Professional Documents
Culture Documents
Science
Science
Uploaded by
jozelpiedraCopyright:
Available Formats
You might also like
- Test Bank For Textbook of Biochemistry With Clinical Correlations 7th Edition Thomas M DevlinDocument3 pagesTest Bank For Textbook of Biochemistry With Clinical Correlations 7th Edition Thomas M DevlinJames Carson100% (36)
- Molecules of LifeDocument8 pagesMolecules of LifeJessamine Romano Aplod100% (1)
- Essentials of Animal PhysiologyDocument594 pagesEssentials of Animal PhysiologyMihalache Alex100% (3)
- Amoeba Sisters BiomoleculesDocument2 pagesAmoeba Sisters Biomoleculesapi-34233421650% (6)
- Mechanism and Theory in Food Chemistry PDFDocument460 pagesMechanism and Theory in Food Chemistry PDFKevin Mendez100% (3)
- Biology Chemistry Physics Core Practical Guide 1575294300769Document211 pagesBiology Chemistry Physics Core Practical Guide 1575294300769crocusozan100% (2)
- BIOMOLECULESeditedDocument28 pagesBIOMOLECULESeditedMA. HAZEL TEOLOGONo ratings yet
- BIOMOLECULESDocument22 pagesBIOMOLECULESchristian josh magtarayoNo ratings yet
- BIOMOLECULESeditedDocument27 pagesBIOMOLECULESeditedMa.Janice GarciaNo ratings yet
- BiomoleculesDocument45 pagesBiomoleculesrenaldNo ratings yet
- Science10q4 Week3-4Document27 pagesScience10q4 Week3-4ClyzuhNo ratings yet
- BiomoleculesDocument27 pagesBiomoleculesFrancis Elijah SalvadoNo ratings yet
- Bio Molecules EditedDocument26 pagesBio Molecules EditedHillary Ashley MartinezNo ratings yet
- Bio MoleculesDocument35 pagesBio MoleculesFerlyn KateNo ratings yet
- BiomoleculesDocument3 pagesBiomoleculesjamesson palermoNo ratings yet
- Week 2 BiochemDocument4 pagesWeek 2 BiochemKawaii TaruNo ratings yet
- Week 2Document4 pagesWeek 2Kawaii TaruNo ratings yet
- Pbio-Lec CarbohydratesDocument13 pagesPbio-Lec CarbohydratesJay JayNo ratings yet
- Chemicals NotesDocument18 pagesChemicals Notesshinde.sampada1923No ratings yet
- BIOCHEM Midterms-ReviewerDocument12 pagesBIOCHEM Midterms-ReviewerJohn Niño CasuelaNo ratings yet
- Chemical Basis of Life in PlantsDocument3 pagesChemical Basis of Life in PlantsMaurice Jane Eunice AyogNo ratings yet
- Biochemistry Lec 1st ShiftDocument12 pagesBiochemistry Lec 1st ShiftNicole RomeroNo ratings yet
- BiomoelculeDocument2 pagesBiomoelculeDaniel VillahermosaNo ratings yet
- Biochem Prelim CompilationDocument22 pagesBiochem Prelim CompilationKezia MadeloNo ratings yet
- Life ProcessesDocument4 pagesLife ProcessesRishabh SinghNo ratings yet
- 2 - BiomoleculesDocument28 pages2 - BiomoleculesBlaire AquinoNo ratings yet
- Macro Tech BrochureDocument2 pagesMacro Tech BrochureaznpowerNo ratings yet
- Molecules of Life - TransesDocument3 pagesMolecules of Life - TransesJenny Ruth TubanNo ratings yet
- Unit 8 Human Biology and HealthDocument171 pagesUnit 8 Human Biology and HealthHymi MarkNo ratings yet
- Biomolecules: Biomolecules, Organic and Inorganic Components, Metabolites, Carbohydrates (Monosaccharide, Disaccharide)Document14 pagesBiomolecules: Biomolecules, Organic and Inorganic Components, Metabolites, Carbohydrates (Monosaccharide, Disaccharide)Prasanna DeshmukhNo ratings yet
- Bio Macromolecules Student ManualDocument70 pagesBio Macromolecules Student ManualMJ Madredijo SadpcsNo ratings yet
- Introduction To Biological Sciences MidtermsDocument6 pagesIntroduction To Biological Sciences MidtermsMatthew SantiagoNo ratings yet
- Biochemistry Midterm ReviewerDocument4 pagesBiochemistry Midterm ReviewerPia JoaquinNo ratings yet
- MACROMOLECULESDocument17 pagesMACROMOLECULESPhoebe Kate YaunNo ratings yet
