1.o 30 . 60 . 1. 00 33.0 100.0 330 3. 666.0
1,Ooo.o
17 f 0 9 . . 78 f 4 3 404 2 98 .
1 f 9 22 f 27 53
* *
06 . 27 . 06 . 20 .
27 f 3 7 .
37 2 19 . 77 f 84 .
88 169 +. 1 . 223 2 3 8 .
63 549 f 1 . 92 576 f 3 . 671 f 28.2
Positive 495 25.5
401 f 2 . 43 7 f 64.7 % 35 916 f 6 .
07 2 . 9 2 f 31 6 . . 3 2 48 1 51 f 38 . 23 162 f 1 . 283 f 24.2
2 2 9
20 .
. 37 f 4 4 68 f 4 0 .
686 f 4 . 64 1053 f 5 1 . 1,116 f 6. 09
30 f 30 f 37 50 f 199 f 381 f
07 . 06 . 1.0 25 . 90 . 3. 30
Trial summary Positive control
a
Positive 452 1 . 86
Positive 367 f 3 . 09
Positive 436 f 2 5
168 f 73 .
Positive
160 f 8 7 .
Positive
Study performed at SRI, International. The detailed protocol is presented in Haworth et aL (1983)with modifications as described by Zeiger et aL (1988). Revertants are presented as mean f standard error from 3 plates. 2-aminoanthracene was used on both strains in the presence o f S9. In the absence of metabolic activation, 4-nitro+-phenylenediamine was tested on TA98,and sodium azide was tested on TA100. Slight toxicity
Genetic Toxicology
141
TABLE C2
Induction of Sister Chromatid Exchanges in Chinese Hamster Ovary Cells
Dose WmL)
by Quercetina
Relative
SCW
Compound
Total cells
Chromo-
No. of
soma
N . of o
SCES
Chromosome8
scw
-11
Hrs SCWChromoin BIW some () %b
Trial 1 Summary: Positive Dimethylsulfoxide Mitomycin-C Quercetin
2. 5 50 8 001 .0 0.010 06 .7 2.00 6.70
50
82 . 1,0500 3 .9 1,044 105
40 1
663 11 8
06 .3 17 .2 09 .9 05 .3 08 .8 10 .3
1. 33 3. 62 2. 08 1. 13 1. 84 2. 17
2. 58 2. 58 25.8 2. 58 2. 58 2. 58
6.4 26 341.47 155.36* 3.4 78. 126.55. 161. 6.4 P<O.001C
2.0 00
50 50 5 50
1,044 1,046 104 1,046
101 ,4 563 92 1,087
+s9
Trial 1 Summary: Positive
Dimethylsulfoxide Cyclophosphamide Quercetin
04 .0
50
1,043 1,048 104 108 ,4 1,043 101 ,4
404
03 .8
81 . 113 1 2. 5.0 3 .3 9 5 3 81 . 3 1. 01 1. 17 1. 19
2. 58 2. 58 2. 58 2. 58 2. 58 2. 58 2.5 46. 4.0 53.
20 .0
50
613 169
506 597
05 .8 16 .2
04 .8 05 .6 05 .7
20 67 . 2. 00
50 50 50
587
48.06.
P<O.ool
T U
Summary: Positive Dimethylsulfoxide Cyclophosphamide Quercetin 04 .0 20 .0
2 5
522 521
103
180
323
-2.30
03 .4 06 .1 22 .3 05 .1 0.58 07 .9
72 . 575 46.04 . 8 1. 09 1. 23 1. 66 7.7 1999 2.
2. 53 2. 53 2. 53 2. 53 2. 53 2. 53 5.4 05. 7.6 04. 100. 3.0 P<O.001
25 5
25 2 5 25
3. 00 4. 50
20.0
524 524 522
272 308 414
Positive (220% increase over solvent control) Study performed at Litton Bionetics, Inc. SCE = sister chromatid exchange; BrdU = bromodeoxyuridine. A detailed description of the SCE protocol is presented by Galloway et d ( 9 5 18, 18) 97. Percent increase in SCEskhromosome of culture exposed to quercetin relative to those of culture exposed to solvent. Values at 0 least 2% above control levels are considered positive. Significance of relative SCWchromosome tested by the linear regression trend test v . log o f the dose s
142
Quercetin, N T P TR 409
TABLEC3 Induction of Chromosomal Aberrations in Chinese Hamster Ovary Cells by Quercetina
439 +59
Abd
Dose WmL)
Total Cells
No. of
Abs
cell
cells with Abs
Percent
Dose
OglmL)
Total cells
N . of o
Abs
Abs/
cell
Percent
Cells
with
Abs
Trial 1 - Harvest time: 20.2 hours Summary: Positive
Dimethylsulfoxide Mitomycin-C
0.05
Trial 1 - h e s t time: 120 hours
Summary: Positive
1.o
26.5 72.0 3.5
200
200
2 95
38
00 .1
0.48 1.52
0.04
Dimethylsulfoxide Cyclophosphamide
7.5
200
200
5
62 42
00 .3 03 .1 1.68
2.5 14.5 56.0 45.0.
0.08
25
2QO
375
Quercetin
25.2 50.3
25
Quercetin
25.2
1. 01
7.6
200 200
7 37 102
01 .9
0.51
1.. 00 2.. 15
P<0.001b
7. 50
20 48
25
27 171
58
0.56 6.84
2.90
100.0.
3.. 33
P<O.ool
Trial 2 - Harvest time: 1 . hours 97 Summary: Positive
Dimethylsulfoxide Mitomycin-C
0.05 0.08
100
100
25
00 .1
0.45 1.00
1.0
30.0 60.0
45 25
Quercetin
25.0 37.5 50.0
100 100 100
25 42
19
0.19 0.25 0.42
1.. 50 2.. 90
P<O.001
7.0.
Positive (PsO.05) Study performed at Litton Bionetics, Inc. A b = abemtions. A detailed presentation of the technique for detecting l 18, 18) 97. chromosomal aberrations i s found in Galloway et a . ( 9 5 Significance of percent cells with aberrations tested by the linear regression trend test vs. log o f the dose
143
AND
TABLE l D TABLE D2
APPENDIX D ORGAN WEIGHTS ORGAN-WEIGHT-TO-BODY-WEIGHT RATIOS
....... ......
144
15 4
OrganWeightsandOrgan-Weight-to-Bodyweight Ratios for Rats at the 6-Month Interim Evaluations in the 2-Year Feed Studies of Quercetin OrganWeightsandOrgan-Weight-to-Body-Weight Ratios for Rats at the 15-Month Interim Evaluations i the 2-Year Feed Studies of Quercetin n
144
Quercetin,
NTP TR 409
TABLE l D
Organ Weights and Organ-Weight-to-Bodyweight Ratios for Rats at the &Month Interim Evaluations i the 2-Year Feed Studies of Quercetin" n
Male
n Necropsy body wt
Brain Absolute Relative R. Kidney Absolute Relative Liver Absolute Relative
9 416 f 10 1.93 f 0.03 4.66 f 0.11 1.17 f 0.05 2.79 f 0.08 12.87 f 0.35b 30.9 f 0 9
9 417 f 7 l.% f 0.02 4.69 f 0.05 1.22 f 0.04b 2.92 f 0.12b 1268 f 0.45 30.3 f 0.8
10 397 f 6 1.72 f 0.11 4.36 f 0.30 1.13 f 0.04 2.83 f 0.09 12.25 f 0.34 30.8 f 0.6
10 401 f 8 l.% f 0.02 4.88 f 0.07 1.29 f 0.02. 3.23 f 0.09.. 13.66 & 0.32 34.1 +. 0.6..
Female
n Necropsy body wt
Brain Absolute Relative R. Kidney Absolute Relative Liver Absolute Relative
10 243 f 5 1.82 2 0.03 7.48 2 0.10
0.68 0.01 2.81 2 0.04
30.0 f 0.7
10 245 f 6 1.83 f 0.03 7.48 f 0.14 0.69 0.02 2.84 f 0.05 7.42 f 0.20 30.3 f 0.5
10 234f4 1.84 f 0.03 7.90 f 0.13.
0.68 2 0.01
10 214 2 5.. 1.87 f 0.02 8.74 f 0.17.. 0.66 3.06
2.89 f 0.03 7.50 f 0.23 32.0 2 0.6.
* 0.02
; t
0.05..
7.31 f 0.25
.
LZ
6.88 0.20 32.1 f 0.4.
