Professional Documents
Culture Documents
ATC News 016 Stop of Sales Activities Concerning Neuro-Surgery Application
ATC News 016 Stop of Sales Activities Concerning Neuro-Surgery Application
Uploaded by
Paulo DoresOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
ATC News 016 Stop of Sales Activities Concerning Neuro-Surgery Application
ATC News 016 Stop of Sales Activities Concerning Neuro-Surgery Application
Uploaded by
Paulo DoresCopyright:
Available Formats
ATC NEWS
No. 016
Stop of sales activities concerning neuro-surgery application
Metzingen, 3.04.2012
Dear Ladies and Gentlemen,
As safety officers we need to inform you about a temporary stop for sales, marketing and offers
to neuro-surgery.
Due to change of European regulations the risk classification of several intra-operative Hitachi-
Aloka probes has changed.
All intra-operative probes which are used with direct contact to the central nervous system
(including brain, spine, etc.) have to be classified as Class III devices. The current classification is
Class IIa.
Hitachi-Aloka in Japan is currently investigating further actions with the Notified Body.
All further required actions, which apply to already sold probes for neuro-surgical purposes will
be informed soon.
As discussed with the European management of Hitachi Medical Systems, we request you to
stop any offer, advertisement, marketing and sales for any neuro-surgical purpose. This in-
cludes handing out brochures.
This stop will be effective from now until further notice.
For further questions please contact the respective safety officer as written below.
Sincerely yours,
Arne Buschhausen Holger Scholl
Regulatory Affairs Specialist, Safety Officer Manager Regulatory Affairs, Safety Officer
Aloka Europe Technical Center Hitachi Medical Systems GmbH
a.buschhausen@hitachi-medical-systems.com h.scholl@hitachi-medical-systems.com
ALOKA EUROPE Technical Center
Carl-Zeiss-Strasse 5, D-72555 Metzingen Tel: +49 7123 9605 0 Fax: +49 7123 9605 388
Eine Zweigniederlassung der Hitachi Medical Systems Europe Holding AG, Sumpfstrasse 13, CH-6300 Zug, Schweiz
www.hitachi-medical-systems.eu
You might also like
- VELAS - 30AorB Medical Diode Laser Systems Operating Manual 0197Document60 pagesVELAS - 30AorB Medical Diode Laser Systems Operating Manual 0197antoniod179237100% (1)
- Automotive Master Technician: Advanced Light Vehicle TechnologyFrom EverandAutomotive Master Technician: Advanced Light Vehicle TechnologyRating: 5 out of 5 stars5/5 (1)
- 4008 E / 4008 B / 4008 H / 4008 S Hemodialysis System Technical ManualDocument350 pages4008 E / 4008 B / 4008 H / 4008 S Hemodialysis System Technical Manualchanlal100% (3)
- EUB 5500 Instruction Q1E EA0612 14Document559 pagesEUB 5500 Instruction Q1E EA0612 14IM50% (2)
- HTTP: - WWW - Hitachi-Medical-Systems - Eu - Products-And-Services - Mri - Airis-Vento-O5-03t.html PDFDocument3 pagesHTTP: - WWW - Hitachi-Medical-Systems - Eu - Products-And-Services - Mri - Airis-Vento-O5-03t.html PDFBasheer AlmetwakelNo ratings yet
- TH-5000 Series Products Instruction Manual V5.2.0.1 (New) PDFDocument269 pagesTH-5000 Series Products Instruction Manual V5.2.0.1 (New) PDFMamdouh Awad0% (1)
- M000C CMS User ManualDocument86 pagesM000C CMS User ManualSándor BerényiNo ratings yet
- OTI-Scan 1000 Manual de ServiçoDocument21 pagesOTI-Scan 1000 Manual de ServiçoCarlos Roberto PereiraNo ratings yet
- Mindray DP 6600 Service Manual PDFDocument71 pagesMindray DP 6600 Service Manual PDFHyacinthe Kossi0% (1)
- Guidelines For Medical Alarm System Software DesignDocument12 pagesGuidelines For Medical Alarm System Software Designhparsaee1504No ratings yet
- WWW - Hitachi-Medical-Systems - Eu - Products-And-Services - Mri - Airis-Vento-O5-03t.htmlDocument3 pagesWWW - Hitachi-Medical-Systems - Eu - Products-And-Services - Mri - Airis-Vento-O5-03t.htmlBasheer Almetwakel0% (1)
- Blink Device Company Announces TwitchView™ Quantitative Monitor For Neuromuscular Blockade Is Now Compatible With Major Electronic Medical RecordsDocument2 pagesBlink Device Company Announces TwitchView™ Quantitative Monitor For Neuromuscular Blockade Is Now Compatible With Major Electronic Medical RecordsPR.comNo ratings yet
- Medical Device Startup Blink Device Company Announces 510 (K) Clearance of TwitchView Quantitative Monitor For Neuromuscular Blockade and Hires VP of Sales and MarketingDocument2 pagesMedical Device Startup Blink Device Company Announces 510 (K) Clearance of TwitchView Quantitative Monitor For Neuromuscular Blockade and Hires VP of Sales and MarketingPR.comNo ratings yet
- Philips M3015ADocument1 pagePhilips M3015APaulinaNo ratings yet
- Echelon SmartDocument5 pagesEchelon SmartTài NguyễnNo ratings yet
