Professional Documents
Culture Documents
DVG Manejo Postoperatorio 2
DVG Manejo Postoperatorio 2
Uploaded by
kradoCopyright:
Available Formats
You might also like
- Case Analysis 2Document4 pagesCase Analysis 2Ivy LupacNo ratings yet
- 39 - MMPI-2 Scales PDFDocument2 pages39 - MMPI-2 Scales PDFSimone Andreotti78% (9)
- Manpower Planning FinalDocument108 pagesManpower Planning FinalDevi PrasannaNo ratings yet
- Gastric Dilation-Volvulus Syndrome in Dogs 2. Surgical and Postoperative ManagementDocument8 pagesGastric Dilation-Volvulus Syndrome in Dogs 2. Surgical and Postoperative ManagementThiara Ayu PangestiNo ratings yet
- Gastropexy GDVDocument5 pagesGastropexy GDVrizalakbarsya100% (1)
- Incisional and Laparoscopy Gastropexy For PreventiDocument7 pagesIncisional and Laparoscopy Gastropexy For PreventiAyu DinaNo ratings yet
- Diverticular Disease and Intestinal ObstructionDocument31 pagesDiverticular Disease and Intestinal ObstructionPauLa Cheneree Peña ÜNo ratings yet
- Fect Eau 2017Document15 pagesFect Eau 2017Allison DiêgoNo ratings yet
- 2018 Diagnostic Approach To The Acute AbdomenDocument15 pages2018 Diagnostic Approach To The Acute AbdomenCamila CalleNo ratings yet
- CANINE-Management Protocol For Acute Gastric Dilatation-Volvulus Syndrom in DogsDocument7 pagesCANINE-Management Protocol For Acute Gastric Dilatation-Volvulus Syndrom in Dogstaner_soysurenNo ratings yet
- Fundoplication For The TreatmentDocument5 pagesFundoplication For The TreatmentDaniel FloreaNo ratings yet
- Iwj 10 138Document7 pagesIwj 10 138Carlos HernándezNo ratings yet
- Management of The Open Abdomen 2018Document6 pagesManagement of The Open Abdomen 2018xcarlosfxNo ratings yet
- 2019 Simplified Minimally Invasive Surgical Approach For Prophylactic Laparoscopic Gastropexy in 21 CasesDocument8 pages2019 Simplified Minimally Invasive Surgical Approach For Prophylactic Laparoscopic Gastropexy in 21 CasesrizalakbarsyaNo ratings yet
- Pretorius Open (2011)Document7 pagesPretorius Open (2011)EVELYN EZEKWENo ratings yet
- Hiatalhernia 181220152813Document34 pagesHiatalhernia 181220152813meftuh abdiNo ratings yet
- Gastric Dilatation Organoaxial Volvulus in A DogDocument6 pagesGastric Dilatation Organoaxial Volvulus in A DogKrlÖz Ändrz Hërëdîa ÖpzNo ratings yet
- Abdomen SurgeryDocument98 pagesAbdomen SurgeryMohammad Al-BadawiNo ratings yet
- Hirschprung DiseaseDocument61 pagesHirschprung DiseaseAdditi Satyal100% (1)
- 4 PDFDocument6 pages4 PDFYigit İskurtNo ratings yet
- 91 Clinical ScenariosDocument198 pages91 Clinical Scenarioszph7100% (1)
- 1 PBDocument6 pages1 PBrizalakbarsyaNo ratings yet
- Hiatal HerniaDocument6 pagesHiatal HerniaElaine Jean UayanNo ratings yet
- AppendectomyDocument5 pagesAppendectomyRoseben SomidoNo ratings yet
- Rectal Computer 2Document5 pagesRectal Computer 2Su-sake KonichiwaNo ratings yet
- Obstetrics - GastrointestinalDocument6 pagesObstetrics - GastrointestinalJonathanNo ratings yet
- Omphalocele and Gastroschisis: Wendy Nguyen and Kumar BelaniDocument8 pagesOmphalocele and Gastroschisis: Wendy Nguyen and Kumar Belaniari naNo ratings yet
- Medical-Surgical NCLEX Practice QUIZ Flashcards - QuizletDocument9 pagesMedical-Surgical NCLEX Practice QUIZ Flashcards - QuizletErika Bacarro100% (1)
- JURNAL Volvulus 2-DikonversiDocument16 pagesJURNAL Volvulus 2-DikonversiLucya WulandariNo ratings yet
- Enteral Tube Feeding in Adults: Continuing Medical EducationDocument6 pagesEnteral Tube Feeding in Adults: Continuing Medical EducationMaca GallardoNo ratings yet
- Gastrointestinal Emergencies: Lillian Aronson, VMD, Daniel Brockman, BVSC, CVR, Csao, and Dorothy Cimino Brown, DVMDocument25 pagesGastrointestinal Emergencies: Lillian Aronson, VMD, Daniel Brockman, BVSC, CVR, Csao, and Dorothy Cimino Brown, DVMKmila RamirezNo ratings yet
- Oral Boards ReviewDocument218 pagesOral Boards ReviewDustin J FanciulloNo ratings yet
- Percutaneous Gastrostomy: EndoscopicDocument3 pagesPercutaneous Gastrostomy: EndoscopicRaluca CondreaNo ratings yet
- Open Versus Laparoscopic Mesh Repair of Ventral Hernias: A Prospective StudyDocument3 pagesOpen Versus Laparoscopic Mesh Repair of Ventral Hernias: A Prospective Study'Adil MuhammadNo ratings yet
- Chronic Gastric Volvulus With Diaphragmatic 2024 International Journal of SDocument5 pagesChronic Gastric Volvulus With Diaphragmatic 2024 International Journal of SRonald QuezadaNo ratings yet
- ContentServer - Asp 9Document7 pagesContentServer - Asp 9AlanGonzalezNo ratings yet
- Gossypiboma - A Rare Cause of Gastric Outlet ObstructionDocument3 pagesGossypiboma - A Rare Cause of Gastric Outlet ObstructionJuniper PublishersNo ratings yet
- Colectomy ManagementDocument10 pagesColectomy ManagementaylinNo ratings yet
- Common Complication: What Is It?Document5 pagesCommon Complication: What Is It?khalidicuNo ratings yet
- Adhesive Intestinal Obstruction. What A Resident Should KnowDocument6 pagesAdhesive Intestinal Obstruction. What A Resident Should Knowrahul ranjanNo ratings yet
- T1503C08Document5 pagesT1503C08zulfikarNo ratings yet
- Journal Mulya BedahDocument4 pagesJournal Mulya BedahMulyaa SudirmanNo ratings yet
- Case Report On Obstructed Obturator Richter Hernia Managed by Open Preperitoneal Mesh RepairDocument7 pagesCase Report On Obstructed Obturator Richter Hernia Managed by Open Preperitoneal Mesh RepairIJAR JOURNALNo ratings yet
- Esophagostomy Feeding Tubes: Procedures Pro Surgery / Critical CareDocument7 pagesEsophagostomy Feeding Tubes: Procedures Pro Surgery / Critical CareEl OnNo ratings yet
- CH 39Document3 pagesCH 39Nailin RamadsNo ratings yet
