Professional Documents
Culture Documents
Chemistry
Chemistry
Uploaded by
Jacosby WorcestershireCopyright:
Available Formats
You might also like
- BIOCHEMISTRY - Summary of PathwaysDocument8 pagesBIOCHEMISTRY - Summary of PathwaysWendy Mae100% (10)
- Talking Fashion: Pierre Cardin Interviewed by Jan KedvesDocument5 pagesTalking Fashion: Pierre Cardin Interviewed by Jan KedvesJanKedves100% (1)
- Amino Acid Metabolism: Inborn ErrorsDocument18 pagesAmino Acid Metabolism: Inborn ErrorsAdedoyin BankoleNo ratings yet
- Soal USM STAN 2014 Kunci JawabanDocument51 pagesSoal USM STAN 2014 Kunci JawabanAnding NdingNo ratings yet
- Biotransformation Reaction: Glucuronide Conjugation OxidationDocument1 pageBiotransformation Reaction: Glucuronide Conjugation OxidationJakareaNo ratings yet
- .Degradasi ProteinDocument29 pages.Degradasi ProteinSeffy Yane SuhandaNo ratings yet
- Thiamine Pyrophosphate (TTP) Thiamine Pyrophosphate (TTP)Document4 pagesThiamine Pyrophosphate (TTP) Thiamine Pyrophosphate (TTP)Lyan SamsonNo ratings yet
- Organic Medicinal Chemistry: FunctionalizationDocument25 pagesOrganic Medicinal Chemistry: FunctionalizationPrincess RonsableNo ratings yet
- Pyidoxine, BiotinDocument19 pagesPyidoxine, Biotinlovi bahunNo ratings yet
- 3 Nucleotide Metabolism FirstDocument25 pages3 Nucleotide Metabolism Firstsampahgordon5No ratings yet
- Niacin: DR. Beenish Zafar BiochemistryDocument32 pagesNiacin: DR. Beenish Zafar BiochemistryShehryar AbbasNo ratings yet
- Nucleotide Structure, Function, Metabolism and DNA Replication (New Curriculum) - StudentsDocument47 pagesNucleotide Structure, Function, Metabolism and DNA Replication (New Curriculum) - StudentsWing Yeng TanNo ratings yet
- Lecture 6 - General and Characteristics. Water Soluble VitaminsDocument29 pagesLecture 6 - General and Characteristics. Water Soluble VitaminsEiad SamyNo ratings yet
- MSB 202: Neurolocomotor: Neurotransmitters - Overview of Metabolism (Anabolism and Catabolism)Document42 pagesMSB 202: Neurolocomotor: Neurotransmitters - Overview of Metabolism (Anabolism and Catabolism)Jacob MasikaNo ratings yet
- Bca Protein Metab 2Document69 pagesBca Protein Metab 2Genina MaylemNo ratings yet
- 1.protein Digestion, Urea Cy, MbbsDocument81 pages1.protein Digestion, Urea Cy, MbbsDebarghya MukherjeeNo ratings yet
- Rate Limiting StepsDocument2 pagesRate Limiting StepsvictoreffiomNo ratings yet
- Biochem 35 Trans 4 Nucleic Acid MetabolismDocument10 pagesBiochem 35 Trans 4 Nucleic Acid MetabolismBeam CanoNo ratings yet
- Proteins LASDocument6 pagesProteins LASelly scatusNo ratings yet
- Amphibolic Nature of Krebs Cycle: How What We Are Is What We EatDocument33 pagesAmphibolic Nature of Krebs Cycle: How What We Are Is What We EatSoloNo ratings yet
- Xenobiotics:: Xenobiotics Phase1 Phase2 Prodrug and Carcinogen Prodrug and Carcinogen Xenobiotics XenobioticsDocument4 pagesXenobiotics:: Xenobiotics Phase1 Phase2 Prodrug and Carcinogen Prodrug and Carcinogen Xenobiotics XenobioticsAsad IslamNo ratings yet
- Lipidosis HepaticaDocument45 pagesLipidosis HepaticaJojoa E WilNo ratings yet
- SupplementaryDocument31 pagesSupplementaryKaranNo ratings yet
- 6 Major Classes of EnzymesDocument6 pages6 Major Classes of EnzymesnoorNo ratings yet
- MicroBio Lec Transes 8 9Document7 pagesMicroBio Lec Transes 8 9Kai BarsanaNo ratings yet
- Vitamins Synonyms Chemistry Coenzyme Form RDA Sources Properties Physiologic Role DeficiencyDocument9 pagesVitamins Synonyms Chemistry Coenzyme Form RDA Sources Properties Physiologic Role Deficiencykristian markus delos santosNo ratings yet
- PDH Complex and TCA CycleDocument20 pagesPDH Complex and TCA CycleDarrion LouisNo ratings yet
- 31NucleotideMetabolism PDFDocument41 pages31NucleotideMetabolism PDFKifayat HussainNo ratings yet