- REPORTBIODocument31 pagesREPORTBIOMichelle LapuzNo ratings yet
- 2DY Bio Macromolecules (Oct 2020)Document31 pages2DY Bio Macromolecules (Oct 2020)B BizzleNo ratings yet
- CARBOHYDRATES HandoutDocument7 pagesCARBOHYDRATES HandoutBernadine EliseNo ratings yet
- NMAT BotanyDocument35 pagesNMAT BotanyXavier AbainzaNo ratings yet
- CM 4Document9 pagesCM 4Michaella “hideyoshi girl” DavisNo ratings yet
- Chapter 5-Macromolecules Part IDocument29 pagesChapter 5-Macromolecules Part Ijanardhan aghavNo ratings yet
- Notes/Background Information Food Is Fuel:: You Are What You Eat!!!!!Document7 pagesNotes/Background Information Food Is Fuel:: You Are What You Eat!!!!!Richard Paul Alcaire CruzNo ratings yet
- Final Term ReviewerDocument27 pagesFinal Term ReviewerBenna Cyril OrbinoNo ratings yet
- Biochem 1st Year College ReviewerDocument16 pagesBiochem 1st Year College ReviewerMeteor 858100% (3)
- Grade 10 Workbook 2021 Topic 1, 2 and 3Document33 pagesGrade 10 Workbook 2021 Topic 1, 2 and 3Lumka KhayembaNo ratings yet
- CHAPTER 18 CARBOHYDRATES ReviewerDocument12 pagesCHAPTER 18 CARBOHYDRATES ReviewerMel MasculinoNo ratings yet
- Biological Molecules 10BDocument41 pagesBiological Molecules 10BShamshad ShaheerNo ratings yet
- Biomolecules NotesDocument73 pagesBiomolecules NotesAllen Rodrigo LambiquitNo ratings yet
- Biological Molecules: Carbohydrates, Proteins & FatsDocument57 pagesBiological Molecules: Carbohydrates, Proteins & FatsSanthoshNo ratings yet
- Biochemistry Lec MidtermsDocument14 pagesBiochemistry Lec MidtermsSandoval, Ma. Carla TayagNo ratings yet
- Chapter 9 BiomoleculesDocument10 pagesChapter 9 Biomoleculesabdulmateenattar2007No ratings yet
- Bio MoleculesDocument26 pagesBio MoleculesClang VelascoNo ratings yet
- Chapter 3 - Molecules of LifeDocument25 pagesChapter 3 - Molecules of LifeCrisha LimNo ratings yet
- IGCSE Biology NotesDocument50 pagesIGCSE Biology NotesSandra AtefNo ratings yet
- BIOCHEM-TRANS With Additional Protein TopicDocument48 pagesBIOCHEM-TRANS With Additional Protein TopicELROSE JAVIERNo ratings yet
- (BioE 402) MolBIo Lecture 1 CarbohydratesDocument12 pages(BioE 402) MolBIo Lecture 1 CarbohydratesJhun Lucky SadsadNo ratings yet
- Grade 10 Unit 1 BioDocument8 pagesGrade 10 Unit 1 Biodharshani shaniNo ratings yet
- Carbohydrates and LipidsDocument4 pagesCarbohydrates and Lipidsbugaspearl0No ratings yet
- Biomolecules - CarbohydrateDocument29 pagesBiomolecules - CarbohydrateCharlene Marri BajuyoNo ratings yet
- PS Week 5 - BiomoleculesDocument40 pagesPS Week 5 - BiomoleculesPrincess AguiNo ratings yet
- Newton BiomoleDocument35 pagesNewton BiomolejasmineblacerNo ratings yet
- GB1 - S1 Properties of Life - Carbs and LipidsDocument74 pagesGB1 - S1 Properties of Life - Carbs and LipidsAndreau GranadaNo ratings yet
- 1 BiologyDocument19 pages1 BiologyAlexandra EscalonaNo ratings yet
- Biosci 106: The Chemical Basis of LifeDocument25 pagesBiosci 106: The Chemical Basis of LifeChengNo ratings yet
- Chapter 5 Nutrition - Lecture NotesDocument4 pagesChapter 5 Nutrition - Lecture Notesapi-3728508100% (1)
- Gen Bio Reviewer Final 1Document5 pagesGen Bio Reviewer Final 1Emmanuelle CalleNo ratings yet
- Saln2022form Aj Ferrer 2023Document2 pagesSaln2022form Aj Ferrer 2023jozelpiedraNo ratings yet
- MusicDocument2 pagesMusicjozelpiedraNo ratings yet
- Meat T.l.e.: Cookery 10Document5 pagesMeat T.l.e.: Cookery 10jozelpiedraNo ratings yet
- MarsoulWare2 0Document3 pagesMarsoulWare2 0jozelpiedraNo ratings yet
- CL ReviewerDocument2 pagesCL ReviewerjozelpiedraNo ratings yet
- Reviewer CLDocument4 pagesReviewer CLjozelpiedraNo ratings yet