Significantly different (PsO.05) from the control group by Williams' or Dunnett's test PrO.O1 Organ weights are given in grams; organ-weight-to-body-weight ratios are given as mg organ weight& body weight (mean f standard error). n-10
Organ Weight Analyses
145
TABLED2
Organ Weights and Organ-Weight-to-Bodyweight Ratios for Rats at the 15-Month Interim Evaluations in the 2-Year Feed Studies of Quercetina
Male
n
NecrolpqrbodyW
10 460 f 12
466f8
10
459 f 14 2.04 f 0.03 4.48 f 0.14 1.44 f 0.04 3.16 f 0.07 15.23 2 0.48 33.2 f 0.5
10
15 456
Brain Absolute Relative R.Kidney Absolute Relative Liver Absolute Relative
2.07 f 0.03 4.53 0.09
2.06 f 0.02 4.44 f 0.08 1.51 f 0.06 3.21 f 0.17 15.12 f 0.63 32.4 f 0.9
4.53
2.05 f 0.03 0.11
1 4 f 0.05 .4 3.15 f 0.09
15.66 2 0.65
34.0 f 1.0
1.59 2 0.05 3.49 f 0 . 0 6 8
17.40 0.75 38.1 f 1 0 .-
Female
n Necropsy body wt Brain Absolute Relative R. Kidney Absolute Relative Liver Absolute Relative
324
10
&
10 337 f 8 1 9 f 0.02 .0 5.65 f 0.13
0.93 f 0.02 2.77 f 0.07
307
10
*6
10 287 zt 6.. 19 . 0 0.02 6.65 f 0.15'' 0.88 f 0.02 3.08 f 0.09** 9.53 0.34 33.2 f 0.8..
5.88
1 9 +. 0.03 .0 0.12
1 8 f 0.02 .9 6.20 f 0.13 0 8 f 0.02 .7 2.85 f 0.04 8.90 29.1
0.89 f 0.03
2.74
0.06
9.21 28.5
* 0.21
2
0.6
9 4 f 0.31 .4 27.9 0.4
* 0.28 * 0.8
* Significantly different (PsO.05) from the control group by Williams' or Dunnett's test * * PsO.01
standard error).
Organ weights are given in grams; organ-weight-to-body-weight ratios are given as mg organ weight/g body weight (mean rt
APPENDIX E HEMATOLOGY, CLINICAL CHEMISTRY, AND URINALYSIS RESULTS
TABLEE l
TABLEE2
Hematology,ClinicalChemistry,andUrinalysisDatafor Rats at the &Month Interim Evaluations in the 2-Year Feed Studies of Quercetin Hematology,ClinicalChemistry,andUrinalysisDatafor Rats at the 15-Month Interim Evaluations in the 2-Year Feed Studies of Quercetin
....... ......
148 150
148
Quercetin,
NTP TR 409
TABLE El
Hematology, Clinical Chemistry, and Urinalysis Data for in the 2-YearFeed Studies o Quercetin' f
Rats at the &Month Interim Evalustions
Male
n
Hematology
10
10
10
Erythrocytes (l@/pL) Leukocytes (ld/pL) Segmented neutrophils (ld/pL) Lymphaytes (1"J Monocytes (ld/hL) Eosinophils (ld/pL) Nucleated erythrocytes (ld/pL)
Clinical chemistry BUN (mg/dL) Creatinine (mg/dL) Sodium (mEq/L) Potassium (mE,q/L) Chloride (mE,q/L) ALT (IU/L) AST (IUL) SDH (IUL) Urinalysis
Urinary potassium (mElq/L)
9.50 f 0.24 5.77 f 0.16 1 4 f 0.12 .1 4.04 f 0.13 0.28 f 0.03
9.39 0.25 5.32 f 0.07. 1.34 f 0.13 3.63 0.18
0.28 f 0.04 0.06 f 0.03 0.01 f 0.01
0.05 f 0.02 0.02 f 0.01
957 f 0.24 4.87 0.17'. 1.40 rt 0.13 3.20 0.200.17 f 0 0 . .3 0.10 f 0.03 0.01 f 0 0 .1
= *
8.78 f 0.25. 5.11 f 0.2014 f 0 1 .5 .4 3.33 f 0.15..
0.21 f 0.03. 0.12 f 0.03 0.00 f 0.00
12.7 f O.Sb 0.70 -C 0.05
147 2 1 3.75 2 0.08 108 2 1 72 f 7 122 f 9
567 f 113b
18.6 & 3.2 0.77 f 0.05 147 f 1 3.83 k 0.08 109 f 1 63 & Sb 119 & lob 558 f 81d
11.9 & 0.5 0.60 f 0 0 .3 148 f 1c 3.79 f 0 l C .l
109 f 1c 67 & 5 115 2 7 792 f 134
10.7 f 0.57 f 144 f 3.67 f
0.3
0.02.
106 f 0 53 f 4 .
0.07
82 f . 4 647 f 104
Urinary sodium (mEqL)
Urinary chloride (mEq/L)
46 f 14 136 f 21 91 & 19
144 rt 23
50 f 10
105 f 1 8
60 f 11 121 f 17
98 f 16
Xematology, Clinical Chemistry, and Urinalysis
149
T ~ L El E Hematology, Clinical Chemistry, and Urinalysis Data in the 2-Year Feed Studies of Quercetin (continued)
for Rats at the &Month Interim Evaluations
Female n Hematology Erythrocyts (l@/pL) Leukocytes (ld/pL) Segmented neutrophils (ld/pL) Lymphocytes (I@/&) Monocytes ( ~ d / p ~ ) Eosinophils (ld/pL) Nucleated erythrocytes (IdlpL) Clinical chemistry BUN (mg/dL) Creatinine (mg/dL) Sodium (mEqL) Potassium (mEqlL) Chloride (mE!qlL) ALT (IUL) AST ( I U L ) SDH (IUL)
10
10
10
10
0.11 f 0.02 0.02 f 0.01 0.01 f 0.01
8.18 f 3.73 0.78 f 2.80
* 0.22 0.10 * 0.19
0.22
8.75 f 0.16 4.07 i 0.22 0.83 f 0.05 295 f 0.19
0.01
0.24 f om** 0.05 f 0.01
* 0.01
8.73 f 0.17 3.80 2 0.26 0.95 f 0.07b 2 5 0 i 0.15 0.17 2 0.02 0.06 f 0.03 0.02 f 0.01
8.56 f 0.19 3.43 f 0.15b 0.74 f 0.07b 2.60 f 0.17 0.13 f O.OZb
0.01 f 0.01 0.01 f 0.01
17.9 f 1.3 0.54 2 0.03 143 f 0 3.04 f 0.06 107 f 0 30 f l b 65 +: 2b 414 23b
21.7 .C 2.0 0.54 f 0.04 144 f 0 3.13 f 0.10
108
33 2 72 f 3 557 f 62.b
IC_
0.47 144 3.21 108 34 83 535
19.9 f 1.1
f
f O* f 0.14 f1
0.03
* qb
f
f 7.b
85e
21.1 f 1.0 0.42 f 0.04. 144 f 0 3.23 f 0.09 108 f 1 42 f 5 76 f 5. 635 f 76b
UriWllgsi
Urinary sodium (mEqL) Urinary potassium (mEqL) Urinary chloride (mEq/L)
43 f 6 99 +. 16 IO f 13
26 f 2. 61 6 45 3
* *
110 f 23 64 f 10
31
*4
123 92
38 f 4b 22
* * 19
Significantly different (PsO.05) from the control group by Dunns or Shirleys test PsO.01 Mean f standard m r . BUN=blood urea nitrogen; ALT=alanine aminotransferase; AST=aspartate aminotransferase, SDH=sorbitol dehydrogenase. n=9 n=lO n=7 n=g
10 5
Quercetin,
NTP TR 409
TABLE E2
Hematology, Clinical Chemistry, and Urinalysis Data in the 2-Year Feed Studies of Quercetin.