- Amino Acid MetabolismDocument7 pagesAmino Acid MetabolismROHITNo ratings yet
- Janes Metro Map Pathways BLANKDocument1 pageJanes Metro Map Pathways BLANKWinston TengNo ratings yet
- Metabolism of TryptophanDocument38 pagesMetabolism of Tryptophanjagan mohan rao vanaNo ratings yet
- Water Soluble VitaminsDocument55 pagesWater Soluble VitaminsDr. M. Prasad Naidu100% (1)
- Bcaromtrans-Protein MetabolismDocument8 pagesBcaromtrans-Protein MetabolismRay Emmanuel Enriquez DomingoNo ratings yet
- ACAWPurineand Pyrimindine Synthesis Presentationfor October 112010Document41 pagesACAWPurineand Pyrimindine Synthesis Presentationfor October 112010Ezekoko ChineseNo ratings yet
- Biochemistry - Enzymes Shuttles Proteins Summary 1Document8 pagesBiochemistry - Enzymes Shuttles Proteins Summary 1Samuel NafNo ratings yet
- BiochemDocument68 pagesBiochemPrashant ChettriNo ratings yet
- Vitmin and MineralDocument1 pageVitmin and Mineralkero R.habibNo ratings yet
- Feline Hepatic LipidosisDocument46 pagesFeline Hepatic LipidosisAndre Suarez FarfanNo ratings yet
- Amino AcidsDocument1 pageAmino AcidsBobet ReñaNo ratings yet
- Tryptophan Metabolism: Dr. Ashok Kumar J International Medical School Management and Science University MalaysiaDocument23 pagesTryptophan Metabolism: Dr. Ashok Kumar J International Medical School Management and Science University MalaysiaShah HuzaifaNo ratings yet
- Chapter 18 - Nucleotide Metabolism: Nucleotides Are Composed of Three ComponentsDocument5 pagesChapter 18 - Nucleotide Metabolism: Nucleotides Are Composed of Three ComponentsSaiful IslamNo ratings yet
- Degradacion Aminoacidos y ProteinasDocument52 pagesDegradacion Aminoacidos y ProteinasEncina Abrigo Andrés PabloNo ratings yet
- Nucleotide Met Postgrad Student 2019 HandoutDocument15 pagesNucleotide Met Postgrad Student 2019 HandoutMark KoshlandNo ratings yet
- IbuprofenDocument36 pagesIbuprofenJelita ThalabNo ratings yet
- Mls 218 Protein-MetDocument45 pagesMls 218 Protein-MetZainabNo ratings yet
- Vitamin and MineralDocument8 pagesVitamin and MineralhobbycontestsNo ratings yet
- Chemistryofproteinswithclinicalapplications 190621192525 PDFDocument181 pagesChemistryofproteinswithclinicalapplications 190621192525 PDFAl-waleed Julkanain100% (1)
- PATHWAYS SummaryDocument5 pagesPATHWAYS Summaryslu.veniegasmb.1144No ratings yet
- Amino Acid Metabolism 2Document29 pagesAmino Acid Metabolism 2Francis GacheruNo ratings yet
- Bioenergetics. Tricarboxylic Acids CycleDocument60 pagesBioenergetics. Tricarboxylic Acids CyclePurwa RaneNo ratings yet
- Biosynthesis of LIpidsDocument21 pagesBiosynthesis of LIpidsJoyce Hanniel CastinoNo ratings yet
- BBB PDFDocument37 pagesBBB PDFaq educateNo ratings yet
- Urea cycle-MBBSDocument31 pagesUrea cycle-MBBSRama SubramanianNo ratings yet
- Food Chemistry - Activity 2Document3 pagesFood Chemistry - Activity 2Leda PeñaNo ratings yet
- BIOCHEMISTRY: Proteins 2: Physical PropertiesDocument7 pagesBIOCHEMISTRY: Proteins 2: Physical PropertiesCon ChinNo ratings yet
- Bio - CO 6Document2 pagesBio - CO 6Jae Bert UbisoftNo ratings yet
- Collection For Digestive Enzymes of Protein (Mansoura Dentistry)Document1 pageCollection For Digestive Enzymes of Protein (Mansoura Dentistry)elsayed barhomeNo ratings yet
- Co- and Post-Translational Modifications of Therapeutic Antibodies and ProteinsFrom EverandCo- and Post-Translational Modifications of Therapeutic Antibodies and ProteinsNo ratings yet
- The Crest of The Tide of Renascence: Sankaradeva's Kirttan-GhosāDocument30 pagesThe Crest of The Tide of Renascence: Sankaradeva's Kirttan-GhosāBhargavNo ratings yet
- JK Civil Sevices Classification Control and AppealpdfDocument35 pagesJK Civil Sevices Classification Control and AppealpdfShuja Malik100% (1)