- Lessonon Holy RosaryDocument5 pagesLessonon Holy RosaryjozelpiedraNo ratings yet
- Recipe Plan TLEDocument3 pagesRecipe Plan TLEjozelpiedraNo ratings yet
- Hoefnagels Essentials4e Ch02 LecturePPT AccessibleDocument91 pagesHoefnagels Essentials4e Ch02 LecturePPT AccessibleYamileth Nino MoranNo ratings yet
- Rituparna Banerjee, Arun K. Verma, Mohammed Wasim Siddiqui - Natural Antioxidants - Applications in Foods of Animal Origin-Apple Academic Press (2017)Document414 pagesRituparna Banerjee, Arun K. Verma, Mohammed Wasim Siddiqui - Natural Antioxidants - Applications in Foods of Animal Origin-Apple Academic Press (2017)wilmerjmorenoNo ratings yet
- Ch5 Food and Humans: Multiple-Choice QuestionsDocument5 pagesCh5 Food and Humans: Multiple-Choice QuestionsYing Hei NgNo ratings yet
- Gaurav Kumar Hemnani - UNIT TEST 2 - MOVEMENT IN AND OUT OF CELLS - BIOLOGICAL MOLECULESDocument7 pagesGaurav Kumar Hemnani - UNIT TEST 2 - MOVEMENT IN AND OUT OF CELLS - BIOLOGICAL MOLECULESSlavic GauravNo ratings yet
- Science10 Chemistry Module3 2023-2024Document17 pagesScience10 Chemistry Module3 2023-2024mjmabini047100% (1)
- ATP-Citrate Lyase A Key Player in Cancer MetabolismDocument7 pagesATP-Citrate Lyase A Key Player in Cancer MetabolismRajni KumariNo ratings yet
- Lipids 1Document4 pagesLipids 1Omhar CeresNo ratings yet
- Organic Chemistry 12 Cheat SheetDocument25 pagesOrganic Chemistry 12 Cheat SheetVanessa MurphyNo ratings yet
- Physical Science DLP M6Document6 pagesPhysical Science DLP M6Ciel LueNo ratings yet
- Biology HL Student GuideDocument134 pagesBiology HL Student GuideYesenia JassoNo ratings yet
- Macro Tech BrochureDocument2 pagesMacro Tech BrochureaznpowerNo ratings yet
- Buenasher Learning Academy IncDocument4 pagesBuenasher Learning Academy IncEl CruzNo ratings yet
- Study Materials: Vedantu Innovations Pvt. Ltd. Score High With A Personal Teacher, Learn LIVE Online!Document12 pagesStudy Materials: Vedantu Innovations Pvt. Ltd. Score High With A Personal Teacher, Learn LIVE Online!M. Amebari NongsiejNo ratings yet
- Barecuatro - BSN1 11L - Pre Finals ActivityDocument12 pagesBarecuatro - BSN1 11L - Pre Finals ActivityAngelica Claire BarecuatroNo ratings yet
- 2015-2016 SQ2 (Chemistry of Lipids and Biological Menbranes)Document11 pages2015-2016 SQ2 (Chemistry of Lipids and Biological Menbranes)Jerrica Charlene GalopeNo ratings yet
- Lab Activity LipidsDocument4 pagesLab Activity LipidsNathaniel PulidoNo ratings yet
- University College CorkDocument133 pagesUniversity College CorkRaihanul HaqueNo ratings yet
- 372 The Best Dyspraxia Program EverDocument1 page372 The Best Dyspraxia Program EverAnupama PouloseNo ratings yet
- Topic 1 Chemistry of LifeDocument31 pagesTopic 1 Chemistry of LifeHapsah Muhammad100% (1)
- Biochemistry (Multiple Choices)Document5 pagesBiochemistry (Multiple Choices)fayeNo ratings yet
- Internship Report Claude FinDocument51 pagesInternship Report Claude FinDusengimana Aminadab100% (1)
- Lab Report Green Peas - Dna ExtractionDocument3 pagesLab Report Green Peas - Dna ExtractionJoshua RomeaNo ratings yet
- Lipid Metabolism - I: Chemistry, Digestion and Absorption of LipidsDocument38 pagesLipid Metabolism - I: Chemistry, Digestion and Absorption of LipidscheckmateNo ratings yet
- Biomolecule - WikipediaDocument11 pagesBiomolecule - WikipediaSumeet MahapatraNo ratings yet
Science
Science
Uploaded by
jozelpiedraCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Science
Science
Uploaded by
jozelpiedraCopyright:
Available Formats
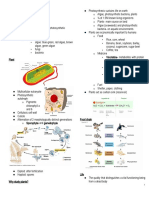
Biomolecules science 10 s.y.