for Rats at the 15-Month Interim Evaluations
Male n Hematology Whrocytes ClO%.L) Leukocytes (Id/&) Segmented neutrophils (Id/&) Lymphocytes (Id/&) Monocytes (ld/gL) Eosinophils (l@/gL) Nucleated erythrocytes (ld/gL) Cinical chemistry BUN (mg/dL) Creatinine(mg/dL) Sodium (mQ/L) Potassium(mEkq/L) Chloride (mQ/L) S D H (IUL) Urinalysis Urinary sodium (mEq/L) Urinary potassium (mEqL) Urinary chloride (mQ/L) 54 A 8 177 f 14 120 f 12 57 f 7 190 f 12 128 f 9 1. f 78 04 f .9 146 2 36 2 .1 110 f 816 f 10 . 00 .5 0 00 .8 1 114b 3. f 24 0.72 f 147 2 37 f .2 94 . 01 .6 1 01 .1 . 1 . f 10 83 04 f 00 .4 .2 147 k 0 3 5 -c 0 0 .4 .8 109 & 1 708 f 73b 1. f 78 0.58 f 147 f 37 f .8 107 345 f 14 . 00 .4 0 00 .6 1 . 349.44 f 0.23 50 f 02 .8 .6 .4 20 f 02 .3 2 7 f 01 .2 .9 0 2 f 00 .4 .4 0 1 f 0.02 .0 00 f 00 .3 .2 96 . 5 02 .1 53 f 0 3 .3 .0 .1 16 f 01 .7 33 f 02 .8 .2 .3 0.20 f 0 0 00 f 00 .8 .3 0 0 f 0.02 .4 1 0 10 10 10
96 f 01 .5 .4 48 f 02 .8 .1 1 5 f 0.16 .6 3 0 f 0.17 .2 0.u) f 0 0 .3 .3 01 2 00 .0 00 f 0 0 .6 .2
.4 9.43 f 0 2 4 9 f 0.33 .9 .9 18 f 01 .9 28 f 01 .7 .8 0 1 f 0.03 .9 0 0 f 0.02 .5 00 .3 00 .2
621 f 70b
108 f 1
63 7b 195 f 13 139 2 gb
%A7 1 1 f 12 4 90 f 10
Hematology, Clinical Chemistry, and Urinalysis
151
TAB= E2
Hematology, Clinical Chemistry, and Urinalysis Data in the 2-Year Feed Studies o f Quercetin (continued)
for Rats at the 15-Month Interim Evaluations
Female
n Hematology Erythrocytes (l@/pL) Leukocytes (ld/hL) segmented neutrophils ( ~ d / p ~ ) Lymphocytes (Id/&) Monocytes (ld/pL) Eosinophils (ld/pL) Nucleated erythrocytes (ld/pL) Clinical chemistry BUN (mg/dL) Creatinine (mg/dL) Sodium (mFiqL) Potassium (mEq/L) Chloride ( m m ) ALT (WL) AST (IUL) SDH (IUL) uriM@iS Urinary sodium (mFq/L) U r i ~ potassium (mEq/L) ~y Urinary chloride (mFiq/L)
10
10
10
10
8.62 f 0.11 3.12 f 0.16 1.05 f 0.06 1.90 f 0.11 0.13 f 0.02 0.04 f 0.01 0.04 f 0.01
8.48 0.12 3.01 f 0.12 0.89 0.04 1.93 f 0.12 0.16 f 0.02 0.03 f 0.01 0.04 f 0.02
8.56 3.10 0.95 1.98 0.15 0.03 0.05
f 0.10
f f f f f
* 0.17
0.07 0.14 0.02 0.01 0.01
8.15 f 0.11.' 3.33 f 0.23 0.91 f 0.10 2 3 f 0.15 2 0.16 f 0.04 0.03 f 0.01 0.04 f 0.01
16.5 1.2 0.55 f 0.04 147 f 0 3.17 f 0.07 110 f 0 29*2 63 f 4 205 f 21
14.0 -t O.Sb 0.62 -c 0.05 147 f 1 3.32 f 0.08 111 f 1 27 f Zb 63 f 2b 214 f 24
15.8 f 0.9 0.54 f 0.02 146 f 1 3.30 f 0.08 111 f 1 29 f 2 67 f 5 180 f 17
17.1 f 1.7 0.59 f 0.04 146 f 0 3.27 2 0.09 111 f 1 33f3 61 3 248 f 35
143 f 7b 110 f 7b
50 f s b
4Of6 113 f 5* 90 f 6.
35 f 7b 121 f 7. 87 6.
29 f 7. 114 f 13.' 78 f 6**b
** PsO.01
a
Significantly different (PsO.05) from the control group by Dunn's or Shirley's test Mean f standard error. BUN=blood urea nitrogen; ALT=alanine aminotransferase; AST=aspartate aminotransferase; SDHtsorbitol dehydrogenase. n=9
153
APPENDIX F CHEMICAL CHARACTERIZATION AND DOSE FORMULATION STUDIES
...... . ................... . ........ ....... . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . Frcum F1 Infrared Absorption Spectrum of Quercetin . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Frcum F2 Nuclear Magnetic Resonance Spectrum of Quercetin . . . . . . . . . . . . . . . . . . . . . . . . . TABLE F1 Preparation and Storage of Dose Formulations i the Feed Studies of Quercetin . . . . . n TABLE F2 Results of Analysis ofDose Formulations in the 2-Year Feed Studies of Quercetin . . .
PROCUREMENT CHARACTERIZATION AND PREPARATION AND ANALYSIS OF DOSE FORMULATIONS TABLE Results of Referee Analysis of Dose Formulations in the 2-Year Feed Studies F3 ofQuercetin
154 155 156 157 158 159 160
.....................................................
14 5
Quercetin,
NTP TR 409
CHEMICAL CHARACTERIZATION AND DOSE FORMULATION STUDIES
PROCUREMEN" AND CHARACTERIZATION Quercetin was obtained in two lots from Freeman Industries (Tuckahoe, NY). Lot no. %93790-05 (anhydrous form) was used during the first year of the studies and lot no. %9-0483-18BL (dihydrate form) was used during the second year of the studies. Identity, purity and stability analyses were conducted by the analytical chemistry laboratory Midwest Research Institute (MRI) (Kansas City, MO). MRI reports on analyses performed in support o f the quercetin studies are on file at the National Institute o f Environmental Health Sciences.
The study chemical, a yellow crystalline powder, was identified as quercetin by infrared, ultravioletbisible, and nuclear magnetic resonance (NMR) spectroscopy. All spectra were consistent with those expected for the structure and with the literature spectra of quercetin, as shown in Figures F1 and F2 (Sudtler Standard Spectra).
The purity o f both lots was determined by elemental analyses, Karl Fischer water analysis, weight loss on drying, NMR, titration, and chromatographic analyses. Titration o f two acid groups was performed in dimethylformamide with 0.1 N tetrabutylammonium hydroxide in methanol:2-propanol (1:9) as the titrant. Thin-layer chromatography was performed withtwosystems: 1) on MN Polyamide-TLC11 plates with methano1:acetylacetone (60.40), and 2) on silica gel plates with to1uene:dioxane:acetic acid:methanol (40:25:2015). After the plates were sprayed with 2,6-dibromoquinonechloroimide, visualization was accomplished with short wave (254 nm) and long wave ( 6 nm) ultraviolet light. 36 2,2',4,4'-Tetrahydroxybenmphenone in absolute ethanol (1 p L of a 10 mglmL solution) was used as the reference standard. High-performance liquid chromatography (HPLC) was performed with a p b n d a p a k C& column and a mobile phase mixture o f two solvents: A) water with pH adjusted to 2.0 with concentrated phosphoric acid and B) methanol with an equal volume of phosphoric acid as added in solvent A. The ratio o f solvents used was 5248 (AB), at a flow rate of 1 mL/minute. Ultraviolet detection was at 254 nm.
For the anhydrous form, elemental analyses for carbon and hydrogen showed carbon was low and hydrogen was slightly high. Weight loss on drying indicated the presence o f 1%to 3% water. NMR quantification indicated the presence of 2.4% water. Titration o f two acid groups indicated a purity o f 100.8 f 1.1%. This method would not necessarily distinguish between quercetin and other nonphenolic acid components or quercetin-like compounds. Thin-layer chromatography indicated a major product spot, a minor spot, and a trace by solvent system 1, and a major spot and two t r a m by solvent system 2. HPLC indicated three impurities with a combined area of 6.6% relative to the major peak. The largest impurity (6.4% by peak area) was identified as ellagic acid by spectroscopy and mass spectrometry. Quantitation against an ellagic acid standard resulted in an estimate of the impurity level of 2.6%(whv). The overall purity is estimated at approximately 95% as the anhydrous form. For the dihydrous form, elemental analyses for carbon and hydrogen showed carbon was slightly high, but the value for hydrogen agreed with the theoretical value. Karl Fischer analysis indicated 11.2 2 0.5% water, which is consistent with the theoretical value for the dihydrous form. Weight loss on drying indicated the presence of9.1 f 0.1% water. Titration of acid groups indicated a purity o f 112.5 f 0.4%. Thin-layer chromatography indicated a major product spot and two t r a m by both solvent systems. HPLC indicated three impurities with areas greater than 0.1% relative to the major peak and a combined relative area o f 3.5%. The largest peak (3.1% by peak area) was identified as ellagic acid by spectroscopy and mass spectrometry. Quantitation against an ellagic acid standard
Chemical Characterization and
D s Formulation oe
155
resulted in an estimate o f the impurity level of1.1%(whv). approximately 98% as the dihydrate.