- Dishonour of Cheques and Negotiable Instruments - Legalsutra - Law Students' Knowledge-Base - Law School Projects, Moot Court Memorials, Class and Case Notes and More!Document8 pagesDishonour of Cheques and Negotiable Instruments - Legalsutra - Law Students' Knowledge-Base - Law School Projects, Moot Court Memorials, Class and Case Notes and More!Himanshu Mene100% (1)
- English Presentations - Useful PhrasesDocument2 pagesEnglish Presentations - Useful PhrasesNitish Nagar100% (1)
- MOD2 Statement of Cash FlowsDocument2 pagesMOD2 Statement of Cash FlowsGemma DenolanNo ratings yet
- Oracle Switch ES1-24 Configuration GuideDocument88 pagesOracle Switch ES1-24 Configuration GuideNoe HernadezNo ratings yet
- Soal Kalimat Prohibition SMP Kelas 7Document3 pagesSoal Kalimat Prohibition SMP Kelas 7yura chanNo ratings yet
- Motor Claim Form THE ORIENTAL INSURANCE CO. LTD.Document4 pagesMotor Claim Form THE ORIENTAL INSURANCE CO. LTD.rajiv.surveyor7145No ratings yet
- 13 Eo Bcat Vaw Barangay Committee On Anti Trafficking and Violence Against WomenDocument2 pages13 Eo Bcat Vaw Barangay Committee On Anti Trafficking and Violence Against WomenOremor RemerbNo ratings yet
- Evaluating Tilting Pad - PaperDocument10 pagesEvaluating Tilting Pad - PaperAsit SuyalNo ratings yet
- ThesisDocument13 pagesThesiszavia_02No ratings yet
- Feminism: Broad Streams of FeminismDocument4 pagesFeminism: Broad Streams of FeminismDexin JoyanNo ratings yet
- Alter Ego 181 PreviewDocument20 pagesAlter Ego 181 PreviewJohn LloydNo ratings yet
- Keyword: 50s Music Title: Great 50s Songs For Different Moods: ContentDocument1 pageKeyword: 50s Music Title: Great 50s Songs For Different Moods: Contentaditya_bb_sharmaNo ratings yet
- CGR Project Report DDGGDDDocument21 pagesCGR Project Report DDGGDDTanvi KodoliNo ratings yet
- DeputationDocument8 pagesDeputationAnand MauryaNo ratings yet
- Main Slokas With MeaningDocument114 pagesMain Slokas With MeaningRD100% (1)
- 1.publications All BranchesDocument25 pages1.publications All BranchesNaresh GollapalliNo ratings yet
- Crane Fabrication Standard Kit: Main Locations GH PhilosophyDocument2 pagesCrane Fabrication Standard Kit: Main Locations GH PhilosophyFiroz PawaskarNo ratings yet
- Services Procurement Data SheetDocument5 pagesServices Procurement Data Sheetrollingstone3mNo ratings yet
- Beyond The Blackboard Reflection PaperDocument3 pagesBeyond The Blackboard Reflection PaperPacatang Evelyn100% (1)
- POSTMODERN, 253s '12Document270 pagesPOSTMODERN, 253s '12Raluca Gîlcă100% (1)
- Spare Parts CatalogueDocument45 pagesSpare Parts CatalogueАлексей ДомнинNo ratings yet
- Apbush DBQDocument2 pagesApbush DBQMaria Ines Carrillo100% (1)
- Performance Correction Chart of Centrifugal Oil Pump For Handling Viscous LiquidsDocument7 pagesPerformance Correction Chart of Centrifugal Oil Pump For Handling Viscous LiquidsalikajbafNo ratings yet
- Hoshizaki Technical Support - Warranty Labor Claim InstructionsDocument12 pagesHoshizaki Technical Support - Warranty Labor Claim InstructionsJohn DuttingerNo ratings yet
- Reflective Competency Statement IIIDocument4 pagesReflective Competency Statement IIIapi-534821343No ratings yet
- The 7 SealDocument11 pagesThe 7 Sealendalkachew gudeta100% (1)
- PROCEEDINGS of The Numismatic and Antiquarian Society of PhiladelphiaDocument304 pagesPROCEEDINGS of The Numismatic and Antiquarian Society of PhiladelphiaRichard CastorNo ratings yet
Chemistry
Chemistry
Uploaded by
Jacosby WorcestershireCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemistry
Chemistry
Uploaded by
Jacosby WorcestershireCopyright:
Available Formats
Epinephrine (+) is 20 times more potent than its (-) enantiomer.