2023 -2024
Biomolecules • Monomer (building block) —
Monosaccharides (Glucose is most
• The molecule that is present in all living common)
organisms and is involved in the maintenance
and metabolic processes. • Polymer — Polysaccharides
(starch, Glycogen, Cellulose, Chitin)
6
• All Biomolecule contain CARBON 𝐶12
• Examples — Chocolate, Bread,
• Carbon is the most versatile and prominent Pasta, Fruits, Vegetables (All from
element of life. plants.)
Other elements:
❖ Sugars that make up Carbohydrates:
➢ Hydrogen (H)
➢ Oxygen (O) ▪ Single sugar: monosaccharide
➢ Nitrogen (N) Ex: glucose, fructose (in fruits)
➢ Sulphur (S)
➢ Sodium (Na) ▪ 2 monosaccharides: disaccharide
➢ Calcium (Ca) Ex: maltose, sucrose.
➢ Magnesium (Mg)
▪ 3+monosaccharides: polysaccharide
• Biomolecules Ex: Starch, Glycogen, Cellulose,
➢ Inorganic and Chitin.
➢ Organic
➢ Carbohydrates Types of polysaccharides:
➢ Lipids
➢ Proteins ▪ Starch:
➢ Nucleic Acids
➢ Enzymes o Used for energy storage in
➢ Vitamins plants.
• These are very large molecules of many o Potatoes, pasta, and rice
ATOMS that are covalently bonded. are starches.
• ENERGY is stored in the COVALENT o They provide a quick form
BONDS. When we eat, we get energy to live of energy for the body.
because chemical reactions within our bodies
break these bonds. ▪ Glycogen:
Types of biomolecules o (I am formed in the Liver!)
1. Carbohydrates o Used for energy storage in
animals.
• Are sugars.
▪ Cellulose:
• We get 4 kilocalories per gram of
carbohydrates that we eat! o Provides structural support
in plants (found in the cell
• The most common organic wall).
molecule.
▪ Chitin:
• Function — Primary energy source
our body needs. o Found in the exoskeletons
of arthropods (insects,
spiders).
• Elements present — C, H, O (1:2:1
ratio)
Florenz Janus mina | 1
Science 10 | 4th quarter // Mr. vince bugarin
o Found in the cell walls of • Unsaturated: There is at least one
some fungi. double or triple bond between
carbons present.
Structure of Carbohydrates:
o Liquid at room temperature
• Remember: The elements are C, H, and O.
o Mainly plant-based fats
• Primarily in a Ring shape (but not always) (olive oil, peanut oil) as well
as oily fish (tuna, sardines).
Lipid Structure:
• Remember: The elements present are C,
H, and O.
• Long strands of carbon and hydrogen.
➢ Called Hydrocarbons
2. Lipids
• Are fats.
• We get 9 kcal per gram of fat that we
consume.
• Function — Store energy, insulate
your body, and make up the Cell
Membrane!
• Elements — C-H-O
• Monomer (building blocks) — 3. Proteins
glycerol and 3 fatty acids
• Builds us.
• Polymer — phospholipids,
• We get 4 kcal per gram of protein that we
triglycerides consume.
• Example — steroids, cholesterol, • Function of proteins
fats, oils, nuts, and waxes make up
part of the cell membrane! • Transport molecules in and out
of the cell.
❖ Lipids can be:
• Control the speed of chemical
• Saturated: The bonds between all reactions.
the carbons are single bonds.
• Used for growth and repair.
o Solid at room temperature.
• Proteins make up the structure
o Mainly animal fats (bacon of living things. Hair, nails, skin,
grease, lard) bones, muscles, etc. are all built
by protein.