The overall purity is estimated at
Stability studies performed by HPLC with the system described above but with a flow rate of 2.0 mL/minute and with acetanilide added as an internal standard indicated that quercetin, when stored protected from light and under a nitrogen headspace, was stable as a bulk chemical for 2 weeks at temperatures up to 60" C. During the 2-year studies, the stability of the bulk chemical was monitored by the study laboratory using HPLC, with the system described above and with a flow rate of 1.0 muminute, and infrared spectral analysis; no degradation of the study material was seen throughout the studies. PREPARATION AND ANALYSIS OF DOSEFORMULATIONS The dose formulations were prepared layering a premix, prepared by grinding equal amounts of quercetin and feed with a mortar and pestle, with the remainder of the feed in a blender, (PattersonKelley N n Shell with intensifier bar) and mixing for 15 minutes (Table Fl). Studies were conducted by the analytical chemistry laboratory to determine homogeneity and stability o f the dosed feed preparations. For homogeneity analyses, the formulations were extracted with methano1:acetic acid (W1) and the absorbance o f the samples was measured versus methanol by ultraviozet spectroscopy at 370 nm. Concentrations were calculated using a standard curve. For the stability studies, a methano1:hydrochloric acid (99.5:OS) solution was used for extraction and the extract injected into an HPLC system equipped with a pBondapak C,, column and a 254 nm detector. The mobile phase was a mixture o f two solvents: A) 1.2 mL phosphoric acid and 800 mL water, with pH approximately 2, and B) 1.2 mL phosphoric acid and 800 mL methanol. The ratio o f solvents used was 4 : 0 (AB) at a flow 06 rate of 2 muminute. Visible detection was at 254 nm. Quercetin at the 10,OOO ppm dose level mixed in rodent feed (NIH-07 Rat and Mouse Ration) produced a homogeneous blend and was found to be stable when stored at temperatures up to 25" C. There was a 3% loss o f chemical in feed stored 2 weeks at 45" C. Periodic analyses o f the dose formulations of quercetin were conducted at the study laboratory and at the analytical chemistry laboratory using ultraviolet spectroscopy. During the 2-year studies, the dose formulations were analyzed at least once every 8 weeks. All formulations were within the specified 10% of the target concentrations. Results o f the dose formulation analyses studies are presented in Table F2. Results o f periodic referee analysis performed by the analytical chemistry laboratory indicated good agreement with the results obtained by the study laboratory (Table F3).
156
Quercetin, NTP TR 409
NOISSIWSNVHl l N 3 3 H 3 d
FIGURE F1 Infrared Absorption Spectrum of Quercetin
Chemical Characterization and
D s Formulation oe
157
kl313WOki133dS UWN ZHVU 09 OSE-YY3
v)
i
b
W
Q Q
FIGURE F2 Nuclear Magnetic Resonance Spectrum of Quercetin
158
Quercetin,
NTP TR 409
TABLE l F
Preparation and Storage of Dose Formulations in the Feed Studies of Quercetin
Preparation Dose formulations prepared w e l . Chemical-feed premix prepared by grinding quercetin and feed with mortar and pestle; premix eky and remaining feed layered in a blender with intensifier bar and mixed for 15 minutes. Chemical Lot Number 969-3790-0s 969-0483-18BL Maximum Storage Time nvo weeks
Storage Conditions Cold room at approximately 4" C, in opaque plastic bags
Chemical Characterization and Dose Formulation
159
TABLE F2 Results of Analysis of Dose Formulations in the 2-Year Feed Studies of Quercetin
Date Prepared Date Analyzed, Target Concentration (PPm) Determined Concentrationa (PPd
97Ob
% Ditference from Target
17 June 1982 17 June 1982
18 June 1982
-3
21 June 1982
40,Oood 17 August 1982 17 August 1982 9 November 1982 9 November 1982 7 December 1982 7 December 1982
1 March 1983
2 3 39,100'
980 9,970 39,900 980 9,980 4,0 0 0 2
990
952' 980d
-5 -2 -2 -3 -2
-2
18 August 1982 19 August 1982 17 November 1982 18 November 1982
8 December 1982
0 0
-2 0 0 -1
0 +1
9 December 1982 2 March 1983
10,Ooo 40,500
990
9,900 39,800 980 10,200 39,200
960 10,500 41,600
-1 -1 -2 +2 -2
+5 +4
0
-4
-1
5 April 1983
7 April 1983
31 M a y 1983
2 June 1983
19 July 1983 19 July 1983 2 September 1983
20 July 1983
21 July 1983
6 September 1983
w-)oo
970 9,950 39,800
9,900
-1
-3 -1 -1 -2
13 December 1983 13 December 1983
14 December 1983
980 39,400
10,100
15 December 1983
+1 -2
160
Quercetin, NTP TR 409
TABLE F2
Results of Analysis of Dose Formulations in the 2-Year Feed Studies of Quercetin (continued)
Date Prepared Date Analyzed Target Concentration (PPW
1Oo ,o l0,Ooo
Determined Concentration (PPm)
1, O o o 10,400 39,700
% Difference
itom Target
14 February 1984
15 February 1984
4, 0 m
13 March 1984
15 March 1984
+4
-1
-4
4Oo 0o ,
15 May 1984
1Oo ,o 10,Ooo 1Oo ,o 10,Ooo
9,900 38,700 970 10,050
%o
-1 -3
-3 +1 -3
17 May 1984
40,Ooo
38,900
Results of duplicateanalyses Sample selection from top left zone o f PK Blender Sample selection from top right zone of PK Blender Sample selection from bottom of PK Blender
TABLE F3
Results of Referee Analysis of Dose Formulations in the 2-Year Feed Studies of Quercetin
Determined Concentration (DDm) Date Mixed 17 June 1982 7 December 1982 31 May 1983 13 December 1983
a
Target Concentration (PPm)
Study Laboratory. 970 10,Ooo 41,600 10.100
Referee Laboratory 1,020 9,980 40,500 9,560
Resultsofduplicateanalysis Results of triplicate analysis
161
APPENDIX G FEED AND COMPOUND CONSUMPTION IN THE 2-YEAR FEED STUDIES
Rats in the 2-Year Feed Study TABLE G1 Feed and Compound Consumption by Male ofQuercetin TABLE 2 Feed and Compound Consumption by Female Rats in the 2-Year Feed Study 6 of Quercetin
l l l l
..................................................... . .......... . .. ............ .......................
162 163
162
Quercetin,
NTP TR 409
TABLE G1
Feed and Compound Consumption by Male Rats in the 2-Year Feed Study of Quercetin
1 2 3 4 5 8 9 12 13 17 21 25 29 30 33 37 41 45 49 53 57 61 65 68 73 81
89 93 97 101 14 0
85
20.0 19.0 17.7 16.6 17.7 19.0 18.8 19.7 18.1 17.6 17.8 20.8 17.7 23.4 18.6 19.1 18.7 21.1 17.7 24.0 24.6 18.2 17.4 19.0 22.4 17.5 21.2 17.2 17.9 18.5 10.9 24.8
18.5
162 195 225 253 271 310 322 354 376 399 416 434 445 456 457
340
469 481 487 478 485
464 484
492 492 485 476 473 479 447
486
464
270
19.8 17.9 18.6 16.8 17.4 18.7 18.3 21.0 18.1 18.0 17.4 24.6 17.3 22.4 18.7 19.1 18.6 18.5 18.6 21.2 24.2 18.5 17.6 18.8 21.0 18.6 20.8 16.7 18.9 19.2 11.8 24.6 18.5
230
255 272
160 1 %
124 91 81 66 62 57 62 52 48 44 60 40 51 41 41 40 39 38 44 49 38 36 38 42 38 43
304
321 337 350 375 399 412 438 438 456 470
64
460 466 486
487 491 487 491 493 497 492
488
477 482 485 45 1 451 270
35
26
39 40
55
73
19.8 18.0 18.5 17.5 17.6 19.3 18.1 19.1 22.7 19.9 18.7 25.1 17.6 23.1 19.1 20.6 20.0 18.3 19.3 21.1 27.1 19.3 18.2 17.7 21.8 18.3 20.1 17.0 17.0 17.4 10.5 23.1 19.0
233 257 272 312 325 334 343 373 395 430 435
203
165
1,198 889 794
680 648
619 559 572
409 448
458
482 481 483 490 491 483 473 465 450 427