Levorphanol (L) has potent narcotic, analgesic and anti-tussive effect. Whereas its mirror
image dextrorphanol (D) has only anti-tussive action.
D-propoxyphene is analgesic, while (L) is anti-tussive.
Pseudoephedrine has lower CNS toxicity and vasopressor activity than ephedrine.
Epimerization is important in drug degradation and inactivation.
Cis-diethylstilbestrol has only 7% estrogenic activity of trans-diethylstilbestrol.
Cis-retinoic acid – isotretinoin used orally and trans-retinoic acid – tretinoin used topically in
the treatment of acne.
Trans conformation of acetylcholine binds to muscarinic receptors while gauche
conformation binds to nicotinic receptors.
Bioisosters are molecules containing groups that are spatially and electrochemically
equivalent. E.g. amide bond and ester bond in procainamide and procaine respectively.
Amino acids have zweitter ion structure (has both positive and negative electrical charge)
which accounts for their high melting points and lower water solubility.
The pH at which zweitter ion exists is called isoelectric point.
The pH at which cation exists is below isoelectric point.
The pH at which anion exists is above isoelectric point.
Α-methyl dopa is zweitter ion, being water insoluble, it is formulated as ethyl ester
hydrochloride for aqueous injection.
Conversion of amino acids
Glycine Purine Base
Glutathione Contain three amino acids
- ɣ - glutamyl
- cysteinyl
- glycine
Creatine Phosphorylated by creatine
Also known as phosphokinase to creatine
-Methyl guanidinoacetate phosphate
Synthesized from
- glycine Creatine lose water and
- arginine form creatinine which is
- methionine rapidly removed from
blood and excreted by
kidney.
Haem Combine with globin =
Glycine + succinylcholine = haemoglobin
Haem
Phenylalanine Through phenylalanine Converted into tyrosine
hydroxylase and
dihydrobiopterin (DHB)
reductase enzymes
Deficiency of this enzymes Cause phenylketonuria
- accumulation of
phenylalanine
Tyrosine precursor of - dopamine
catecholamines - norepinephrine
- epinephrine
Epinephrine - structure active relationship
Meta -OH group Essential for activity
Removal of para -OH group Only has Selectivity for α-receptor
Addition of methyl group on α – carbon Improve oral activity
Larger N-substitution than methyl on α-carbon Has selectivity for β-receptor, e.g.