- Filia D. Yaputri A34 Barium Enema IDocument3 pagesFilia D. Yaputri A34 Barium Enema ITatyannah Alexa Maristela100% (1)
- Gastric Dilation-Volvulus Syndrome in Dogs 1 PathoDocument5 pagesGastric Dilation-Volvulus Syndrome in Dogs 1 PathoKrlÖz Ändrz Hërëdîa ÖpzNo ratings yet
- Esophageal Surgery in Newborns, Infants and Children: and K.L.N. RaoDocument5 pagesEsophageal Surgery in Newborns, Infants and Children: and K.L.N. RaoDonny Artya KesumaNo ratings yet
- Answers To Activity 4: Collaborative ManagementDocument3 pagesAnswers To Activity 4: Collaborative ManagementEsmareldah Henry SirueNo ratings yet
- Stamm Gastrostomy (Complete)Document6 pagesStamm Gastrostomy (Complete)robelNo ratings yet
- Atresia Esofágica Con Fistula Traqueoesofágica DistalDocument24 pagesAtresia Esofágica Con Fistula Traqueoesofágica DistalChristian PA100% (1)
- J Thorsurg 2018 07 001Document7 pagesJ Thorsurg 2018 07 001Gabriel RangelNo ratings yet
- The Abdomen 98 QDocument78 pagesThe Abdomen 98 QNahidNo ratings yet
- Intestinal Atresia: A Case ReportDocument3 pagesIntestinal Atresia: A Case ReportOvamelia JulioNo ratings yet
- Appendix Part 2Document7 pagesAppendix Part 2Abdullah EssaNo ratings yet
- Gavage, Enteral Feeding or Tube Feeding. Placement May Be Temporary For TheDocument5 pagesGavage, Enteral Feeding or Tube Feeding. Placement May Be Temporary For TheWillen May Delopere Lomarda100% (1)
- Open Abdomen in Critical 1Document25 pagesOpen Abdomen in Critical 1GenyNo ratings yet
- Neonatal Feeding After Gastrointestinal SurgeryDocument4 pagesNeonatal Feeding After Gastrointestinal SurgeryVianNo ratings yet
- Management of The Patient With The Open AbdomenDocument7 pagesManagement of The Patient With The Open Abdomenfabidol13No ratings yet
- Case Report: The Combination of Gastroschisis, Jejunal Atresia, and Colonic Atresia in A NewbornDocument5 pagesCase Report: The Combination of Gastroschisis, Jejunal Atresia, and Colonic Atresia in A NewbornAnonymous PmfX227No ratings yet
- Explor LaparotomyDocument14 pagesExplor LaparotomyGracia NievesNo ratings yet
- Dysphagia, A Simple Guide To The Condition, Treatment And Related ConditionsFrom EverandDysphagia, A Simple Guide To The Condition, Treatment And Related ConditionsRating: 5 out of 5 stars5/5 (1)
- LaryngoceleDocument7 pagesLaryngoceleM Grecu CeptureanuNo ratings yet
- Weldon Owen 2015 CatalogDocument97 pagesWeldon Owen 2015 CatalogWeldon Owen PublishingNo ratings yet
- CureDocument338 pagesCureThiago Cavalli Azambuja100% (1)
- Adj12077 PDF JsessionidDocument2 pagesAdj12077 PDF JsessionidhayiarNo ratings yet
- MSDS Oxalic AcidDocument5 pagesMSDS Oxalic AcidAmber BurgessNo ratings yet
- Guidance On Drug Master File SystemDocument23 pagesGuidance On Drug Master File SystemKhushman AsodariyaNo ratings yet
- The Great Aloe BookDocument96 pagesThe Great Aloe BookAli AzzaliNo ratings yet
- Example:: Healthy Life Sebastian Oliva Task 1Document1 pageExample:: Healthy Life Sebastian Oliva Task 1Sebastian OlivaNo ratings yet
- Interventions Homework RefusalDocument5 pagesInterventions Homework Refusalafetvdqbt100% (1)
- Ayurvedic Healing A Comprehensive Guide David Frawley.15003 Table of ContentsDocument4 pagesAyurvedic Healing A Comprehensive Guide David Frawley.15003 Table of ContentsPolate Solate0% (1)
- A 27-Year-Old Woman Presented With Three-Day History of Generalized Mild Abdominal PainDocument144 pagesA 27-Year-Old Woman Presented With Three-Day History of Generalized Mild Abdominal PainAldredNo ratings yet
- Home Aquarium CatalogDocument48 pagesHome Aquarium CatalogDjordjevic DraganNo ratings yet
- Zinc in Wound Healing Theoretical, ExperimentalDocument15 pagesZinc in Wound Healing Theoretical, ExperimentalRifky Budi TriyatnoNo ratings yet
- 4 Day Power Muscle Burn Workout Split - Muscle & StrengthDocument26 pages4 Day Power Muscle Burn Workout Split - Muscle & Strengthsupermirko78100% (2)
- Arie History of SurgeryDocument20 pagesArie History of SurgeryArie RezaNo ratings yet
- Your Disability Doesn't Disqualify You From Personal GrowthDocument8 pagesYour Disability Doesn't Disqualify You From Personal GrowthMarjorie BasabeNo ratings yet
- History of PenicillinDocument11 pagesHistory of PenicillinAilsa StephensonNo ratings yet
- Activity IntoleranceDocument6 pagesActivity IntoleranceDenvEr CabaniLlasNo ratings yet
- DementiaDocument4 pagesDementiaRegine Lorenzana Mey-AngNo ratings yet
- TamsulosinDocument27 pagesTamsulosinAnna WidiaNo ratings yet
- Designed, Tested and Trusted by Operating Theatre ProfessionalsDocument5 pagesDesigned, Tested and Trusted by Operating Theatre ProfessionalsYusuf Selim ErkenNo ratings yet
- Medical Leave of Absence (Mloa) Treatment Provider Report: SECTION I: To Be Completed by The StudentDocument4 pagesMedical Leave of Absence (Mloa) Treatment Provider Report: SECTION I: To Be Completed by The StudentShubho DasNo ratings yet
- Chikitsa ChatuspadDocument2 pagesChikitsa ChatuspadAnand YadavNo ratings yet
- Topic 18. Secondary Lesions of The SkinDocument2 pagesTopic 18. Secondary Lesions of The SkinLara Paz100% (1)
- 2019-02-27T205056.849Document3 pages2019-02-27T205056.849writer topNo ratings yet
- Compartment ModelsDocument67 pagesCompartment ModelsSunil100% (3)
- Hormonal Secretion of PancreasDocument17 pagesHormonal Secretion of PancreasNazia WasimNo ratings yet
- MastoiditisDocument37 pagesMastoiditisAkanksha EkkaNo ratings yet
DVG Manejo Postoperatorio 2
DVG Manejo Postoperatorio 2
Uploaded by
kradoOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
DVG Manejo Postoperatorio 2
DVG Manejo Postoperatorio 2
Uploaded by
kradoCopyright:
Available Formats
Downloaded from inpractice.bmj.com on April 24, 2014 - Published by group.bmj.
