Professional Documents
Culture Documents
A Level Chemistry 2
A Level Chemistry 2
Uploaded by
leslieofori257Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
A Level Chemistry 2
A Level Chemistry 2
Uploaded by
leslieofori257Copyright:
Available Formats
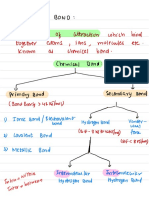
Vander Waals forces /London sores
Temporary dipoles can induce other molecules to become dipoles .
induced induced induces
dipole- Dipole e dipole- >
Dipole
St # F J
DIs
NN-m ----
mub
/IWIL I
Indon fores
have london
All molecules forces
Strength of London gorces
Strength increase going down the group
e
g
.
weak
F
strong
Cl
Br
I
This is because going down the group the number of elections increases
the electrons the electrons will
more less likely the be
evenly disbursed there
gove there are more chances
of creating a
temporary dipole
You might also like
- CAPE Chemistry Forces of AttractionDocument8 pagesCAPE Chemistry Forces of AttractionDaniel Walsh100% (1)
- Chapter 14 - Intermolecular ForcesDocument8 pagesChapter 14 - Intermolecular ForcesRenny Romero LuzadaNo ratings yet
- IMF PogilDocument6 pagesIMF PogilsungminindiaNo ratings yet
- Electronegativity: 1 UNIT-2: Highest El Negativity DiffDocument6 pagesElectronegativity: 1 UNIT-2: Highest El Negativity DiffDP GamerNo ratings yet
- KMT of Solids and Liquids KMT of Solids and LiquidsDocument6 pagesKMT of Solids and Liquids KMT of Solids and LiquidsBea Dacillo BautistaNo ratings yet
- Learning Services Student Resources Intermolecular ForceDocument1 pageLearning Services Student Resources Intermolecular ForceAdrianne Jericho ValdezNo ratings yet
- Intermolecular ForceDocument3 pagesIntermolecular ForceNoor FathiNo ratings yet
- Intermolecular Forces DemoDocument25 pagesIntermolecular Forces DemoAlbert SubistaNo ratings yet
- KMT AND Intermolecular Forces 1Document17 pagesKMT AND Intermolecular Forces 1F. Andrea CieloNo ratings yet
- Intermolecular Forces A.K.A. Van Der Waal's ForcesDocument22 pagesIntermolecular Forces A.K.A. Van Der Waal's ForcesJunel Dave SalapantanNo ratings yet
- Intermolecular ForcesDocument4 pagesIntermolecular ForcesLily RyderNo ratings yet
- Lesson 2 Intermolecular Forces of AttractionDocument26 pagesLesson 2 Intermolecular Forces of AttractionKC MasedmanNo ratings yet
- Intermediate Bonding and Intermolecualr ForcesDocument32 pagesIntermediate Bonding and Intermolecualr ForceskrishnaviNo ratings yet
- Intermolecular ForcesDocument2 pagesIntermolecular ForcesSiaoNo ratings yet
- IMF Lecture 1 - Types of IMF'sDocument35 pagesIMF Lecture 1 - Types of IMF'sGoatNo ratings yet
- IMF Lecture 1 - Types of IMF'sDocument35 pagesIMF Lecture 1 - Types of IMF'sPrivacy NutNo ratings yet
- Unit 1 Intermolecular Forces of Solids & LiquidsDocument4 pagesUnit 1 Intermolecular Forces of Solids & LiquidsMoira Nadeen NicolasoraNo ratings yet
- 2 26 Intermolecular BondingDocument10 pages2 26 Intermolecular BondingAliya RahmanNo ratings yet
- General Chemistry-Iii-071020Document77 pagesGeneral Chemistry-Iii-071020MochamadIqbalJaelaniNo ratings yet
- Learning Activity Sheets: Ganilyn D. Ponciano. Stem 12 - A General Chemistry 2Document7 pagesLearning Activity Sheets: Ganilyn D. Ponciano. Stem 12 - A General Chemistry 2Ganilyn Ponciano0% (3)
- Gen Chem 2 Pointers To Review ReviewerDocument6 pagesGen Chem 2 Pointers To Review ReviewerAlkin RaymundoNo ratings yet
- UntitledDocument28 pagesUntitledMARVIN HILARIONo ratings yet
- States of MatterDocument30 pagesStates of MatterSwapnil MandalNo ratings yet
- Genchem2 Reviewer FinalDocument4 pagesGenchem2 Reviewer FinalMyco Reggie DadoNo ratings yet
- 4 - States of Matter - Theory - (1-41)Document43 pages4 - States of Matter - Theory - (1-41)eerannaNo ratings yet
- Together: I of BindDocument15 pagesTogether: I of BindShreyas PrabhuNo ratings yet