- dobutamine
- terbutaline
Tyrosine (…continue) Many tyrosine residues Thyroid hormones
iodinated to form - Thyroglobulin as a
thyroglobulin precursor
Melanin Tyrosine to dopa to melanin
Tryptophan Precursor of serotonin and melatonin
nicotinic acid (niacin)
indole and skatole
Glutamic acid Glutamate CNS neurotransmitter
Glutathione synthesis
Folic acid synthesis Dihydrofolic acid
A mixture of
- Pteridine base
- PABA
- Glutamic acid
Synthesis of GABA from By L-glutamic acid
glutamic acid decarboxylase enzyme
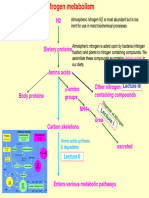
Arginine Urea Arginine and citrulline
involve in urea cycle in liver
Creatine
Nitric oxide (NO) Arginine by NO synthase =
NO + citrulline
Aspartate - Precursor of Purine & pyrimidine
(also act as excitatory synthesis
neurotransmitter)
Asparagine A part of oxytocin
Urea cycle
Carbohydrates
- Polyhydroxy aldehydes or ketones
Monosaccharides Polyhydroxy aldehydes Glucose
Polyhydroxy ketones Fructose
Aldehyde monosaccharides Reducing agents Used previously with
Benedict’s solution
Tes-tape test Glucose
-reagent strips with - glucose oxidase Glucuronic acid + H2O2
enzymes + orthotolidine dye - peroxidase Convert orthotolidine dye to
intense blue color
Oligosaccharides Short chain of Only hydrolysed by acid not
monosaccharides by base
Sucrose Glucose + fructose
Maltose 2 – glucose
Lactose Glucose + galactose
Lactulose A synthetic disaccharide
Lactulose Broken by bacteria in colon So, drop in pH and thus
- also can act as an into Acetic acid + lactic acid Conversion of toxic NH3
osmotic laxative into non-toxic and non-
absorbable NH4+ ion
Reduction in NH3 level Favouring more diffusion of
cause concentration NH3 from blood and so on,
gradient in colon So used in treatment of
hepatic encephalopathy due
to hyperammonemia
Polysaccharides Also called glycans Classified as homo- and
hetero- polysaccharides
Homopolysaccharides Starch Insoluble in water due to
- contain one type of highly branched chain
monomeric units
Glycogen Main storage of
polysaccharides
Dextran Used as plasma expanders
in patient who experienced
loss of blood
Heteropolysaccharides Heparin acid mucopolysaccharide
- two or more type of Used to treat blood clot (sulphate of N-acetyl
monomeric units glucosamine and iduronate)
Hyaluronic acid Consists of altering units of
Present in vitreous and N-acetyl glucosamine and
synovial fluid N-acetyl muramic acid
Fatty acids
Saturated or unsaturated long chain carboxylic acids Is fatty acid
Oxidation of double bond Of unsaturated FA Called rancidity
FA + glycerol = ester Called triacylglycerol Stored in fatty tissues and
used as energy store
Examples of FA
Formic acid One carbon Actually, not a FA
Acetic acid Two carbon
Stearic acid 18 carbons
Oleic acid 18 carbons & unsaturated Cis form
Ricinoleic acid 16 carbons & -OH group sub
Castor oil (ricinolein) Ester of ricinoleic acid with Presence of -OH, make it
glycerol polar & miscible with
alcohol, immiscible with
mineral oils
White wax Ester of high MW Not a FA
(molecular weight) of Derived by bleaching
alcohol and acids beeswax (yellow wax)
Carnauba wax Higher MW than white wax So, used in polishing
gives it hardness mixture of coated tablet
Cetyl palmitate Ester of palmitic acid and Obtained from the head of
Cetyl alcohol sperm whale
Mineral oils A mixture of liquid Being non-polar,
- specific gravity is less hydrocarbons, obtained Immiscible with water,
than 1 – lighter than from petroleum alcohol and castor oil.
water.
Petroleum Mixture of liquid aliphatic Non-polar
hydrocarbons with solid immiscible with alcohol
hydrocarbons
10 ppm vit. E (tocopherol), Added to unsaturated To prevent oxidation,
an antioxidant mineral oil So prevent from undesirable
odour and taste
Prostaglandins & Synthesised from The main precursor in
leukotrienes arachidonic acid humans
Arachidonic acid Derived from phospholipid
membrane by
phospholipase A2
Cyclooxygenase enzymes Prostaglandins
COX 1 & COX 2
5-lipooxygenase enzyme Leukotrienes
Saponification: - the reaction of fat (triglycerides) or oil with an inorganic alkali (KOH) to
prepare soap and glycerol.
e.g. sodium stearate, potassium stearate
zinc stearate – water repellent powder
- used as a tablet lubricant
- but cause pulmonary inflammation
Opioids: - prototype is morphine.
Morphine has 5 asymmetric centres and naturally occurs as L(-) enantiomers.