com
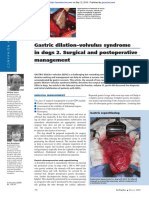
COM PA N I O N A N I M A L PR ACT I CE Appropriate treatment of areas
of gastric necrosis, which can
occur secondarily to gastric
dilation–volvulus, as shown
here, is vital to maximise a
patient’s chance of survival
Gastric dilation–volvulus syndrome
in dogs 2. Surgical and postoperative
management MICKEY TIVERS AND DAN BROCKMAN
GASTRIC dilation–volvulus (GDV) is a challenging but rewarding condition to treat. Appropriate
decision making and management can help to achieve a success rate of up to 95 per cent in cases
uncomplicated by gastric necrosis. This article, the second of two reviewing the management of canine
gastric dilation–volvulus syndrome (GDVs), describes the surgical and postoperative management of
GDVs in dogs. An article in the February issue (In Practice, volume 31, pp 66-69) discussed the diagnosis
and initial stabilisation of patients with GDVs.
Mickey Tivers
graduated from
Bristol in 2002. He SURGICAL MANAGEMENT Repeated gastric lavage with warm tap water will help to
is currently a staff complete gastric emptying. Complete gastric decompres-
clinician in small
animal surgery at
The aims of surgical management of acute GDV include: sion makes repositioning of the stomach easier and facili-
the Royal Veterinary ■ Gastric decompression and repositioning; tates all subsequent gastric manipulations. Assuming that
College (RVC). He
holds the RCVS
■ Assessment of the viability of abdominal organs;
certificate in small ■ Removal of devitalised tissue;
animal surgery and ■ Gastropexy.
is a diplomate of the Gastric repositioning
European College of Time should be taken to fully evaluate and treat an
Veterinary Surgeons. affected dog surgically. It has been shown that, provid-
ing all other aspects of preanaesthetic stabilisation and
anaesthetic administration are appropriate, increased
surgical time does not put an animal at additional
risk. The surgeon must be thorough and methodical
to optimise the chance of recovery.
The dog should be prepared aseptically for surgery
and a ventral midline coeliotomy performed. Care should
be taken when entering the peritoneal cavity to avoid
puncturing the dilated stomach. The incision should be
Dan Brockman
graduated from
as long as necessary to allow adequate inspection of all
Liverpool in 1987. abdominal organs and facilitate gastric decompression
He is currently
and gastropexy (typically from the xiphoid to beyond the
professor of small
animal surgery at umbilicus). Abdominal retractors (Balfour or Gosset
the RVC. He holds retractors) can be used to aid abdominal exposure.
RCVS certificates in
veterinary radiology
and in small animal Gastric decompression and repositioning
orthopaedics. He is
a diplomate of the A distended stomach is immediately obvious on entering
American College of the peritoneal cavity. In dogs with a 180 to 360º clock-
Veterinary Surgeons
and the European
wise gastric rotation, the stomach will have entered the
College of Veterinary omental bursa and will, therefore, be covered by an
Surgeons. omental leaf. Despite preoperative gastric decompres-
sion, further decompression during surgery is usually
helpful. This should be performed by an assistant passing Exploratory laparotomy in a dog with GDV. The dilated
stomach is covered by omentum, which is indicative of
In Practice (2009) an orogastric tube and is facilitated by the surgeon manu- a clockwise torsion
31, 114-121 ally guiding the tube through the cardiac sphincter.
114 In Practice ● MARCH 20 09
Downloaded from inpractice.bmj.com on April 24, 2014 - Published by group.bmj.com
the surgeon is standing on the dog’s right-hand side, the
pylorus is identified and grasped with the right hand Timing of surgical intervention
(typically on the dog’s left-hand side). Downward pres-
The timing of surgical intervention remains controversial. Some clinicians sug-
sure on the right side of the visible portion of the stomach
gest a period of prolonged stabilisation before surgery. The authors prefer a
with the left hand, coupled with gentle traction on the
shorter period of stabilisation followed by rapid surgical exploration. This may
pylorus, will aid gastric repositioning (see box below).
allow identification and treatment of the small number of dogs suffering from
If the passage of a stomach tube is not possible before
gastric necrosis or ongoing haemorrhage and, hence, improve the prognosis in
derotation, intraoperative needle gastrocentesis using
this group of patients.
a 16 gauge needle or intravenous catheter can be
performed before gastric repositioning.
Assessment of gastric viability If the stomach wall appears grey/green, purple or
and gastric resection black, has areas of seromuscular tearing or is much thin-
Gastric necrosis is reported in 10 to 37 per cent of dogs ner than the adjacent stomach wall, ischaemia is likely
with GDV (Glickman and others 1998, De Papp and oth- and subsequent tissue necrosis is probable. Obviously,
ers 1999). It is associated with a poor prognosis and this is a subjective assessment. Gentle palpation for pul-
appropriate treatment is vital to maximise the chance of sations in the gastric and splenic vessels can also be
survival. Once the stomach has been decompressed and helpful. If there is still some doubt about the viability
repositioned, it should be checked carefully for areas of of the stomach, it should be assessed by looking for
ischaemia and necrosis. If the stomach appears grossly active bleeding following partial-thickness incisions in
normal, an assistant should lavage it with warm water the gastric wall.
via the stomach tube. A gastrotomy may occasionally be
necessary to remove impacted food or foreign bodies.