Florenz Janus mina | 2
Science 10 | 4th quarter // Mr. vince bugarin
4. Nucleic Acids
• Elements — C-H-O-N • These biomolecules are not necessarily from
food.
o Nitrogen is present now.
• Function:
• Monomer (Building Block) — amino acids
o Provide our genetic information.
(20 different ones!)
o Holds the instructions to make
• Polymer — proteins (tons) proteins.
• Examples of proteins — • Elements — C-H-O-N-P
include hemoglobin in red blood cells,
albumin in eggs, enzymes that control • Monomer — nucleotides
reactions in the body, and antibodies.
o A nucleotide is made up of:
• Found in — fish, eggs, meat.
➢ Sugar
Protein Structure: ➢ Phosphate
➢ Nitrogen Base: A, T, G,
• Remember — The elements are C, H, O, and C, or U.
N.
• Polymers: DNA, RNA, and ATP
• “R” groups represent one of the 20 amino DNA — Genetic code
acids! (So, each amino acid has something RNA — Recipe for proteins
different in that spot.) ATP — Energy carrier
Nucleic Acids Structure:
❖ Why are amino acids important?
• When groups of amino acids are joined
together, a protein is formed.
• There are 20 kinds of amino acids.
• They consist of a carboxyl group (COOH)
and an amino group (NH2).
• Peptide bonds form between amino
acids.
(polypeptide = many peptide bonds = protein)
Florenz Janus mina | 3
Gas laws science 10 s.y. 2023 -2024
Standard units of measures and Volume:
measures converting units For example:
Temperature = Kelvin (K)
Pressure = Pascal (Pa) and Atmosphere (atm) 1L = 1000 mL
Volume = Liters (L) L=?
Temperature: 10
300mL = 300 × 10, then
1000𝑚𝐿
Celsius > Kelvin Kelvin > Celsius
300mL = 0.3L divide.
K = °C + 273.15 K = °K - 273.15
L = 100
For example:
mL = ?
°10.5 + 273.15
K = 10.5 + 273.15
1000𝑚𝐿
100L = 100 × 1000, then
K = 263.65 1𝐿
100L = 100000mL divide.
Pressure: Boyle’s law
Pascal (Pa) > Atmosphere (atm) Atmosphere > Pascal • The volume and pressure have an inverse
relationship.
Atm = Pa ÷ 10135 Pa = atm × 10135
Standard value:
Pa > mmHg (millimeters mercury) / Torr P1 V1 = P2 V2
Torr / mmHg = Pa × 0.0075
V2: P2:
𝑃1 𝑉1 𝑃1 𝑉1
𝑃2
= V2
𝑉2
= P2
mmHg > Pa
Pa = mmHg ÷ 0.0075
P1: V1:
𝑃2 𝑉2 𝑃2 𝑉2
P1 = V1 =
For example: 𝑉1 𝑃1
Pa = 10 > atm
For example:
atm = Pa ÷ 10135
V1 = 14.0L
atm = 10 ÷ 10135
P1 = 760mmHg > 0.0075
atm = 0.00099 (rounded off from 0.0000987)
P2 = 400mmHg > 0.0039
Hi po! V2 = ?
Hiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiii
Florenz Janus mina | 1
Science 10 | 4th quarter // Mr. vince bugarin
𝑃1 𝑉1
V2 =
𝑃2
.0075𝑎𝑡𝑚 ×14L
V2 =
.0039𝑎𝑡𝑚
V2 = 26.92L
Charles’ law
• V is inversely proportional to T.