Morphine analogue Chemical name
Codeine Methylmorphine
Heroin Diacetyl morphine
Numorphan Oxymorphone
Dilaudid Hydromorphone
Dionin Ethylmorphine
Pentazocine Mixed opioid agonist – antagonist
- used for analgesia
Naloxone An opioid antagonist
Pentazocine + naloxone To reduce potential abuse effect of
pentazocine &
to decrease its respiratory depressive effect
You might also like
- BIOCHEMISTRY - Summary of PathwaysDocument8 pagesBIOCHEMISTRY - Summary of PathwaysWendy Mae100% (10)
- Talking Fashion: Pierre Cardin Interviewed by Jan KedvesDocument5 pagesTalking Fashion: Pierre Cardin Interviewed by Jan KedvesJanKedves100% (1)
- Amino Acid Metabolism: Inborn ErrorsDocument18 pagesAmino Acid Metabolism: Inborn ErrorsAdedoyin BankoleNo ratings yet
- Soal USM STAN 2014 Kunci JawabanDocument51 pagesSoal USM STAN 2014 Kunci JawabanAnding NdingNo ratings yet
- Biotransformation Reaction: Glucuronide Conjugation OxidationDocument1 pageBiotransformation Reaction: Glucuronide Conjugation OxidationJakareaNo ratings yet
- .Degradasi ProteinDocument29 pages.Degradasi ProteinSeffy Yane SuhandaNo ratings yet
- Thiamine Pyrophosphate (TTP) Thiamine Pyrophosphate (TTP)Document4 pagesThiamine Pyrophosphate (TTP) Thiamine Pyrophosphate (TTP)Lyan SamsonNo ratings yet
- Organic Medicinal Chemistry: FunctionalizationDocument25 pagesOrganic Medicinal Chemistry: FunctionalizationPrincess RonsableNo ratings yet
- Pyidoxine, BiotinDocument19 pagesPyidoxine, Biotinlovi bahunNo ratings yet
- 3 Nucleotide Metabolism FirstDocument25 pages3 Nucleotide Metabolism Firstsampahgordon5No ratings yet
- Niacin: DR. Beenish Zafar BiochemistryDocument32 pagesNiacin: DR. Beenish Zafar BiochemistryShehryar AbbasNo ratings yet
- Nucleotide Structure, Function, Metabolism and DNA Replication (New Curriculum) - StudentsDocument47 pagesNucleotide Structure, Function, Metabolism and DNA Replication (New Curriculum) - StudentsWing Yeng TanNo ratings yet
- Lecture 6 - General and Characteristics. Water Soluble VitaminsDocument29 pagesLecture 6 - General and Characteristics. Water Soluble VitaminsEiad SamyNo ratings yet
- MSB 202: Neurolocomotor: Neurotransmitters - Overview of Metabolism (Anabolism and Catabolism)Document42 pagesMSB 202: Neurolocomotor: Neurotransmitters - Overview of Metabolism (Anabolism and Catabolism)Jacob MasikaNo ratings yet
- Bca Protein Metab 2Document69 pagesBca Protein Metab 2Genina MaylemNo ratings yet
- 1.protein Digestion, Urea Cy, MbbsDocument81 pages1.protein Digestion, Urea Cy, MbbsDebarghya MukherjeeNo ratings yet
- Rate Limiting StepsDocument2 pagesRate Limiting StepsvictoreffiomNo ratings yet
- Biochem 35 Trans 4 Nucleic Acid MetabolismDocument10 pagesBiochem 35 Trans 4 Nucleic Acid MetabolismBeam CanoNo ratings yet
- Proteins LASDocument6 pagesProteins LASelly scatusNo ratings yet
- Amphibolic Nature of Krebs Cycle: How What We Are Is What We EatDocument33 pagesAmphibolic Nature of Krebs Cycle: How What We Are Is What We EatSoloNo ratings yet
- Xenobiotics:: Xenobiotics Phase1 Phase2 Prodrug and Carcinogen Prodrug and Carcinogen Xenobiotics XenobioticsDocument4 pagesXenobiotics:: Xenobiotics Phase1 Phase2 Prodrug and Carcinogen Prodrug and Carcinogen Xenobiotics XenobioticsAsad IslamNo ratings yet
- Lipidosis HepaticaDocument45 pagesLipidosis HepaticaJojoa E WilNo ratings yet
- SupplementaryDocument31 pagesSupplementaryKaranNo ratings yet
- 6 Major Classes of EnzymesDocument6 pages6 Major Classes of EnzymesnoorNo ratings yet
- MicroBio Lec Transes 8 9Document7 pagesMicroBio Lec Transes 8 9Kai BarsanaNo ratings yet
- Vitamins Synonyms Chemistry Coenzyme Form RDA Sources Properties Physiologic Role DeficiencyDocument9 pagesVitamins Synonyms Chemistry Coenzyme Form RDA Sources Properties Physiologic Role Deficiencykristian markus delos santosNo ratings yet