- 1 - KMTDocument45 pages1 - KMTEllysa Jade VelascoNo ratings yet
- Ion-Dipole Inter Action: TraitsDocument1 pageIon-Dipole Inter Action: TraitsRichelle CastanedaNo ratings yet
- Attracted To Intermolecular Forces Part A: Frayer Model of Liquids and SolidsDocument6 pagesAttracted To Intermolecular Forces Part A: Frayer Model of Liquids and SolidsGanilyn PoncianoNo ratings yet
- Inorganic As LevelDocument63 pagesInorganic As Levelchan chanNo ratings yet
- Intermolecular Forces:: Attraction in Molecular LevelDocument34 pagesIntermolecular Forces:: Attraction in Molecular LevelKelly MarceloNo ratings yet
- Topic 7 Unit 07 Lp01ps - Van Der Waal Forces OkkkkaaaaayyyyyyyyDocument26 pagesTopic 7 Unit 07 Lp01ps - Van Der Waal Forces OkkkkaaaaayyyyyyyyRodjhen Anne P. BarquillaNo ratings yet
- Intermolecular ForcesDocument15 pagesIntermolecular ForcesGEORGIA AILEEN SORNELNo ratings yet
- Quibot, Mikaela (BSCE 1A) - Discussion Post No. 2Document1 pageQuibot, Mikaela (BSCE 1A) - Discussion Post No. 2Mikaela QuibotNo ratings yet
- Magnetic Material 1st AssignmentDocument11 pagesMagnetic Material 1st Assignment00786luqman.1No ratings yet
- handoutsDocument3 pageshandoutscalainejynnecabusao25No ratings yet
- 3.1 Intermolecular Forces-2Document11 pages3.1 Intermolecular Forces-2faridaisepicNo ratings yet
- Lesson 3 - Intermolecular Forces of AttractionDocument43 pagesLesson 3 - Intermolecular Forces of AttractionFreshieeNo ratings yet
- IMFADocument41 pagesIMFAShaila DelatorreNo ratings yet
- IntermolecularDocument8 pagesIntermolecularChris Michole Tabura AbabaNo ratings yet
- Chapter 1Document28 pagesChapter 1yaqoobNo ratings yet
- Intermolecular ForcesDocument2 pagesIntermolecular ForcesMukil MNo ratings yet
- Unit 07 LP01PS - Van Der Waal Forces v06Document26 pagesUnit 07 LP01PS - Van Der Waal Forces v06jaymart villartaNo ratings yet
- Gen Chem ReviewerDocument10 pagesGen Chem ReviewerLawrence Angelo Mana-ayNo ratings yet
- Example: Individual Therapy: Intermolecular Forces Are The Forces BetweenDocument8 pagesExample: Individual Therapy: Intermolecular Forces Are The Forces BetweenRochelleCasador180No ratings yet
- Science Fair NewsletterDocument9 pagesScience Fair NewsletterbuenafefloresNo ratings yet
- 11.4C Group 7 ElementsDocument19 pages11.4C Group 7 ElementsЕлнур ИкимбаевNo ratings yet
- Intermolecular Forces and SolubilityDocument32 pagesIntermolecular Forces and Solubilityliana.mirlohi4No ratings yet
- Lecture03f - Van Der WaalsDocument36 pagesLecture03f - Van Der WaalsKarla B. CamperoNo ratings yet
- Solute Solvent Interactions PPI BPII - IDocument32 pagesSolute Solvent Interactions PPI BPII - IYuppie Raj100% (2)
- MoleculesDocument3 pagesMoleculesMoktar EsmailNo ratings yet
- 6 Polarity and Intermolecular ForcesDocument41 pages6 Polarity and Intermolecular ForcesMarielle GutierrezNo ratings yet
- PolarityDocument20 pagesPolarityYsabela MataNo ratings yet
- Intermolecular Forces of AttractionDocument3 pagesIntermolecular Forces of AttractionJose Ferdinand Marcos Jay SaligumbaNo ratings yet
- Intermolecular ForcesDocument16 pagesIntermolecular ForcesSHEENA MAE DALGUNTASNo ratings yet
- La Casa Fuerza::the Captivating Differences of Attraction and Its Intermolecular ForcesDocument5 pagesLa Casa Fuerza::the Captivating Differences of Attraction and Its Intermolecular ForcesRamNo ratings yet
- Lesson 3 FORCES OF ATTRACTIONDocument23 pagesLesson 3 FORCES OF ATTRACTIONLala CkeeNo ratings yet
- Attracted To Intermolecular Forces Part A: Frayer Model of Liquids and SolidsDocument6 pagesAttracted To Intermolecular Forces Part A: Frayer Model of Liquids and SolidsGanilyn PoncianoNo ratings yet
- Condensed Phases: Liquids and Solids: Intermolecular ForcesDocument11 pagesCondensed Phases: Liquids and Solids: Intermolecular ForcesSarah FeyNo ratings yet