464 464
535 474 613 410 532 426 450 431 395
663
400
488 484
438 555 399 378 361
444
379 418 359 247 525
480
366 386
440
272
18.7 16.7 18.0 19.2 18.4 19.6 18.5 19.9 22.8 17.8 17.5 23.7 17.5 22.6 18.1 21.0 21.3 19.7 21.1 22.5 27.2 19.8 18.6 18.7 22.0 20.2 22.6 19.0 19.6 20.6 12.2 24.8 19.1
252 259
228
167 198
309
320 328 343 382 393 413 426 432 439 442 453 453
364
409
457 453 458 458 451 436 426 418 402 403
460
444
4,495 337 3,145 3,047 2,837 2,529 2310 2,425 2,656 1,954 1,833 2,408 1,6% 2,210 1,701 1,946 1,943 1,784 18 4 ,6 1,989 5369 1,733 1,639 1,631 1.921 1,792 2,037 1,742 1,835 1,972 1,213 5463 5981
weeks 1-13
Mean
736
25 47
Weeks 14-52: Mean 19.2
Weeks 53-104: Mean 19.5
a
440
19.3
440
44
20.2
436
467
20.0
415
1,934
479
19.4
483
40
19.1
472
404
20.6
440
1,872
GCUIISof feed consumed per animal per day
Milligrams of quercetin consumed per day per kilogram of body weight
Feed and Compound Consumption
163
TABLE 2 6 Feed and Compound Consumption by Female Rats i the 2-Year Feed Study of Quercetin n
1 2 5 6 7 8 12 13 17 2 1 25 29 33 37 41 45 49 53 57 61 65 69 73 77
85 89 93 97 101 104
81
1. 23 1. 17 1. 26 1. 24 1. 27 1. 36 1. 44 1. 46 1. 50 1. 38 13.4 1. 55 12.9 1. 42 14.0 1. 44 14.8 1. 46 1. 53 1. 45 1. 54 14.6 1. 49 1. 53
15.5 1. 55 1. 67 1. 06 1. 14 11.5
15.0
138 153 177 187 191
215 215
225
199
233 244 255 257
268
279 292 301 311 319 327 336 343 350 355
365 369 369 360 365 357
184
362
1. 29 1. 26 1. 26 1. 31 1. 30 1. 29 1. 44 14.7 1. 47 13.0 1. 34 1. 43 1. 25 1. 51 1. 53 1. 55 1. 46 1. 53 15.5 1. 61 1. 52 1. 56 1. 49 1. 70 1. 46 15.5 1. 48 1. 80 99 . 1. 15 1. 21 1. 33
141 155 178 186 193 199 214 219 226 233
246
257 263 276 288 299 317 329 337 344 349 355 364
91 81 71 70 67 65 67 67 65 56 55 55 48
55
305
367 371 376 367 368 360
368
53 52 48 48 47 48 44 45 42 47 40 42 40 48 27 3 1 34 72
123 11.6 1. 23 122 124 124 125 1. 44 1. 35 1. 28 13.0 1. 31 1. 18 1. 32 1. 37 13.7 1. 32 13.4 1. 41 14.4 1. 48 1. 37 1. 43 15.5 1. 39 14.9 1. 52 1. 61 8.2 1. 23 1. 25 1. 25
139 152 177 183 189 194 202 217 222 232 237 242 252 261 273 279 290 299 310 320 331 335 340
348 352 355 340 351 349
207
345
886 769 692 668 656 642 619 700 621 579 561 554 488 524 526 502 474 460 471 465 463 416 426 455
405
429 431 453 242 350 359 704
1. 13 1. 21 1. 24 1. 26 121 1. 16 1. 29 1. 38 1. 22 11.7 1. 15 122 1. 16 1. 22 1. 31 13.4 1. 25 13.4 1. 48 15.7 14.8 1. 53 1. 42 1. 54 1. 47 1. 54 1. 44 1. 79 10.1 1. 20 12.2 1. 23
141 152 176 181 186 190 195 192 203
209
220
220
225 231 239 246 248 256 265 277
3,225 3,171 2,813 2,771 259 ,9 2,445 266 ,3 2,885 2, 2,242 2,092 2,207 2,059 2,113
2,m
285 291
296
303 308 311 314 318 312 317 31 1 177
2,185 2,027 2,098 2,244 2,274 2,074 2,100 1,928 2,031 1,913 1,983 1,837 2,249 1,295 1,511 1,564 2,818
W ~ k 1-13: s Mean 1. 31 Weeks 14-52 Mean 1. 42 W ~ k 53-104: s Mean 14.4
a
186
180
261
1. 43
266
54
1. 31
246
537
1. 23
227
2,170
349
14.7
355
42
13.8
333
416
1. 43
297
1,936
Grams of feed consumed per animal per day Milligrams of quercetin consumed per day per kilogram of body weight
165
APPENDIX H INGREDIENTS, NUTRIENT COMPOSITION, AND CONTAMINANT LEVELS IN NIH-07 RAT AND MOUSE RATION
TABLE H1 TABLE H2 TABLE H3 TABLE H4 Ingredients of NIH-07 Rat and Mouse Ration Vitamins and Minerals in NIH-07 Rat and Mouse Ration Nutrient Composition of NIH-07 Rat and Mouse Ration Contaminant Levels in NIH-07 Rat and Mouse Ration
............................. .................... .....................
......................
166 166 167 168
166
Quercetin, NTP
TR 409
TABLE l H Ingredients of NIH-07 Rat and Mouse Ration'
Ingredientsb
Ground #2 yellow shelled corn Ground hard winter wheat Soybean meal (49% protein) FBh meal (60% protein) Wheat middlings Dried skim milk Alfalfa meal (dehydrated, 17% protein) Corn gluten meal (60% protein) Say oil Dried brewer's yeast Dry molasses Dicalcium phosphate Ground limestone Salt Premixes (vitamin and mineral)
a NCI, 1976; NIH, 1978
Percent by Weight
24.50 23.00 12.00 10.00 10.00 5.00 4.00 3.00 2.50 200 1.50 1.25 0.50 0.50 0.25
Ingredients ground to pass through a U.S. Standard Screen No. 16 before being mixed
TABLE 2 H Vitamins and Minerals in NIH-07 Rat and Mouse Ration'
Vitpmine
A
du-Tocopheryl acetate Choline Folic acid Niacin d-Pantothenic acid Riboflavin Thiamine Bl2 Pyridoxine Biotin
K3
D3
5,500,Ooo IU 4,600,OOO IU 2.8 g
20,Ooo IU 560.0 g 2.2 g 3. g 00 18.0 g 3.4 g 10.0 g 4O O l(g ,O 1.7 g 140.0 mg
120.0 g 60.0 g 16.0 g 4.0 g 1.4 g 0.4 g
Stabilized vitamin A palmitate or acetate D-activated animal sterol Menadione Choline chloride d-Calcium pantothenate Thiamine mononitrate Pyridoxine hydrochloride d-Biotin
Mlaerpls
Iron Manganese
Zinc
Iron sulfate Manganous oxide
Zinc oxide Copper sulfate Calcium iodate Cobalt carbonate
Copper Iodine Cobalt Per ton (2,OOO Ib) of finished product
'
Feed Analyses
167
TABLE H3
Nutrient Composition of NIH-07 Rat and Mouse Ration
Nutrients
Protein (% by weight) Crude fat (% by weight) Crude fiber ( I by weight) Ash (% by weight)
Amlno Achls (% of
Mean f Standard
Deviation
Range
21.2-25.9 4258 .-. 2845 .-. 6371 .-. 1.310-1.390 0.218-0.400 1.060-1.210 0.531-0.603 0.881-0.944 1.850-1.990 1.200-1.370 0.665-1.050 0.8244898 0.156-0.671 0.564-0.769 1.050-1.170 1.830-2.520 0.210-0.308 4,u)o-22,OOO 3,ooo-6,300 31.1-48.0 12.0-31.0 6.10-8.20 65.0-150.0 23.0-34.0 1.80-3.70 0.19-0.32 10.638.0 2,40&3,430 10-.3 .414 0.90-1.10 0.772-0.971 0.3804.635 0.258-0.371 0,151-0.181 0.268-0.420 262.0-523.0 81.70-99.40 46.10-58.20 8.09-15.39 1.52-3.82 1.44-2.09 0.490-0.780
5.60-8.80 0.3060.699
Number d Samples
26 26 26 26
5 5 5 5 5 5 5
.9 2 . 5 f 11 29 5.08 f 0 4 .6 .0 35 f 06 .0 6.66 f 0 2 .1 1.320 f 0.072 0.319 f 0.088 1 1 6 f 0.063 .4 0 5 1 f 0.026 .7 0.914 f 0 0 0 .3 1.946 f 0.056 1 2 0 f 0.067 .8 .6 0.436 f 0 1 5 0.938 f 0 1 8 .5 0.855 f 0.035 0 2 7 -C 0.221 .7 0.618 f 0.086 1 1 8 f 0.043 .0 2.290 f 0.313 0.258 f 0.040 11,565 f 4,450 f 43.58 f 18.46 76 f . 4,265 1,382 69 .2 3.89 08 .5 97.8 f 3 . 8 16 30.06 4.31 .1 7.68 1 3 2.62 0.89 0.254 0,053 24.21 f 1 . 6 26 3,122 f 416.8
tow diet)
Arginine Cystine Glycine Histidine Isoleucine Leucine Lysine Methionine Phenylalanine Threonine Tryptophan "Qmsine Valine Essential Fatty Acids (% of totel diet) Linoleic Linolenic
vitamins
5 5 5 5 5 5
5 5
Vitamin A (IU/kg) Vitamin D (IU/kg) a-Tocopherol (ppm) Thiamine (ppm) Riboflavin (ppm) Niacin (ppm) Pantothenic acid (ppm) Pyridoxine (ppm) Folic acid (ppm) Biotin (ppm) Vitamin B (ppb) , Choline (ppm)
Minerals
26 4 5 26 5 5 5 5 5
5 5 5
Calcium (%) Phosphorus ( I ) Potassium ( I ) Chloride (%) Sodium (%) Magnesium (%) Sulfur (%) Iron (PPm) Manganese (ppm) Zinc (PP@ Copper (PP@ Iodine (ppm) Chromium (ppm) (PP4
O.% f 0 0 .5 0.900 f 0.098
1.26
* 0.10 *
.1 0.513 0 1 4 0.323 f 0.043 0,167 f 0.012
0.304 f 0.064
26 26 3 5 5 5
5 5
410.3 f 94.04 90.29 f 7 1 .5 52.78 f 4.94 .6 1.2 f 27 07 2 9 f 10 .5 .5 18 f 0 2 .5 .5 0.681 f 0 1 .4
5 5 5
4 5 4
1% 6
Quercetin, NTP
TR 409
TABLE H4
Contaminant Levels in NIH-07 Rat and Mouse Ration
Contaminants
Mean f Standard
Deviation'
Range