The junction between the fundus and the gastric body
along the greater curvature of the stomach is the most
common site for necrosis following GDV and should be
thoroughly examined. In cases of GDV, the serosa is often
bruised, so five to 10 minutes should be allowed for it to
recover before a full assessment is carried out. The most
practical indicators of gastric wall condition are:
■ Colour;
■ Wall thickness;
■ Presence of pulses in local vessels;
■ Bleeding from partial thickness (seromuscular) inci-
Necrotic gastric wall
sions in the gastric wall; seen during exploratory
■ Presence of thrombi in the gastric vasculature. laparotomy
Repositioning of the twisted stomach pictured The dilated stomach in a normal position
on the left following derotation
In Practice ● MARCH 20 09 115
Downloaded from inpractice.bmj.com on April 24, 2014 - Published by group.bmj.com
In dogs suspected of having an area of gastric wall
devitalisation or in cases of necrosis, a partial gastrectomy
must be performed. Gastric necrosis without partial
gastrectomy has been associated with unacceptably high
mortality. A safe partial gastrectomy can be performed
manually but is much easier using surgical stapling equip-
ment. This alternative method has the advantage of reduc-
ing operating time, which potentially offsets the increased
cost of the procedure. The authors recommend the use of
Partial gastric resection either a gastrointestinal anastomosis stapler (GIA-50 or
due to gastric wall necrosis. GIA-90; US Surgical/Tyco) or a thoracoabdominal stapler
The necrotic portion of the
stomach is resected using a (TA-90; US Surgical/Tyco) with a 4·8 mm (green) staple
GIA-90 stapler (US Surgical/ cartridge. The staple lines should be reinforced using a
Tyco)
continuous Cushing or Lembert suture pattern.
The gastric cardia and distal oesophagus should also
be carefully evaluated for necrosis. Successful resection
Open resection and reconstruction of this area is extremely challenging
and the prognosis for dogs with this injury is grave.
■ Place stay sutures in the stomach wall using 2 or 3 metric polypropylene to
Gastric necrosis occasionally leads to perforation and
allow manipulation
contamination of the peritoneal cavity. Treatment is as for
■ Pack the stomach off from the rest of the abdomen with large, moist laparot-
dogs requiring gastric resection with additional therapy
omy sponges
for peritonitis. Typically, such dogs are more severely
■ Sharply excise the affected portion of the gastric wall and continue the resec-
affected in a global sense and are therefore at greater risk
tion until the cut edges are actively bleeding. This should ensure healing without
of additional disease processes such as systemic inflam-
further necrosis
matory response syndrome (SIRS) and disseminated
■ Close the stomach in two or three layers. A simple continuous suture pattern
intravascular coagulation (DIC). These animals are the
in the submucosa should be followed by a simple interrupted pattern in the mus-
most challenging to treat.
cularis and serosa. Oversewing the suture line with a continuous or interrupted
inverting pattern (eg, Cushing or Lembert) can reinforce this closure. Size 2 or 3
Assessment of the spleen and splenectomy
metric polydioxanone (PDS; Ethicon) or polyglactin 910 (Vicryl; Ethicon) are suit-
Once repositioned, the spleen should be assessed for
able suture materials
evidence of vascular compromise. The splenic vessels
Incisional gastropexy
A B
(A) Identify the pyloric antrum following gastric (B) Make a 5 cm seromuscular incision longitudinally in the
decompression pyloric antrum, taking care not to enter gastric lumen
E F
Suture the edges of gastric wall incision to the edges of the body wall incision with two simple continuous sutures using
3 metric polydioxanone. (E) Place the first suture dorsally on the cranial border of the incisions. (F) Appose the cranial
borders of the incisions with a simple continuous suture
116 In Practice ● MARCH 20 09
Downloaded from inpractice.bmj.com on April 24, 2014 - Published by group.bmj.com
should also be inspected for avulsion and thrombosis. If these reasons, this technique can be particularly useful
the splenic vein or artery contains palpable thrombi, in dogs with chronic GD or those that have undergone
both vessels should be ligated and the spleen removed gastric resection.
promptly to prevent the release of thrombi into the sys- Following surgery, the abdomen should be lavaged,
temic or portal circulation. If no intravascular thrombi ideally with a balanced and buffered electrolyte solution,
are present, the spleen should be allowed to recover and closed in a routine fashion.
while perfusion returns to normal. Black areas of spleen
are consistent with infarction, so a partial or complete
splenectomy should be performed, depending on the POSTOPERATIVE MANAGEMENT
amount of spleen affected. The authors do not recom-
mend attempting a partial splenectomy without surgical Frequent, careful monitoring of a patient’s physical
stapling equipment. If the spleen has undergone com- parameters, including pulse rate and quality, respiratory
plete torsion, a splenectomy should be performed before rate, temperature, mucous membrane colour, capillary
reducing the twist to prevent the risk of releasing toxins, refill time (CRT) and evidence of abdominal distension,
myocardial active substances and thromboemboli into will enable early detection of complications. Packed cell
the circulation. volume (PCV) and serum total solids (TS) should also
be measured every two to four hours initially. As men-
Gastropexy tioned in Part 1, frequent quantification of urine produc-
A gastropexy should be performed in all cases of GDV tion and evaluation of urine specific gravity is easy to
or gastric dilation (GD) to prevent recurrent GDV. perform and gives extremely useful information about
Recurrence of GDV is common in dogs that do not the perfusion state of an animal. Ideally, 1 to 2 ml/kg/
undergo gastropexy. Most gastropexy techniques aim to hour of urine should be produced. Additional monitor-
fix the pylorus, which is the most mobile part of the ing, including continuous electrocardiography, invasive
stomach, to the right abdominal wall. A gastropexy can or non-invasive blood pressure measurement, central
be carried out using incisional (see box below), belt-loop, venous pressure assessment and urine output, is helpful,
circumcostal and tube (see box on pages 118 to 119) if available.
techniques. Incisional, belt-loop and tube gastropexies Fluid therapy is maintained by intravenous adminis-
are all quick to perform and create strong adhesions. A tration of a balanced electrolyte solution at a rate of 4 to
tube gastropexy has the advantage of providing enteral 10 ml/kg/hour for the first 24 hours, depending on the
access for postoperative nutrition and allows gastric assessment of cardiovascular parameters. The intra-
decompression should postoperative dilation occur. For venous fluid rate and composition should be regularly
C D
(C) and (D) Make a matching incision in a ventrodorsal direction in the peritoneum and transverse abdominal muscle
on the right abdominal wall, caudal to the last rib
G
(G) Place another suture dorsally at the caudal border
H
of the incisions and appose the caudal borders of the
incisions with a simple continuous suture (H) The finished gastropexy
In Practice ● MARCH 20 09 117
Downloaded from inpractice.bmj.com on April 24, 2014 - Published by group.bmj.com
assessed and tailored to the perceived needs of the ani- COMPLICATIONS AND PROGNOSIS
mal, based on changes in subjective (mucous membrane
colour, CRT, pulse rate and quality) and objective (arte- The prognosis for dogs treated for GD and GDV is excel-
rial blood pressure, PCV/TS, urine output) data. After 24 lent and good, respectively. Many reports cite a survival
hours, if the patient is progressing well, fluid therapy can rate of almost 100 per cent for dogs with GDV that do not
be gradually withdrawn. require gastric resection. For patients requiring partial
Postoperative analgesia is an important aid to recov- gastrectomy, the survival rate is up to 70 per cent.