Standard Value:
𝑉1 𝑉2
T1 ( = 𝑇2 ) T1
𝑇1
𝑉2 ×T1 𝑉2
V1 = T1 =
𝑇2 𝑇2 ×V1
𝑉1 ×T2 𝑉1 ×T1
V2 = T2 =
𝑇1 𝑉2 ×T1
Hello
Chat mo ‘ko pleaseee
djk (DJK)
Florenz Janus mina | 2
You might also like
- Test Bank For Textbook of Biochemistry With Clinical Correlations 7th Edition Thomas M DevlinDocument3 pagesTest Bank For Textbook of Biochemistry With Clinical Correlations 7th Edition Thomas M DevlinJames Carson100% (36)
- Molecules of LifeDocument8 pagesMolecules of LifeJessamine Romano Aplod100% (1)
- Essentials of Animal PhysiologyDocument594 pagesEssentials of Animal PhysiologyMihalache Alex100% (3)
- Amoeba Sisters BiomoleculesDocument2 pagesAmoeba Sisters Biomoleculesapi-34233421650% (6)
- Mechanism and Theory in Food Chemistry PDFDocument460 pagesMechanism and Theory in Food Chemistry PDFKevin Mendez100% (3)
- Biology Chemistry Physics Core Practical Guide 1575294300769Document211 pagesBiology Chemistry Physics Core Practical Guide 1575294300769crocusozan100% (2)
- BIOMOLECULESeditedDocument28 pagesBIOMOLECULESeditedMA. HAZEL TEOLOGONo ratings yet
- BIOMOLECULESDocument22 pagesBIOMOLECULESchristian josh magtarayoNo ratings yet
- BIOMOLECULESeditedDocument27 pagesBIOMOLECULESeditedMa.Janice GarciaNo ratings yet
- BiomoleculesDocument45 pagesBiomoleculesrenaldNo ratings yet
- Science10q4 Week3-4Document27 pagesScience10q4 Week3-4ClyzuhNo ratings yet
- BiomoleculesDocument27 pagesBiomoleculesFrancis Elijah SalvadoNo ratings yet
- Bio Molecules EditedDocument26 pagesBio Molecules EditedHillary Ashley MartinezNo ratings yet
- Bio MoleculesDocument35 pagesBio MoleculesFerlyn KateNo ratings yet
- BiomoleculesDocument3 pagesBiomoleculesjamesson palermoNo ratings yet
- Week 2 BiochemDocument4 pagesWeek 2 BiochemKawaii TaruNo ratings yet
- Week 2Document4 pagesWeek 2Kawaii TaruNo ratings yet
- Pbio-Lec CarbohydratesDocument13 pagesPbio-Lec CarbohydratesJay JayNo ratings yet
- Chemicals NotesDocument18 pagesChemicals Notesshinde.sampada1923No ratings yet
- BIOCHEM Midterms-ReviewerDocument12 pagesBIOCHEM Midterms-ReviewerJohn Niño CasuelaNo ratings yet
- Chemical Basis of Life in PlantsDocument3 pagesChemical Basis of Life in PlantsMaurice Jane Eunice AyogNo ratings yet
- Biochemistry Lec 1st ShiftDocument12 pagesBiochemistry Lec 1st ShiftNicole RomeroNo ratings yet
- BiomoelculeDocument2 pagesBiomoelculeDaniel VillahermosaNo ratings yet
- Biochem Prelim CompilationDocument22 pagesBiochem Prelim CompilationKezia MadeloNo ratings yet
- Life ProcessesDocument4 pagesLife ProcessesRishabh SinghNo ratings yet
- 2 - BiomoleculesDocument28 pages2 - BiomoleculesBlaire AquinoNo ratings yet
- Macro Tech BrochureDocument2 pagesMacro Tech BrochureaznpowerNo ratings yet
- Molecules of Life - TransesDocument3 pagesMolecules of Life - TransesJenny Ruth TubanNo ratings yet
- Unit 8 Human Biology and HealthDocument171 pagesUnit 8 Human Biology and HealthHymi MarkNo ratings yet
- Biomolecules: Biomolecules, Organic and Inorganic Components, Metabolites, Carbohydrates (Monosaccharide, Disaccharide)Document14 pagesBiomolecules: Biomolecules, Organic and Inorganic Components, Metabolites, Carbohydrates (Monosaccharide, Disaccharide)Prasanna DeshmukhNo ratings yet
- Bio Macromolecules Student ManualDocument70 pagesBio Macromolecules Student ManualMJ Madredijo SadpcsNo ratings yet
- Introduction To Biological Sciences MidtermsDocument6 pagesIntroduction To Biological Sciences MidtermsMatthew SantiagoNo ratings yet
- Biochemistry Midterm ReviewerDocument4 pagesBiochemistry Midterm ReviewerPia JoaquinNo ratings yet