- PDH Complex and TCA CycleDocument20 pagesPDH Complex and TCA CycleDarrion LouisNo ratings yet
- 31NucleotideMetabolism PDFDocument41 pages31NucleotideMetabolism PDFKifayat HussainNo ratings yet
- Amino Acid MetabolismDocument7 pagesAmino Acid MetabolismROHITNo ratings yet
- Janes Metro Map Pathways BLANKDocument1 pageJanes Metro Map Pathways BLANKWinston TengNo ratings yet
- Metabolism of TryptophanDocument38 pagesMetabolism of Tryptophanjagan mohan rao vanaNo ratings yet
- Water Soluble VitaminsDocument55 pagesWater Soluble VitaminsDr. M. Prasad Naidu100% (1)
- Bcaromtrans-Protein MetabolismDocument8 pagesBcaromtrans-Protein MetabolismRay Emmanuel Enriquez DomingoNo ratings yet
- ACAWPurineand Pyrimindine Synthesis Presentationfor October 112010Document41 pagesACAWPurineand Pyrimindine Synthesis Presentationfor October 112010Ezekoko ChineseNo ratings yet
- Biochemistry - Enzymes Shuttles Proteins Summary 1Document8 pagesBiochemistry - Enzymes Shuttles Proteins Summary 1Samuel NafNo ratings yet
- BiochemDocument68 pagesBiochemPrashant ChettriNo ratings yet
- Vitmin and MineralDocument1 pageVitmin and Mineralkero R.habibNo ratings yet
- Feline Hepatic LipidosisDocument46 pagesFeline Hepatic LipidosisAndre Suarez FarfanNo ratings yet
- Amino AcidsDocument1 pageAmino AcidsBobet ReñaNo ratings yet
- Tryptophan Metabolism: Dr. Ashok Kumar J International Medical School Management and Science University MalaysiaDocument23 pagesTryptophan Metabolism: Dr. Ashok Kumar J International Medical School Management and Science University MalaysiaShah HuzaifaNo ratings yet
- Chapter 18 - Nucleotide Metabolism: Nucleotides Are Composed of Three ComponentsDocument5 pagesChapter 18 - Nucleotide Metabolism: Nucleotides Are Composed of Three ComponentsSaiful IslamNo ratings yet
- Degradacion Aminoacidos y ProteinasDocument52 pagesDegradacion Aminoacidos y ProteinasEncina Abrigo Andrés PabloNo ratings yet
- Nucleotide Met Postgrad Student 2019 HandoutDocument15 pagesNucleotide Met Postgrad Student 2019 HandoutMark KoshlandNo ratings yet
- IbuprofenDocument36 pagesIbuprofenJelita ThalabNo ratings yet
- Mls 218 Protein-MetDocument45 pagesMls 218 Protein-MetZainabNo ratings yet
- Vitamin and MineralDocument8 pagesVitamin and MineralhobbycontestsNo ratings yet
- Chemistryofproteinswithclinicalapplications 190621192525 PDFDocument181 pagesChemistryofproteinswithclinicalapplications 190621192525 PDFAl-waleed Julkanain100% (1)
- PATHWAYS SummaryDocument5 pagesPATHWAYS Summaryslu.veniegasmb.1144No ratings yet
- Amino Acid Metabolism 2Document29 pagesAmino Acid Metabolism 2Francis GacheruNo ratings yet
- Bioenergetics. Tricarboxylic Acids CycleDocument60 pagesBioenergetics. Tricarboxylic Acids CyclePurwa RaneNo ratings yet
- Biosynthesis of LIpidsDocument21 pagesBiosynthesis of LIpidsJoyce Hanniel CastinoNo ratings yet
- BBB PDFDocument37 pagesBBB PDFaq educateNo ratings yet
- Urea cycle-MBBSDocument31 pagesUrea cycle-MBBSRama SubramanianNo ratings yet
- Food Chemistry - Activity 2Document3 pagesFood Chemistry - Activity 2Leda PeñaNo ratings yet
- BIOCHEMISTRY: Proteins 2: Physical PropertiesDocument7 pagesBIOCHEMISTRY: Proteins 2: Physical PropertiesCon ChinNo ratings yet
- Bio - CO 6Document2 pagesBio - CO 6Jae Bert UbisoftNo ratings yet
- Collection For Digestive Enzymes of Protein (Mansoura Dentistry)Document1 pageCollection For Digestive Enzymes of Protein (Mansoura Dentistry)elsayed barhomeNo ratings yet
- Co- and Post-Translational Modifications of Therapeutic Antibodies and ProteinsFrom EverandCo- and Post-Translational Modifications of Therapeutic Antibodies and ProteinsNo ratings yet