ery. Initially, morphine at a dose of 0·2 to 0·4 mg/kg intra-
muscularly or methadone at the same dose intravenously, Hypoperfusion and hypotension
every four hours, is recommended. After the first 24 Dogs occasionally remain hypotensive and, therefore,
hours, buprenorphine at 0·01 to 0·03 mg/kg intravenously hypoperfused after surgery. This hypotension is most
should be adequate. Non-steroidal anti-inflammatory commonly due to inadequate fluid therapy in the periop-
drugs (NSAIDs) should generally be avoided initially erative period. Hypotension may also occur secondarily to
because of their potentially devastating effects on renal ongoing haemorrhage because of inadequate primary sur-
and gastric mucosal blood flow in hypotensive animals. gical haemostasis. In addition, it can be caused by reduced
Once a dog has recovered from surgery and remains colloid osmotic pressure and subsequent loss of fluid from
euvolaemic, NSAIDs can be prescribed to provide further the intravascular space or abnormal fluid distribution as a
postoperative analgesia. If recovery is uneventful, water result of altered peripheral vascular tone. Poor cardiac
and small amounts of food can be offered orally the day function can sometimes lead to hypotension (see below).
after surgery and the fluid rate reduced. Patients should Pulse rate and quality, CRT and urine output should all
remain hospitalised for three to five days following be monitored carefully. Poor pulse quality, tachycardia,
surgery to ensure a complication-free recovery. slow CRT and the production of a reduced volume of con-
Tube gastropexy
A B
A large (24 to 26 gauge) Foley catheter or de Prezzer mushroom-tipped catheter (the authors prefer
the latter in most cases) should be used for the tube. (A) Make a stab incision in the body wall
approximately 2 to 5 cm lateral to the ventral midline and 2 cm caudal to the last rib on the right-
hand side. (B) and (C) Pass the tube through the stab incision into the abdominal cavity with the
aid of forceps
C
G
F
(G) and (H) Preplace four pexy sutures using 3 metric polydioxanone
(F) Tightly tie the purse-string suture around the catheter and, if using a around the gastric and abdominal wall incisions (a box suture), taking
Foley catheter, inflate the bulb with saline. care to avoid including the end of the catheter in these sutures
118 In Practice ● MARCH 20 09
Downloaded from inpractice.bmj.com on April 24, 2014 - Published by group.bmj.com
centrated urine are all indicative of hypotension and hypo- and electrolytes (especially potassium and, if possible,
volaemia. Direct or indirect arterial blood pressure moni- magnesium), and correct any underlying abnormality as
toring provides an objective measurement of hypotension well as addressing the arrhythmia.
and is a useful indicator of response to treatment. PCV and The impact of an arrhythmia on a dog’s cardiac func-
TS should be measured in patients responding poorly to tion should be evaluated before considering treatment.
fluid therapy. In a hypotensive animal, PCV and TS sug- Assessment of hypoperfusion as described above will
gestive of haemoconcentration indicate the need for more help to determine the significance of the arrhythmia.
aggressive fluid therapy (boluses of balanced electrolyte Persistent hypoperfusion despite adequate fluid therapy
solution). If the PCV and TS are low in a hypotensive ani- suggests that the arrhythmia is affecting cardiac per-
mal, the administration of blood products or synthetic col- formance and should be treated. Dogs with ventricular
loids may be indicated. Patients should be re-evaluated arrhythmias should be treated with lidocaine in the first
frequently following changes in fluid therapy. instance. A bolus of 1 to 2 mg/kg is given intravenously,
slowly, over 30 seconds. This dose can be repeated up to
Cardiac arrhythmias a total of 8 mg/kg. Rapid bolus injection often results in
Cardiac arrhythmias are reported in approximately 40 to vomiting. Overdosage causes seizure activity, although
50 per cent of dogs following an acute episode of GDV. this is typically short lived. If a favourable response is
They are most frequently ventricular in origin, ranging seen to lidocaine therapy (ie, conversion to sinus rhythm,
from intermittent ventricular premature conductions to slowing of the ventricular rate), a constant-rate infusion
sustained ventricular tachycardia. Arrhythmias can be should be started at a rate of 50 to 80 μg/kg/minute. As
due to myocardial injury, persistence of circulating myo- lidocaine is proarrhythmic at low doses, this dose should
cardial active substances or electrolyte imbalances. It is not be tapered, but stopped abruptly when therapy is no
therefore important to check a dog’s acid–base status longer required.
D E
(D) Surgeon’s view of the abdominal contents, with the pylorus in the centre of the image. The tube can be seen entering through the right abdominal
wall. Place stay sutures in the stomach to allow manipulation. (E) Preplace a purse-string suture in the pyloric antrum using 3 metric polydioxanone.
Make a stab incision into the stomach through the suture and place the catheter into the lumen
I
(I) Omentalise the pexy site. (J) Draw up the balloon or
mushroom tip to the stomach wall and secure the tube
on the outside of the skin with a Chinese finger-trap
suture. Place an abdominal bandage postoperatively to
protect the tube. The gastropexy tube should remain in
place for seven to 10 days, and should be kept clean and
protected with a bandage. Remove the tube by traction
H and leave the stoma to heal by secondary intention J
In Practice ● MARCH 20 09 119
Downloaded from inpractice.bmj.com on April 24, 2014 - Published by group.bmj.com
Although arrhythmias are a potentially serious com-
plication, they do not appear to influence survival if Client education
treated appropriately.
Owners of dogs suffering from GDV should be warned that the condition could
recur. The signs associated with recurrence should be made clear and the clients
Aspiration pneumonia
advised to seek veterinary attention as soon as possible if they are concerned.
Retching and vomiting before surgery, perioperative and
Advice on feeding remains unclear. The authors recommend dividing food up
postoperative regurgitation and oesophagitis place dogs
into several small meals and advise owners to keep mealtimes as stress-free as
with GDV at risk of developing aspiration pneumonia.
possible.