- MACROMOLECULESDocument17 pagesMACROMOLECULESPhoebe Kate YaunNo ratings yet
- REPORTBIODocument31 pagesREPORTBIOMichelle LapuzNo ratings yet
- 2DY Bio Macromolecules (Oct 2020)Document31 pages2DY Bio Macromolecules (Oct 2020)B BizzleNo ratings yet
- CARBOHYDRATES HandoutDocument7 pagesCARBOHYDRATES HandoutBernadine EliseNo ratings yet
- NMAT BotanyDocument35 pagesNMAT BotanyXavier AbainzaNo ratings yet
- CM 4Document9 pagesCM 4Michaella “hideyoshi girl” DavisNo ratings yet
- Chapter 5-Macromolecules Part IDocument29 pagesChapter 5-Macromolecules Part Ijanardhan aghavNo ratings yet
- Notes/Background Information Food Is Fuel:: You Are What You Eat!!!!!Document7 pagesNotes/Background Information Food Is Fuel:: You Are What You Eat!!!!!Richard Paul Alcaire CruzNo ratings yet
- Final Term ReviewerDocument27 pagesFinal Term ReviewerBenna Cyril OrbinoNo ratings yet
- Biochem 1st Year College ReviewerDocument16 pagesBiochem 1st Year College ReviewerMeteor 858100% (3)
- Grade 10 Workbook 2021 Topic 1, 2 and 3Document33 pagesGrade 10 Workbook 2021 Topic 1, 2 and 3Lumka KhayembaNo ratings yet
- CHAPTER 18 CARBOHYDRATES ReviewerDocument12 pagesCHAPTER 18 CARBOHYDRATES ReviewerMel MasculinoNo ratings yet
- Biological Molecules 10BDocument41 pagesBiological Molecules 10BShamshad ShaheerNo ratings yet
- Biomolecules NotesDocument73 pagesBiomolecules NotesAllen Rodrigo LambiquitNo ratings yet
- Biological Molecules: Carbohydrates, Proteins & FatsDocument57 pagesBiological Molecules: Carbohydrates, Proteins & FatsSanthoshNo ratings yet
- Biochemistry Lec MidtermsDocument14 pagesBiochemistry Lec MidtermsSandoval, Ma. Carla TayagNo ratings yet
- Chapter 9 BiomoleculesDocument10 pagesChapter 9 Biomoleculesabdulmateenattar2007No ratings yet
- Bio MoleculesDocument26 pagesBio MoleculesClang VelascoNo ratings yet
- Chapter 3 - Molecules of LifeDocument25 pagesChapter 3 - Molecules of LifeCrisha LimNo ratings yet
- IGCSE Biology NotesDocument50 pagesIGCSE Biology NotesSandra AtefNo ratings yet
- BIOCHEM-TRANS With Additional Protein TopicDocument48 pagesBIOCHEM-TRANS With Additional Protein TopicELROSE JAVIERNo ratings yet
- (BioE 402) MolBIo Lecture 1 CarbohydratesDocument12 pages(BioE 402) MolBIo Lecture 1 CarbohydratesJhun Lucky SadsadNo ratings yet
- Grade 10 Unit 1 BioDocument8 pagesGrade 10 Unit 1 Biodharshani shaniNo ratings yet
- Carbohydrates and LipidsDocument4 pagesCarbohydrates and Lipidsbugaspearl0No ratings yet
- Biomolecules - CarbohydrateDocument29 pagesBiomolecules - CarbohydrateCharlene Marri BajuyoNo ratings yet
- PS Week 5 - BiomoleculesDocument40 pagesPS Week 5 - BiomoleculesPrincess AguiNo ratings yet
- Newton BiomoleDocument35 pagesNewton BiomolejasmineblacerNo ratings yet
- GB1 - S1 Properties of Life - Carbs and LipidsDocument74 pagesGB1 - S1 Properties of Life - Carbs and LipidsAndreau GranadaNo ratings yet
- 1 BiologyDocument19 pages1 BiologyAlexandra EscalonaNo ratings yet
- Biosci 106: The Chemical Basis of LifeDocument25 pagesBiosci 106: The Chemical Basis of LifeChengNo ratings yet
- Chapter 5 Nutrition - Lecture NotesDocument4 pagesChapter 5 Nutrition - Lecture Notesapi-3728508100% (1)
- Gen Bio Reviewer Final 1Document5 pagesGen Bio Reviewer Final 1Emmanuelle CalleNo ratings yet
- Saln2022form Aj Ferrer 2023Document2 pagesSaln2022form Aj Ferrer 2023jozelpiedraNo ratings yet
- MusicDocument2 pagesMusicjozelpiedraNo ratings yet
- Meat T.l.e.: Cookery 10Document5 pagesMeat T.l.e.: Cookery 10jozelpiedraNo ratings yet