0.18474 0.10-0.20 0.27-2.93 0.21445 250-19.0 0.10-6.10 200-20.0 1.00-13.0 <3.0-m <3.00-15.00 <3.00-150.00 08-03 .03.0
0.50-30.00 6,~20,ooO
Number of Samples
26
Arsenic (ppm) Cadmium (ppm) (PPW Merculy (PPm) Selenium (ppm) Aflatoxins (ppb) Nitrate nitrogen (ppm Nitrite nitrogen (ppm) BHA (PPmY BHT (PPm)C Aerobic plate count (CFU/g)d Coliform (MPN$)~ E. coli (MPN/g) E. coli (MPNg) Total nitrosoarnines (ppb)g N-Nitrosodimethylamine (ppb)g N-Nitrosopyrmlidine (ppb)g
CO.05
0.51 f 0.14 0.12 f 0.04 0.65 f 0.52 0.31 f 0.06
9.66 f 4.49 1.43 f 1.50 4.04 f 4.98 2.92 f 259 146,527 f 143,387 585 f 859 3.83 f 2.6% 9.42 f 28.79 5.30 f 5.98 4.47 f 5.91 0.81 f: 0.65 eo.01 CO.02 <0.01 eo.01 <0.01 eo.01 <0.01 <0.01 <0.01 <0.01 <0.01 <0.01
co.05
<5.0
0.30-2.20
26 26 26 26 26 26 26 26 26 26 26 25 26 26 26
26
Pesticides (ppm)
8-BHC I-BHC 6-BHC Heptachlor Aldrin Heptachlor epoxide DDE DDD DDT HCB Mirex Methoxychloj Dieldrin' Endrin Telodrin Chlordane Toxaphene Estimated PCBs Ronnel Ethion Trithion Diazinon Methyl parathion Ethyl parathion Malathiod Endosulfan I Endosulfan I1 Endosulfan sulfate
c L - ~ ~ ~ h
26 26 26
26 26 26
26 26 26 26
26 0.06
<0.01 <0.01 <0.01 <0.1
<0.2
0.02
<0.05
<0.01 <0.02
<0.05
<0.1 eo.02 0.15 f 0.17 co.01 <0.01 <0.03
<0.02
0.054.81
26 26 26 26 26 26 26 26 26 26 26 26 26 26 26 26 26 26
Feed Analyses
169
TABLE H4
a
Contaminant Levels in NIH-07 Rat and Mouse Ration (continued)
For values less than the limit o f detection, the detection limit is given for the mean. Sources of contamination: alfalfa, grains, and fiih meal Sources of contarnination: say oil and f i h meal CFU = colony-forming unit e MPN = most probable number Excludes one high value of 150 MPN/g obtained from the lot milled on 26 August 1982. g AU values were corrected for percent recovery. B H C = hexachlorocyclohexane or benzene hexachloride i Value and date of one obsewation which was above the detection limit is given under the range. All other values were less than the detection limit. Fifteen lots contained more than 0.05 ppm.
171
APPENDIX I SENTINEL ANIMAL PROGRAM
METHODS
...............................................................
172
172
Quercetin, NTP
TR 409
SENTINEL ANIMAL PROGRAM
Rodents used in the Carcinogenesis Program of the National Toxicology Program are produced in optimally clean facilities to eliminate potential pathogens that may affect study results. The Sentinel Animal Program is part o f the periodic monitoring o f animal health that occurs during the toxicologic evaluation of chemical compounds. Under this program, the disease state o f the rodents is monitored via serology on sera from extra (sentinel) animals in the study rooms. These animals are untreated, and these animals and the study animals are subject to identical environmental conditions. The sentinel animals come from the same production source and weanling groups as the animals used for the studies of chemical compounds. Fifteen F4N rats of each sex were selected at the time of randomization and allocation o f the animals 34 to the various study groups. Five animals o f each designated sentinel group were killed at 6, 12, and 18 months on study. Samples for viral screening at 24 months were collected from 10 diet control animals, 5 per sex. The blood from each animal was collected and clotted, and the serum was separated. The serum was cooled on ice and shipped to Microbiological Associates Comprehensive Animal Diagnostic Service for determination of the antibody titers. The following tests were performed: Method of Analvsis Hemagglutination Inhibition P V M (pneumonia virus of mice) Sendai KRV (Kilham rat virus) H-1 (Toolans H-1 virus) ELISA RCV/SDA (rat corona virus/sialodacryoadenitis virus)
All test results for sentinel animals were negative.
METHODS
Time o f Analvsis 6, 12, 18, and 24 months
6, 12, 18, and 24 months
NATIONAL TOXICOLOGY PROGRAM TECHNICAL REPORTS PRINTED As OF AUGUST 1992
TR No.
201
CHEMICAL
2,3,7,8-Tetrachlorodibenzo-pdiaxin (Dermal) 1,2-Dibromo-3chloropropane Cytembena FD & C Yellow No. 6 2,3,7,8-Tetrachlorodibenzo-p-dioxin(Gavage) 1,2-Dibromoethane C.I. Acid Orange 10 Di(2-ethylhexyl)adipte Butyl Benzyl Phthalate Caprolactam Bisphenol A 11-Aminoundecanoic Acid
TR No.
274 275 276 277 278 279
CHEMICAL
Tris(zethylhslyl)phosphate 2-Chlomthanol 8-Hydroxyquinoline Tremolite 2,6-Xylidine Amosite Asbestos Crocidolite Asbestos HC Red No. 3 Chlorodibromomethane Diallylphthalate (Rats) C.I. Basic Red 9 Monohydrochloride Dimethyl Hydrogen Phosphite 1J-Butadiene Benzene Isophorone HC Blue No. 2 Chlorinated Trisodium Phosphate Chrysotile Asbestos (Rats) Tetrakis(hydroxymethy1) phosphonium Sulfate & Tetrakis(hydroxymethy1) phosphonium Chloride Dimethyl Morpholinophosphoramidate C I . Disperse Blue 1 3-Chloro-2-methylpropene o-Phenylphenol 4-Vinylcyclohexene Chlorendic Acid Chlorinated Parafins (C&, 43% chlorine) Dichloromethane (Methylene Chloride) Ephedrine Sulfate Chlorinated Paraffins (C12, 60% chlorine) Decabromodiphenyl Oxide Marine Diesel Fuel and JP-5 Navy Fuel Tetrachloroethylene (Inhalation) n-Butyl Chloride Mirex Methyl Methacrylate Oxytetracycline Hydrochloride 1-Chloro-2-methylpropene Chlorpheniramine Maleate Ampicillin Trihydrate 1,4-Dichlorobenzene Rotenone Bromodichloromethane Phenylephrine Hydrochloride Dimethyl Methylphosphonate Boric Acid Pentachloronitrobenne Ethylene Oxide Xylenes (Mixed) Methyl Carbamate 1,2-Epaxybutane 4-Hexylresorcinol Malonaldehyde, Sodium Salt 2-Mercaptobenzothiale N-Phenyl-2-naphthylamine 2-Amino-5-nitrophenoI C.I. Acid Orange 3 Penicillin VK Nitrofurazone
207 210 211 212 213 214 215 216 217 219 220 221 222 223 224 225 226 227 228 229
230
206
208 2Q9
280 281 282
284
231 232 233
236
234 235
237 238 239 240 242 243 244 245 246 247 248 249 250 251 252 253 254 255 257 259 21 6 263
266
Di(2ethylhexyl)phthalate 2,6-Dichloro-p-phenylenediamine C.I. Acid Red 14 Locust Bean Gum C.I. Disperse Yellow 3 Eugenol Tara Gum D & C Red No. 9 C.I. Solvent Yellow 14 Gum Arabic Vinylidene Chloride Guar Gum Agar Stannous Chloride Pentachloroethane 2-Biphenylamine Hydrochloride Allyl Isothiocyanate Zearalenone D-Mannitol 1,1,1,2-Tetrachloroethane Ziram
285 287
288
289 291 293 294 295 2%
298 299
267 269 271 272 273
Bis(2chloro-1-methylethy1)ether Propyl Gallate Diallyl Phthalate (Mice) Trichloroethylene (Rats and Mice) Polybrominated Biphenyl Mixture Melamine Chrysotile Asbestos (Hamsters) L-Ascorbic Acid 4,4'-Methylenedianiline Dihydrochloride Amosite Asbestos (Hamsters) Benzyl Acetate 2,4- & 2,6-Toluene Diisocyanate Geranyl Acetate A l l y l Isovalerate Dichloromethane (Methylene Chloride) 1,2-Dichlorobenzene Diglycidyl Resorcinol Ether Ethyl Acrylate Chlorobenzene 1,2-Dichloropropane Monuron , 1,2-Propylene Oxide Telone I I e (1,3-Dichloropropene) HC Blue No. 1 Propylene Trichloroethylene (Four Rat Strains)
301 303 304 305 306 307 308 309 310 311 312 313 314 315 316 317
300
318 319 320 321 322 323 324
325 326 327 328 329
330 331 332 333 334 335 336 337
NATIONAL TOXICOLOGY PROGRAM TECHNICAL REPORTS
PRINTED AS OF AUGUST 1 9 92
TRNO.