An abnormal respiratory rate and pattern, crackles on
thoracic auscultation, depression and pyrexia are sugges-
tive of pneumonia. Thoracic radiographs, arterial blood
gas analysis and tracheal or bronchoalveolar lavage with Prophylactic gastropexy
cytology and culture will help to confirm this clinical
suspicion. Dogs with aspiration pneumonia should be Some consideration should be given to prophylactic gastropexy in dog breeds
treated with appropriate antibiotics, nebulisation and that are predisposed to GD and GDV. Dogs with relatives that have experienced
coupage, and supplemental oxygen, as required. GDV are significantly more likely to be affected themselves and there may be
good reason for prophylaxis in these animals. Gastropexy can be performed
Abnormal gastric motility as an elective procedure or preferably at the time of an ovariohysterectomy or
Following successful GDV surgery, some dogs, especially other abdominal procedure. The risks and complications related to the surgery
those having undergone partial gastrectomy, may suffer must be weighed up against the risk of GDV, which remains uncommon (prev-
from reduced or absent gastric motility. Such dogs may alence of 0·8 to 2·8 per cent). Laparoscopic gastropexy may facilitate surgery
vomit or remain anorexic. Metaclopramide (1 to 2 mg/kg/ (Rawlings and others 2002).
day intravenous infusion) or a very low dose of erythro-
mycin (0·5 to 1·0 mg/kg every eight hours) may help to
improve motility. Dogs at risk of SIRS and DIC should be monitored
Dogs that have suffered mucosal damage may benefit closely. Coagulation parameters, acid–base status and
from the administration of a proton pump inhibitor such blood gas levels should be determined before further
as omeprazole (1 mg/kg intravenously once daily) or a treatment. Prolonged prothrombin and activated partial
gastric protectant such as sucralfate (2·5 to 5 ml orally thromboplastin times, low platelet count, decreased
every eight hours). fibrinogen and increased fibrin degradation products or
D-dimers are indicators of DIC. Treatment with fresh
Gastric necrosis and perforation frozen plasma to replace coagulation factors may be
Gastric necrosis and perforation can occur up to five days indicated in these patients. The prognosis for animals
postoperatively despite careful intraoperative gastric wall with these complications is very poor.
assessment. It may be due to dehiscence of a gastrectomy
repair and highlights the importance of careful assess- References
DE PAPP, E., DROBATZ, K. J. & HUGHES, D. (1999) Plasma lactate
ment at the time of surgery and good surgical technique. concentration as a predictor of gastric necrosis and survival among
This complication is suspected in dogs that deteriorate or dogs with gastric dilatation–volvulus: 102 cases (1995-1998).
Journal of the American Veterinary Medical Association 215, 49-52
fail to improve following surgery or show signs of sepsis. GLICKMAN, L. T., LANTZ, G. C., SCHELLENBERG, D. B. &
Radiography, ultrasonography and cytological evaluation GLICKMAN, N. W. (1998) A prospective study of survival and
of peritoneal fluid can help to confirm the diagnosis. recurrence following the acute gastric dilatation–volvulus
syndrome in 136 dogs. Journal of the American Animal Hospital
Surgical treatment is necessary and involves an explora- Association 34, 253-259
tory coeliotomy, debridement and repair of the gastric RAWLINGS, C. A., MAHAFFEY, M. B., BEMENT, S. & CANALIS, C.
(2002) Prospective evaluation of laparoscopic-assisted gastropexy
wall defect, and appropriate management of peritonitis. in dogs susceptible to gastric dilatation. Journal of the American
The prognosis is grave in cases with postoperative gastric Veterinary Medical Association 221, 1576-1581
TIVERS, M. & BROCKMAN, D. (2009) Gastric dilation–volulus
necrosis, perforation and septic peritonitis. syndrome in dogs 1. Pathophysiology, diagnosis and stabilisation.
In Practice 31, 66-69
Systemic inflammatory response syndrome,
Further reading
sepsis and disseminated intravascular BROCKMAN, D. J. & HOLT, D. E. (2000) Management protocol for
coagulation acute gastric dilatation–volvulus syndrome in dogs. Compendium on
Continuing Education for the Practicing Veterinarian 22, 1025-1034
Dogs suffering severe problems such as GDV are at risk BROCKMAN, D. J., HOLT, D. E. & WASHABAU, R. J. (2000)
of SIRS. This is the clinical manifestation of systemic Pathogenesis of acute canine gastric dilatation–volvulus
inflammation in response to a variety of serious insults. syndrome: is there a unifying hypothesis? Compendium on
Continuing Education for the Practicing Veterinarian 22, 1108-1113
Patients showing persistent hypoperfusion or hypoten- BROCKMAN, D. J., WASHABAU, R. J. & DROBATZ, K. J. (1995)
sion despite adequate volume resuscitation, pyrexia or Canine gastric dilatation–volvulus syndrome in a veterinary
critical care unit: 295 cases (1986-1992). Journal of the American
decreased mentation should be monitored carefully. Veterinary Medical Association 207, 460-464
SIRS increases the risk of multiorgan dysfunction and BUBER, T., SARAGUSTY, J., RANEN, E., EPSTEIN, A., BDOLAH-
ABRAM, T. & BRUCHIM, Y. (2007) Evaluation of lidocaine
every effort should be made to stabilise these animals treatment and risk factors for death associated with gastric
promptly. The presence of a documented infection in the dilatation and volvulus in dogs: 112 cases (1997-2005). Journal of
presence of SIRS is classified as sepsis. the American Veterinary Medical Association 230, 1334-1339
MONNET, E., LHERMETTE, P. & SOBEL, D. (2008) Rigid endoscopy:
DIC may also be seen in severely affected dogs. The laparoscopy. In BSAVA Manual of Canine and Feline Endoscopy
systemic release of inflammatory mediators causes and Endosurgery. Eds P. Lhermette and D. Sobel. Quedgeley,
BSAVA Publications. pp 158-174
widespread endothelial damage and activates the coagu- RASMUSSEN, L. (2003) Stomach. In Textbook of Small Animal Surgery,
lation system and microvascular thrombosis. Eventual 3rd edn. Ed D. Slatter. Philadelphia, Saunders Elsevier. pp 592-643
WILLIAMS, J. M. (2005) Gastric dilatation and volvulus. In BSAVA
depletion of coagulation factors results in coagulopathy Manual of Canine and Feline Abdominal Surgery. Eds J. M.
and clinical signs of bleeding. Williams and J. D. Niles. Quedgeley, BSAVA Publications. pp 80-95
In Practice ● MARCH 20 09 121
Downloaded from inpractice.bmj.com on April 24, 2014 - Published by group.bmj.com
Gastric dilation−volvulus syndrome in dogs:
2. Surgical and postoperative management
Mickey Tivers and Dan Brockman
In Practice 2009 31: 114-121
doi: 10.1136/inpract.31.3.114
Updated information and services can be found at:
http://inpractice.bmj.com/content/31/3/114
These include:
Email alerting Receive free email alerts when new articles cite this article. Sign up in
service the box at the top right corner of the online article.