- MarsoulWare2 0Document3 pagesMarsoulWare2 0jozelpiedraNo ratings yet
- CL ReviewerDocument2 pagesCL ReviewerjozelpiedraNo ratings yet
- Reviewer CLDocument4 pagesReviewer CLjozelpiedraNo ratings yet
- Lessonon Holy RosaryDocument5 pagesLessonon Holy RosaryjozelpiedraNo ratings yet
- Recipe Plan TLEDocument3 pagesRecipe Plan TLEjozelpiedraNo ratings yet
- Hoefnagels Essentials4e Ch02 LecturePPT AccessibleDocument91 pagesHoefnagels Essentials4e Ch02 LecturePPT AccessibleYamileth Nino MoranNo ratings yet
- Rituparna Banerjee, Arun K. Verma, Mohammed Wasim Siddiqui - Natural Antioxidants - Applications in Foods of Animal Origin-Apple Academic Press (2017)Document414 pagesRituparna Banerjee, Arun K. Verma, Mohammed Wasim Siddiqui - Natural Antioxidants - Applications in Foods of Animal Origin-Apple Academic Press (2017)wilmerjmorenoNo ratings yet
- Ch5 Food and Humans: Multiple-Choice QuestionsDocument5 pagesCh5 Food and Humans: Multiple-Choice QuestionsYing Hei NgNo ratings yet
- Gaurav Kumar Hemnani - UNIT TEST 2 - MOVEMENT IN AND OUT OF CELLS - BIOLOGICAL MOLECULESDocument7 pagesGaurav Kumar Hemnani - UNIT TEST 2 - MOVEMENT IN AND OUT OF CELLS - BIOLOGICAL MOLECULESSlavic GauravNo ratings yet
- Science10 Chemistry Module3 2023-2024Document17 pagesScience10 Chemistry Module3 2023-2024mjmabini047100% (1)
- ATP-Citrate Lyase A Key Player in Cancer MetabolismDocument7 pagesATP-Citrate Lyase A Key Player in Cancer MetabolismRajni KumariNo ratings yet
- Lipids 1Document4 pagesLipids 1Omhar CeresNo ratings yet
- Organic Chemistry 12 Cheat SheetDocument25 pagesOrganic Chemistry 12 Cheat SheetVanessa MurphyNo ratings yet
- Physical Science DLP M6Document6 pagesPhysical Science DLP M6Ciel LueNo ratings yet
- Biology HL Student GuideDocument134 pagesBiology HL Student GuideYesenia JassoNo ratings yet
- Macro Tech BrochureDocument2 pagesMacro Tech BrochureaznpowerNo ratings yet
- Buenasher Learning Academy IncDocument4 pagesBuenasher Learning Academy IncEl CruzNo ratings yet
- Study Materials: Vedantu Innovations Pvt. Ltd. Score High With A Personal Teacher, Learn LIVE Online!Document12 pagesStudy Materials: Vedantu Innovations Pvt. Ltd. Score High With A Personal Teacher, Learn LIVE Online!M. Amebari NongsiejNo ratings yet
- Barecuatro - BSN1 11L - Pre Finals ActivityDocument12 pagesBarecuatro - BSN1 11L - Pre Finals ActivityAngelica Claire BarecuatroNo ratings yet
- 2015-2016 SQ2 (Chemistry of Lipids and Biological Menbranes)Document11 pages2015-2016 SQ2 (Chemistry of Lipids and Biological Menbranes)Jerrica Charlene GalopeNo ratings yet
- Lab Activity LipidsDocument4 pagesLab Activity LipidsNathaniel PulidoNo ratings yet
- University College CorkDocument133 pagesUniversity College CorkRaihanul HaqueNo ratings yet
- 372 The Best Dyspraxia Program EverDocument1 page372 The Best Dyspraxia Program EverAnupama PouloseNo ratings yet
- Topic 1 Chemistry of LifeDocument31 pagesTopic 1 Chemistry of LifeHapsah Muhammad100% (1)
- Biochemistry (Multiple Choices)Document5 pagesBiochemistry (Multiple Choices)fayeNo ratings yet
- Internship Report Claude FinDocument51 pagesInternship Report Claude FinDusengimana Aminadab100% (1)
- Lab Report Green Peas - Dna ExtractionDocument3 pagesLab Report Green Peas - Dna ExtractionJoshua RomeaNo ratings yet
- Lipid Metabolism - I: Chemistry, Digestion and Absorption of LipidsDocument38 pagesLipid Metabolism - I: Chemistry, Digestion and Absorption of LipidscheckmateNo ratings yet
- Biomolecule - WikipediaDocument11 pagesBiomolecule - WikipediaSumeet MahapatraNo ratings yet