372 373 374 375 376 377 378 379 380 361 382
TR No.
338 339 340 341 342 343 344 345 346 347 348 349 350 351 352 353 354 355
385
356
357 358 359 360 361 362 363 364 365 366 367 368 369 370 371
386 387 388 389 390 391 392 393
395
396 397 399 401 403
405
406 407 410 412 415 419
These N T P T e " Reports arc available for sale from the National Technical Information s r c , U.S. Depertment of ev e i Commace, 5285 Port Royal Road, Springfi", VA 22161 (7034874650). S i copies of this Tecbnii RepMt arc availabk without charge ( dw h i i suppliea last) from the Public Health savice, National Tarriodogy Pmgram,central Data Management, a P.O. Box 12233, MD AO-01, Research Triangle Park, NC 27709
DEPARTMENT OF HEALTH B HUMAN SERYICES
National Toaicology Program Central Data Management P.O. b x 12233, MD A M 1
Public Health Service
SPECIAL FOURTH-CLAZ:; M A I L
DHHS /NIX
Research Trianglt P, &
NC m 7 0 9
.'
NIH P u b l i ~ t i O ~ 923140 NO. September 1992
You might also like
- MYMAXICARE REVISED BROCHURE 2023 - FinalDocument16 pagesMYMAXICARE REVISED BROCHURE 2023 - FinalAna Feliciano100% (1)
- TR 321Document185 pagesTR 321shakar azizNo ratings yet
- Chlorpheniramine MaleatDocument200 pagesChlorpheniramine MaleatAchmad Fachry100% (1)
- Preclinical Acute Toxicity Studies and Rodent-Based Dosimetry Estimates of The Novel Sigma-1 Receptor Radiotracer (F) FPSDocument13 pagesPreclinical Acute Toxicity Studies and Rodent-Based Dosimetry Estimates of The Novel Sigma-1 Receptor Radiotracer (F) FPSirfanjadoonNo ratings yet
- Supporting Online Material For: Linking Long-Term Dietary Patterns With Gut Microbial EnterotypesDocument22 pagesSupporting Online Material For: Linking Long-Term Dietary Patterns With Gut Microbial EnterotypesRitaSantosNo ratings yet
- Quercetin: 1.1 Chemical and Physical DataDocument19 pagesQuercetin: 1.1 Chemical and Physical DataDian Ayu UtamiNo ratings yet
- Artículo BiodisponibilidadDocument9 pagesArtículo BiodisponibilidadLuis Enrique SanchezNo ratings yet
- Kukanich 2020Document10 pagesKukanich 2020Gianne K. SantosNo ratings yet
- Literature Review On Higher Plants For Toxicity TestingDocument8 pagesLiterature Review On Higher Plants For Toxicity Testingc5p8vze7No ratings yet
- Thiabendazole (WHO Food Additives Series 39)Document7 pagesThiabendazole (WHO Food Additives Series 39)hellen86150No ratings yet
- Gastric Lavage - Position Statement 2013Document7 pagesGastric Lavage - Position Statement 2013UrgenciasCol FoamNo ratings yet
- Current Databases On Biological Variation: Pros, Cons and ProgressDocument10 pagesCurrent Databases On Biological Variation: Pros, Cons and ProgressCarlos PardoNo ratings yet
- Effects of LifePak Supplementation On Antioxidant Status and LDL-Oxidation in Healthy Non-SmokersDocument12 pagesEffects of LifePak Supplementation On Antioxidant Status and LDL-Oxidation in Healthy Non-SmokersCherry San DiegoNo ratings yet
- Anthropometrical Parameters and Markers of Obesity in RatsDocument9 pagesAnthropometrical Parameters and Markers of Obesity in RatsSammer BurgosNo ratings yet
- Rosenkrantz 1988Document12 pagesRosenkrantz 1988NoemiNo ratings yet
- Artikel Penelitian Sistem EndokrinDocument9 pagesArtikel Penelitian Sistem EndokrinMusyamuNo ratings yet
- PIIS0015028207002786 Phytoestrogens in Clinical PracticeDocument7 pagesPIIS0015028207002786 Phytoestrogens in Clinical PracticeGeorge CarpNo ratings yet
- Faecal Microbial Flora and Disease Activity in Rheumatoid Arthritis During A Vegan DietDocument5 pagesFaecal Microbial Flora and Disease Activity in Rheumatoid Arthritis During A Vegan DietHendra SudaryonoNo ratings yet
- Invited ReviewDocument24 pagesInvited ReviewMadhav 360No ratings yet
- Fin 2104Document6 pagesFin 2104Prima Artya KurniawanNo ratings yet
- Adverse Effects of Incorporating Ketoprofen Into Established Rodent StudiesDocument11 pagesAdverse Effects of Incorporating Ketoprofen Into Established Rodent StudiesVivi FatimatuzzuhroNo ratings yet
- Animal-Derived Surfactants Versus Past and Current Synthetic Surfactants: Current StatusDocument33 pagesAnimal-Derived Surfactants Versus Past and Current Synthetic Surfactants: Current StatusSiska LesnussaNo ratings yet
- A Vegan Diet Alters The Human Colonic Faecal MicrobiotaDocument8 pagesA Vegan Diet Alters The Human Colonic Faecal MicrobiotaWilver Edson Otalora AnturianoNo ratings yet
- Barlow 1999Document11 pagesBarlow 1999asdsffggeettrgbfbfbftggrg ergrtertererefrerrNo ratings yet
- Hypobaric Hypoxia Causes Impairment of Spermatogenesis in Developing Rats at Pre-PubertyDocument7 pagesHypobaric Hypoxia Causes Impairment of Spermatogenesis in Developing Rats at Pre-PubertychristianNo ratings yet
- JMPR - Azinphos-Methyl - 2007Document34 pagesJMPR - Azinphos-Methyl - 2007Noé Amargo BarbosaNo ratings yet
- Lopinavir Ritonavir Pharmacokinetic Profile Impact of Sex and Other CovariatesDocument9 pagesLopinavir Ritonavir Pharmacokinetic Profile Impact of Sex and Other CovariatesLuciana OliveiraNo ratings yet
- Clinical Medicine Insights: Ear, Nose, ThroatDocument5 pagesClinical Medicine Insights: Ear, Nose, ThroatagustinadianasariaguNo ratings yet
- Acm 2010 0572 PDFDocument6 pagesAcm 2010 0572 PDFRiley RilanNo ratings yet
- Acm 2010 0572 PDFDocument6 pagesAcm 2010 0572 PDFRiley RilanNo ratings yet
- Portec 2.Document8 pagesPortec 2.henryNo ratings yet
- Cytotoxicity CompundDocument8 pagesCytotoxicity Compunddeepak lourembamNo ratings yet
- Pengaruh Makanan Pada CaptoprilDocument5 pagesPengaruh Makanan Pada CaptoprilNiluh putu Satria maharaniNo ratings yet
- Ding Et AlDocument4 pagesDing Et AlNguyễn Quang HiếuNo ratings yet
- Articulo ParcialDocument10 pagesArticulo ParcialKarina TillnerNo ratings yet
- Genomics and Risk Assessment: Mini-MonographDocument15 pagesGenomics and Risk Assessment: Mini-MonographNola AyundaNo ratings yet
- Antiproliferative Potential of Escitalopram in Dimethylhydrazine (DMH) Induced Colon Cancer in Swiss Albino MiceDocument7 pagesAntiproliferative Potential of Escitalopram in Dimethylhydrazine (DMH) Induced Colon Cancer in Swiss Albino MiceIJAR JOURNALNo ratings yet
- 2253Document6 pages2253Rani JeyaramanNo ratings yet
- Infection Status of Hospitalized Diarrheal Patients With Gastrointestinal Protozoa, Bacteria, and Viruses in The Republic of KoreaDocument9 pagesInfection Status of Hospitalized Diarrheal Patients With Gastrointestinal Protozoa, Bacteria, and Viruses in The Republic of KoreaMelia Kusuma WardaniNo ratings yet
- JournalDocument31 pagesJournalNandini VermaNo ratings yet