Notes
To request permissions go to:
http://group.bmj.com/group/rights-licensing/permissions
To order reprints go to:
http://journals.bmj.com/cgi/reprintform
To subscribe to BMJ go to:
http://group.bmj.com/subscribe/
You might also like
- Case Analysis 2Document4 pagesCase Analysis 2Ivy LupacNo ratings yet
- 39 - MMPI-2 Scales PDFDocument2 pages39 - MMPI-2 Scales PDFSimone Andreotti78% (9)
- Manpower Planning FinalDocument108 pagesManpower Planning FinalDevi PrasannaNo ratings yet
- Gastric Dilation-Volvulus Syndrome in Dogs 2. Surgical and Postoperative ManagementDocument8 pagesGastric Dilation-Volvulus Syndrome in Dogs 2. Surgical and Postoperative ManagementThiara Ayu PangestiNo ratings yet
- Gastropexy GDVDocument5 pagesGastropexy GDVrizalakbarsya100% (1)
- Incisional and Laparoscopy Gastropexy For PreventiDocument7 pagesIncisional and Laparoscopy Gastropexy For PreventiAyu DinaNo ratings yet
- Diverticular Disease and Intestinal ObstructionDocument31 pagesDiverticular Disease and Intestinal ObstructionPauLa Cheneree Peña ÜNo ratings yet
- Fect Eau 2017Document15 pagesFect Eau 2017Allison DiêgoNo ratings yet
- 2018 Diagnostic Approach To The Acute AbdomenDocument15 pages2018 Diagnostic Approach To The Acute AbdomenCamila CalleNo ratings yet
- CANINE-Management Protocol For Acute Gastric Dilatation-Volvulus Syndrom in DogsDocument7 pagesCANINE-Management Protocol For Acute Gastric Dilatation-Volvulus Syndrom in Dogstaner_soysurenNo ratings yet
- Fundoplication For The TreatmentDocument5 pagesFundoplication For The TreatmentDaniel FloreaNo ratings yet
- Iwj 10 138Document7 pagesIwj 10 138Carlos HernándezNo ratings yet
- Management of The Open Abdomen 2018Document6 pagesManagement of The Open Abdomen 2018xcarlosfxNo ratings yet
- 2019 Simplified Minimally Invasive Surgical Approach For Prophylactic Laparoscopic Gastropexy in 21 CasesDocument8 pages2019 Simplified Minimally Invasive Surgical Approach For Prophylactic Laparoscopic Gastropexy in 21 CasesrizalakbarsyaNo ratings yet
- Pretorius Open (2011)Document7 pagesPretorius Open (2011)EVELYN EZEKWENo ratings yet
- Hiatalhernia 181220152813Document34 pagesHiatalhernia 181220152813meftuh abdiNo ratings yet
- Gastric Dilatation Organoaxial Volvulus in A DogDocument6 pagesGastric Dilatation Organoaxial Volvulus in A DogKrlÖz Ändrz Hërëdîa ÖpzNo ratings yet
- Abdomen SurgeryDocument98 pagesAbdomen SurgeryMohammad Al-BadawiNo ratings yet
- Hirschprung DiseaseDocument61 pagesHirschprung DiseaseAdditi Satyal100% (1)
- 4 PDFDocument6 pages4 PDFYigit İskurtNo ratings yet
- 91 Clinical ScenariosDocument198 pages91 Clinical Scenarioszph7100% (1)
- 1 PBDocument6 pages1 PBrizalakbarsyaNo ratings yet
- Hiatal HerniaDocument6 pagesHiatal HerniaElaine Jean UayanNo ratings yet
- AppendectomyDocument5 pagesAppendectomyRoseben SomidoNo ratings yet
- Rectal Computer 2Document5 pagesRectal Computer 2Su-sake KonichiwaNo ratings yet
- Obstetrics - GastrointestinalDocument6 pagesObstetrics - GastrointestinalJonathanNo ratings yet
- Omphalocele and Gastroschisis: Wendy Nguyen and Kumar BelaniDocument8 pagesOmphalocele and Gastroschisis: Wendy Nguyen and Kumar Belaniari naNo ratings yet
- Medical-Surgical NCLEX Practice QUIZ Flashcards - QuizletDocument9 pagesMedical-Surgical NCLEX Practice QUIZ Flashcards - QuizletErika Bacarro100% (1)
- JURNAL Volvulus 2-DikonversiDocument16 pagesJURNAL Volvulus 2-DikonversiLucya WulandariNo ratings yet
- Enteral Tube Feeding in Adults: Continuing Medical EducationDocument6 pagesEnteral Tube Feeding in Adults: Continuing Medical EducationMaca GallardoNo ratings yet
- Gastrointestinal Emergencies: Lillian Aronson, VMD, Daniel Brockman, BVSC, CVR, Csao, and Dorothy Cimino Brown, DVMDocument25 pagesGastrointestinal Emergencies: Lillian Aronson, VMD, Daniel Brockman, BVSC, CVR, Csao, and Dorothy Cimino Brown, DVMKmila RamirezNo ratings yet
- Oral Boards ReviewDocument218 pagesOral Boards ReviewDustin J FanciulloNo ratings yet
- Percutaneous Gastrostomy: EndoscopicDocument3 pagesPercutaneous Gastrostomy: EndoscopicRaluca CondreaNo ratings yet
- Open Versus Laparoscopic Mesh Repair of Ventral Hernias: A Prospective StudyDocument3 pagesOpen Versus Laparoscopic Mesh Repair of Ventral Hernias: A Prospective Study'Adil MuhammadNo ratings yet
- Chronic Gastric Volvulus With Diaphragmatic 2024 International Journal of SDocument5 pagesChronic Gastric Volvulus With Diaphragmatic 2024 International Journal of SRonald QuezadaNo ratings yet
- ContentServer - Asp 9Document7 pagesContentServer - Asp 9AlanGonzalezNo ratings yet
- Gossypiboma - A Rare Cause of Gastric Outlet ObstructionDocument3 pagesGossypiboma - A Rare Cause of Gastric Outlet ObstructionJuniper PublishersNo ratings yet
- Colectomy ManagementDocument10 pagesColectomy ManagementaylinNo ratings yet
- Common Complication: What Is It?Document5 pagesCommon Complication: What Is It?khalidicuNo ratings yet
- Adhesive Intestinal Obstruction. What A Resident Should KnowDocument6 pagesAdhesive Intestinal Obstruction. What A Resident Should Knowrahul ranjanNo ratings yet
- T1503C08Document5 pagesT1503C08zulfikarNo ratings yet
- Journal Mulya BedahDocument4 pagesJournal Mulya BedahMulyaa SudirmanNo ratings yet
- Case Report On Obstructed Obturator Richter Hernia Managed by Open Preperitoneal Mesh RepairDocument7 pagesCase Report On Obstructed Obturator Richter Hernia Managed by Open Preperitoneal Mesh RepairIJAR JOURNALNo ratings yet
- Esophagostomy Feeding Tubes: Procedures Pro Surgery / Critical CareDocument7 pagesEsophagostomy Feeding Tubes: Procedures Pro Surgery / Critical CareEl OnNo ratings yet