- The Crest of The Tide of Renascence: Sankaradeva's Kirttan-GhosāDocument30 pagesThe Crest of The Tide of Renascence: Sankaradeva's Kirttan-GhosāBhargavNo ratings yet
- JK Civil Sevices Classification Control and AppealpdfDocument35 pagesJK Civil Sevices Classification Control and AppealpdfShuja Malik100% (1)
- Dishonour of Cheques and Negotiable Instruments - Legalsutra - Law Students' Knowledge-Base - Law School Projects, Moot Court Memorials, Class and Case Notes and More!Document8 pagesDishonour of Cheques and Negotiable Instruments - Legalsutra - Law Students' Knowledge-Base - Law School Projects, Moot Court Memorials, Class and Case Notes and More!Himanshu Mene100% (1)
- English Presentations - Useful PhrasesDocument2 pagesEnglish Presentations - Useful PhrasesNitish Nagar100% (1)
- MOD2 Statement of Cash FlowsDocument2 pagesMOD2 Statement of Cash FlowsGemma DenolanNo ratings yet
- Oracle Switch ES1-24 Configuration GuideDocument88 pagesOracle Switch ES1-24 Configuration GuideNoe HernadezNo ratings yet
- Soal Kalimat Prohibition SMP Kelas 7Document3 pagesSoal Kalimat Prohibition SMP Kelas 7yura chanNo ratings yet
- Motor Claim Form THE ORIENTAL INSURANCE CO. LTD.Document4 pagesMotor Claim Form THE ORIENTAL INSURANCE CO. LTD.rajiv.surveyor7145No ratings yet
- 13 Eo Bcat Vaw Barangay Committee On Anti Trafficking and Violence Against WomenDocument2 pages13 Eo Bcat Vaw Barangay Committee On Anti Trafficking and Violence Against WomenOremor RemerbNo ratings yet
- Evaluating Tilting Pad - PaperDocument10 pagesEvaluating Tilting Pad - PaperAsit SuyalNo ratings yet
- ThesisDocument13 pagesThesiszavia_02No ratings yet
- Feminism: Broad Streams of FeminismDocument4 pagesFeminism: Broad Streams of FeminismDexin JoyanNo ratings yet
- Alter Ego 181 PreviewDocument20 pagesAlter Ego 181 PreviewJohn LloydNo ratings yet
- Keyword: 50s Music Title: Great 50s Songs For Different Moods: ContentDocument1 pageKeyword: 50s Music Title: Great 50s Songs For Different Moods: Contentaditya_bb_sharmaNo ratings yet
- CGR Project Report DDGGDDDocument21 pagesCGR Project Report DDGGDDTanvi KodoliNo ratings yet
- DeputationDocument8 pagesDeputationAnand MauryaNo ratings yet
- Main Slokas With MeaningDocument114 pagesMain Slokas With MeaningRD100% (1)
- 1.publications All BranchesDocument25 pages1.publications All BranchesNaresh GollapalliNo ratings yet
- Crane Fabrication Standard Kit: Main Locations GH PhilosophyDocument2 pagesCrane Fabrication Standard Kit: Main Locations GH PhilosophyFiroz PawaskarNo ratings yet
- Services Procurement Data SheetDocument5 pagesServices Procurement Data Sheetrollingstone3mNo ratings yet
- Beyond The Blackboard Reflection PaperDocument3 pagesBeyond The Blackboard Reflection PaperPacatang Evelyn100% (1)
- POSTMODERN, 253s '12Document270 pagesPOSTMODERN, 253s '12Raluca Gîlcă100% (1)
- Spare Parts CatalogueDocument45 pagesSpare Parts CatalogueАлексей ДомнинNo ratings yet
- Apbush DBQDocument2 pagesApbush DBQMaria Ines Carrillo100% (1)
- Performance Correction Chart of Centrifugal Oil Pump For Handling Viscous LiquidsDocument7 pagesPerformance Correction Chart of Centrifugal Oil Pump For Handling Viscous LiquidsalikajbafNo ratings yet
- Hoshizaki Technical Support - Warranty Labor Claim InstructionsDocument12 pagesHoshizaki Technical Support - Warranty Labor Claim InstructionsJohn DuttingerNo ratings yet
- Reflective Competency Statement IIIDocument4 pagesReflective Competency Statement IIIapi-534821343No ratings yet
- The 7 SealDocument11 pagesThe 7 Sealendalkachew gudeta100% (1)
- PROCEEDINGS of The Numismatic and Antiquarian Society of PhiladelphiaDocument304 pagesPROCEEDINGS of The Numismatic and Antiquarian Society of PhiladelphiaRichard CastorNo ratings yet