- DR +Niket+Sonpal+-+Research+AbstractsDocument7 pagesDR +Niket+Sonpal+-+Research+AbstractsamiqtNo ratings yet
- Red Meat CYP2E1Document6 pagesRed Meat CYP2E1'Alivia Nabdakh ClocheNo ratings yet
- Part 3 - Huang Qin Tang at Yale Medical SchoolDocument3 pagesPart 3 - Huang Qin Tang at Yale Medical SchoolCarleta StanNo ratings yet
- Pancreatic Enzyme Extract Improves Survival in Murine Pancreatic CancerDocument12 pagesPancreatic Enzyme Extract Improves Survival in Murine Pancreatic CancerRexGoliathNo ratings yet
- Hanoo 2Document20 pagesHanoo 2mr samoNo ratings yet
- Method and Measurement in Pharmacology: BioassayDocument11 pagesMethod and Measurement in Pharmacology: BioassaydoctorjbrittoNo ratings yet
- Defense Energy Quantum Article - Jun:2020Document20 pagesDefense Energy Quantum Article - Jun:2020Luciano AraújoNo ratings yet
- Sezer 2020Document1 pageSezer 2020KarlaPadrelananNo ratings yet
- Evaluation of OrganWeights For Rodent and Non-Rodent ToxicityDocument9 pagesEvaluation of OrganWeights For Rodent and Non-Rodent Toxicityromaricarmel13No ratings yet
- Toluene 0118 - SummaryDocument33 pagesToluene 0118 - SummarysukantaenvNo ratings yet
- Shin, Seol, Son - Interpretation of Animal Dose and Human Equivalent Dose For Drug Development - 2010 PDFDocument7 pagesShin, Seol, Son - Interpretation of Animal Dose and Human Equivalent Dose For Drug Development - 2010 PDFHendra Wana Nur'aminNo ratings yet
- Effect of Daily Intake of Yoghurt and Bread Enriched With Biologically Active Substances On Blood Lipids and Vitamin A in Adolescents and Young AdultsDocument9 pagesEffect of Daily Intake of Yoghurt and Bread Enriched With Biologically Active Substances On Blood Lipids and Vitamin A in Adolescents and Young AdultsEsmeralda SantillanNo ratings yet
- Multivitamin and Micronutrient TreatmentDocument7 pagesMultivitamin and Micronutrient Treatmentanand809No ratings yet
- The Application of in Vitro Data in The Derivation of The Acceptable Daily Intake of Food AdditivesDocument6 pagesThe Application of in Vitro Data in The Derivation of The Acceptable Daily Intake of Food AdditivesyolandatyasNo ratings yet
- Scientific Article - Cuarto ExamenDocument7 pagesScientific Article - Cuarto ExamenPaolo MayoNo ratings yet
- 5 Fu 2Document10 pages5 Fu 2Luiis LimaNo ratings yet
- Metabolomic Profiling of Amoebic and Pyogenic Liver Abscesses An in Vitro NMR StudyDocument16 pagesMetabolomic Profiling of Amoebic and Pyogenic Liver Abscesses An in Vitro NMR Studysantosh091283No ratings yet
- Jaalas 2022000113Document19 pagesJaalas 2022000113izensienNo ratings yet
- Higher Urinary Heavy Metal, Phthalate, and Arsenic But Not Parabens Concentrations in People With High Blood Pressure, U.S. NHANES, 2011-2012Document11 pagesHigher Urinary Heavy Metal, Phthalate, and Arsenic But Not Parabens Concentrations in People With High Blood Pressure, U.S. NHANES, 2011-2012Ryan JonesNo ratings yet
- Care of Clients With Cellular Aberrations: NCM 106/ NCM 112Document22 pagesCare of Clients With Cellular Aberrations: NCM 106/ NCM 112Janelle Cabida SupnadNo ratings yet
- Medical-Surgical Nursing Assessment and Management of Clinical Problems 9e Chapter 16Document15 pagesMedical-Surgical Nursing Assessment and Management of Clinical Problems 9e Chapter 16sarasjunk100% (1)
- Wiki - Week 1 - The Nervous System - CourseraDocument3 pagesWiki - Week 1 - The Nervous System - Courseracharles luisNo ratings yet
- PATHology Questions MGR Medical UniversityDocument40 pagesPATHology Questions MGR Medical UniversityPONNUSAMY P50% (2)
- TR 421Document287 pagesTR 421daeron42No ratings yet
- Jinnah Sindh Medical University: Foundation-2 Module Study Guide - MBBS Year-3, 2023 Spiral II Module TitleDocument28 pagesJinnah Sindh Medical University: Foundation-2 Module Study Guide - MBBS Year-3, 2023 Spiral II Module TitleKamlesh KumarNo ratings yet
- Tumor Phyloides JurnalDocument5 pagesTumor Phyloides JurnalSuzika DewiNo ratings yet
- NO 2. Triphasic Computed Tomography (CT) Scan in Focal Tumoral Liver Le-1Document7 pagesNO 2. Triphasic Computed Tomography (CT) Scan in Focal Tumoral Liver Le-1hermanusNo ratings yet
- MEDSURG - Cellular AberrationDocument10 pagesMEDSURG - Cellular AberrationLeslie CruzNo ratings yet
- Poroma of The Hip and ButtockDocument3 pagesPoroma of The Hip and ButtockDeba P SarmaNo ratings yet
- Nodular Swelling of The Buccal Mucosa: Clinical PresentationDocument7 pagesNodular Swelling of The Buccal Mucosa: Clinical Presentationotoralla1591No ratings yet
- Benign Gynecologic TumorsDocument57 pagesBenign Gynecologic TumorsDexter IanNo ratings yet
- 1 - Biochemistry of CancerDocument18 pages1 - Biochemistry of CancerJoo Se HyukNo ratings yet
- Imaging of Kidney Cancer - GuermaziDocument439 pagesImaging of Kidney Cancer - GuermaziNar RungrojanarakNo ratings yet
- 18 Cellular AberrationsDocument70 pages18 Cellular AberrationsBea Bianca CruzNo ratings yet
- Sched 3rd YrDocument19 pagesSched 3rd YrKeshab Raj NeupaneNo ratings yet
- Atlas of Gastrointestinal Endoscopy in Dogs and CatsDocument8 pagesAtlas of Gastrointestinal Endoscopy in Dogs and CatsDama Ayu RaniNo ratings yet
- Fish Diseases (Jorge C. Eiras, Helmut Segner, Thomas Wahli Etc.) (Z-Library)Document1,327 pagesFish Diseases (Jorge C. Eiras, Helmut Segner, Thomas Wahli Etc.) (Z-Library)Iryna ZapekaNo ratings yet
- NeoplasiaDocument51 pagesNeoplasiaElstella Eguavoen Ehicheoya100% (1)
- Benign and Premalignant LesionsDocument25 pagesBenign and Premalignant LesionsAyush PaudelNo ratings yet
- English Final CoursDocument223 pagesEnglish Final CourspopeataNo ratings yet
- What Is NeoplasmsDocument38 pagesWhat Is NeoplasmsRashid HussainNo ratings yet
- Assignment 1 (Cancer)Document8 pagesAssignment 1 (Cancer)Leo Aurelio MarcelinoNo ratings yet
- Neoplastic Thyroid Disease - Thyroid Nodules Goiter and Thyroid CancerDocument57 pagesNeoplastic Thyroid Disease - Thyroid Nodules Goiter and Thyroid CancerNavya SreeNo ratings yet
- Benigntumorsofjaw2 130421054527 Phpapp01Document118 pagesBenigntumorsofjaw2 130421054527 Phpapp01orthomedNo ratings yet
- Skeletal and Skin TractionDocument38 pagesSkeletal and Skin TractionCORROS JASMIN MARIE100% (1)
- LEARNING TASK # 3 Parathyroid Cancer Awareness (Llorin, Jenny-An Pauline)Document2 pagesLEARNING TASK # 3 Parathyroid Cancer Awareness (Llorin, Jenny-An Pauline)Llorin, Jenny-An Pauline B.No ratings yet
- Neoplasia IDocument53 pagesNeoplasia INooria ButtNo ratings yet
- 1.06 General Pathology - Neoplasia (Part 1) - Dr. Annette SallilasDocument17 pages1.06 General Pathology - Neoplasia (Part 1) - Dr. Annette SallilasCherry RahimaNo ratings yet