- CH 39Document3 pagesCH 39Nailin RamadsNo ratings yet
- Filia D. Yaputri A34 Barium Enema IDocument3 pagesFilia D. Yaputri A34 Barium Enema ITatyannah Alexa Maristela100% (1)
- Gastric Dilation-Volvulus Syndrome in Dogs 1 PathoDocument5 pagesGastric Dilation-Volvulus Syndrome in Dogs 1 PathoKrlÖz Ändrz Hërëdîa ÖpzNo ratings yet
- Esophageal Surgery in Newborns, Infants and Children: and K.L.N. RaoDocument5 pagesEsophageal Surgery in Newborns, Infants and Children: and K.L.N. RaoDonny Artya KesumaNo ratings yet
- Answers To Activity 4: Collaborative ManagementDocument3 pagesAnswers To Activity 4: Collaborative ManagementEsmareldah Henry SirueNo ratings yet
- Stamm Gastrostomy (Complete)Document6 pagesStamm Gastrostomy (Complete)robelNo ratings yet
- Atresia Esofágica Con Fistula Traqueoesofágica DistalDocument24 pagesAtresia Esofágica Con Fistula Traqueoesofágica DistalChristian PA100% (1)
- J Thorsurg 2018 07 001Document7 pagesJ Thorsurg 2018 07 001Gabriel RangelNo ratings yet
- The Abdomen 98 QDocument78 pagesThe Abdomen 98 QNahidNo ratings yet
- Intestinal Atresia: A Case ReportDocument3 pagesIntestinal Atresia: A Case ReportOvamelia JulioNo ratings yet
- Appendix Part 2Document7 pagesAppendix Part 2Abdullah EssaNo ratings yet
- Gavage, Enteral Feeding or Tube Feeding. Placement May Be Temporary For TheDocument5 pagesGavage, Enteral Feeding or Tube Feeding. Placement May Be Temporary For TheWillen May Delopere Lomarda100% (1)
- Open Abdomen in Critical 1Document25 pagesOpen Abdomen in Critical 1GenyNo ratings yet
- Neonatal Feeding After Gastrointestinal SurgeryDocument4 pagesNeonatal Feeding After Gastrointestinal SurgeryVianNo ratings yet
- Management of The Patient With The Open AbdomenDocument7 pagesManagement of The Patient With The Open Abdomenfabidol13No ratings yet
- Case Report: The Combination of Gastroschisis, Jejunal Atresia, and Colonic Atresia in A NewbornDocument5 pagesCase Report: The Combination of Gastroschisis, Jejunal Atresia, and Colonic Atresia in A NewbornAnonymous PmfX227No ratings yet
- Explor LaparotomyDocument14 pagesExplor LaparotomyGracia NievesNo ratings yet
- Dysphagia, A Simple Guide To The Condition, Treatment And Related ConditionsFrom EverandDysphagia, A Simple Guide To The Condition, Treatment And Related ConditionsRating: 5 out of 5 stars5/5 (1)
- LaryngoceleDocument7 pagesLaryngoceleM Grecu CeptureanuNo ratings yet
- Weldon Owen 2015 CatalogDocument97 pagesWeldon Owen 2015 CatalogWeldon Owen PublishingNo ratings yet
- CureDocument338 pagesCureThiago Cavalli Azambuja100% (1)
- Adj12077 PDF JsessionidDocument2 pagesAdj12077 PDF JsessionidhayiarNo ratings yet
- MSDS Oxalic AcidDocument5 pagesMSDS Oxalic AcidAmber BurgessNo ratings yet
- Guidance On Drug Master File SystemDocument23 pagesGuidance On Drug Master File SystemKhushman AsodariyaNo ratings yet
- The Great Aloe BookDocument96 pagesThe Great Aloe BookAli AzzaliNo ratings yet
- Example:: Healthy Life Sebastian Oliva Task 1Document1 pageExample:: Healthy Life Sebastian Oliva Task 1Sebastian OlivaNo ratings yet
- Interventions Homework RefusalDocument5 pagesInterventions Homework Refusalafetvdqbt100% (1)
- Ayurvedic Healing A Comprehensive Guide David Frawley.15003 Table of ContentsDocument4 pagesAyurvedic Healing A Comprehensive Guide David Frawley.15003 Table of ContentsPolate Solate0% (1)
- A 27-Year-Old Woman Presented With Three-Day History of Generalized Mild Abdominal PainDocument144 pagesA 27-Year-Old Woman Presented With Three-Day History of Generalized Mild Abdominal PainAldredNo ratings yet
- Home Aquarium CatalogDocument48 pagesHome Aquarium CatalogDjordjevic DraganNo ratings yet
- Zinc in Wound Healing Theoretical, ExperimentalDocument15 pagesZinc in Wound Healing Theoretical, ExperimentalRifky Budi TriyatnoNo ratings yet
- 4 Day Power Muscle Burn Workout Split - Muscle & StrengthDocument26 pages4 Day Power Muscle Burn Workout Split - Muscle & Strengthsupermirko78100% (2)
- Arie History of SurgeryDocument20 pagesArie History of SurgeryArie RezaNo ratings yet
- Your Disability Doesn't Disqualify You From Personal GrowthDocument8 pagesYour Disability Doesn't Disqualify You From Personal GrowthMarjorie BasabeNo ratings yet
- History of PenicillinDocument11 pagesHistory of PenicillinAilsa StephensonNo ratings yet
- Activity IntoleranceDocument6 pagesActivity IntoleranceDenvEr CabaniLlasNo ratings yet
- DementiaDocument4 pagesDementiaRegine Lorenzana Mey-AngNo ratings yet
- TamsulosinDocument27 pagesTamsulosinAnna WidiaNo ratings yet
- Designed, Tested and Trusted by Operating Theatre ProfessionalsDocument5 pagesDesigned, Tested and Trusted by Operating Theatre ProfessionalsYusuf Selim ErkenNo ratings yet
- Medical Leave of Absence (Mloa) Treatment Provider Report: SECTION I: To Be Completed by The StudentDocument4 pagesMedical Leave of Absence (Mloa) Treatment Provider Report: SECTION I: To Be Completed by The StudentShubho DasNo ratings yet
- Chikitsa ChatuspadDocument2 pagesChikitsa ChatuspadAnand YadavNo ratings yet
- Topic 18. Secondary Lesions of The SkinDocument2 pagesTopic 18. Secondary Lesions of The SkinLara Paz100% (1)
- 2019-02-27T205056.849Document3 pages2019-02-27T205056.849writer topNo ratings yet
- Compartment ModelsDocument67 pagesCompartment ModelsSunil100% (3)
- Hormonal Secretion of PancreasDocument17 pagesHormonal Secretion of PancreasNazia WasimNo ratings yet
- MastoiditisDocument37 pagesMastoiditisAkanksha EkkaNo ratings yet