- 225126leading Ten Ideas To Build Your Anesthesia MakerDocument2 pages225126leading Ten Ideas To Build Your Anesthesia Makerr2vfbqz240No ratings yet
- Somatom - Session 22Document82 pagesSomatom - Session 222vjNo ratings yet
- Philips Healthcare PDFDocument6 pagesPhilips Healthcare PDFAnonymous KKSGFIZP7PNo ratings yet
- Multifiltrate OP 08 08 11 SW 04 01 EN PDFDocument274 pagesMultifiltrate OP 08 08 11 SW 04 01 EN PDFJelena RepicNo ratings yet
- IT1032484+Philips+Nederland+FSN+CLE16 034+ +brilliance Ingenuity en Tcm294 379910Document3 pagesIT1032484+Philips+Nederland+FSN+CLE16 034+ +brilliance Ingenuity en Tcm294 379910Anonymous KKSGFIZP7PNo ratings yet
- Amendment Instruction - Q1E-EA1503-3Document1 pageAmendment Instruction - Q1E-EA1503-3IMINo ratings yet
- Instrulife Multiparameter Monitor M9500 User's ManualDocument166 pagesInstrulife Multiparameter Monitor M9500 User's ManualInstrulife OostkampNo ratings yet
- it+2030345+Siemens+Healthcare+GmbH+XP028 19 S+ (FSN) +and+XP029 19 S+ (FSCA) +Ysio+Max,+Luminos+dRF+Max,+Agile+Max,+Omnia+MaxDocument3 pagesit+2030345+Siemens+Healthcare+GmbH+XP028 19 S+ (FSN) +and+XP029 19 S+ (FSCA) +Ysio+Max,+Luminos+dRF+Max,+Agile+Max,+Omnia+MaxEric MedinaNo ratings yet
- Rev 1 - FONA ART Plus Operator Manual GB PDFDocument32 pagesRev 1 - FONA ART Plus Operator Manual GB PDFjose_mario1128No ratings yet
- Fgai4h I 036Document93 pagesFgai4h I 036sundarNo ratings yet
- Wsquare Consultants Profile (Hospitals)Document6 pagesWsquare Consultants Profile (Hospitals)waqasPRDNo ratings yet
- Cardiowise - Manual en XRCISESTRESS 20171001Document78 pagesCardiowise - Manual en XRCISESTRESS 20171001Muddassir DurweshNo ratings yet
- 1.5T 8CH Breast Coil by Sentinelle - SM - Doc1111870 - 1Document72 pages1.5T 8CH Breast Coil by Sentinelle - SM - Doc1111870 - 1service iyadMedicalNo ratings yet
- MRI Issue 5Document91 pagesMRI Issue 5Irinel Busca100% (1)
- Biotronik Internship Report Material STRDocument275 pagesBiotronik Internship Report Material STRsakshi chauhanNo ratings yet
- U.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Document15 pagesU.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993khawar mukhtarNo ratings yet
- Analog Input Module AI 4xi 2-/4-Wire ST (6ES7134-6GD01-0BA1)Document40 pagesAnalog Input Module AI 4xi 2-/4-Wire ST (6ES7134-6GD01-0BA1)TCP.MT.2014 TCP.MT.2014No ratings yet
- Eups 70Document25 pagesEups 70Rigoberto Quintana MontenegroNo ratings yet
- IT1032530+Siemens+Healthcare+-+FSN+AX052 16 S+-+AXIOM+Artis+Systems++SW+VB35E EN tcm294-379916Document2 pagesIT1032530+Siemens+Healthcare+-+FSN+AX052 16 S+-+AXIOM+Artis+Systems++SW+VB35E EN tcm294-379916CeasarNo ratings yet
- M9500 Patient Monitor User's Manual: Guangdong Biolight Meditech Co., LTDDocument166 pagesM9500 Patient Monitor User's Manual: Guangdong Biolight Meditech Co., LTDJose Ivan Carvajal CortizosNo ratings yet
- Inter-Operative Safety GuidelinesDocument34 pagesInter-Operative Safety GuidelinesKang MadaNo ratings yet
- Micro Drives pdc1000 Manual en-US en-USDocument36 pagesMicro Drives pdc1000 Manual en-US en-USPezhmandmNo ratings yet
- Electromedical Ray XDocument13 pagesElectromedical Ray XLuis MuñozNo ratings yet
- SA7913 V6.0 BioGraph Infiniti Getting StartedDocument39 pagesSA7913 V6.0 BioGraph Infiniti Getting StartedAnonymous haOGKg8JUVNo ratings yet
- MAGLIFE Light: User Manual Version 1.2 / October 2006Document51 pagesMAGLIFE Light: User Manual Version 1.2 / October 2006Heidi BlueNo ratings yet
- Toshiba Activion CT System - Press Release EcrDocument2 pagesToshiba Activion CT System - Press Release EcrRashid KhNo ratings yet
- Noblus - Ecograf.f31 Brochure en 300913 RZDocument4 pagesNoblus - Ecograf.f31 Brochure en 300913 RZrca ieftinNo ratings yet
- OchureDocument4 pagesOchurerca ieftinNo ratings yet
- Important Customer Information: Medical Instruments Production + Trading GMBHDocument2 pagesImportant Customer Information: Medical Instruments Production + Trading GMBHGiovanni BalderramaNo ratings yet
- Et200s 2 4 Ai RTD ST Manual en-US en-USDocument32 pagesEt200s 2 4 Ai RTD ST Manual en-US en-USachniajosiNo ratings yet
- Blink Device Company Receives CE Mark For TwitchView™ Quantitative Monitor For Neuromuscular Blockade Debuts at European Society of Anaesthesiology MeetingDocument2 pagesBlink Device Company Receives CE Mark For TwitchView™ Quantitative Monitor For Neuromuscular Blockade Debuts at European Society of Anaesthesiology MeetingPR.comNo ratings yet
- E IA Diagnostic Atmos Cube 2012-07-04Document32 pagesE IA Diagnostic Atmos Cube 2012-07-04RogerNo ratings yet
- Field Safety Corrective Action ReportDocument4 pagesField Safety Corrective Action ReportChala DabalaNo ratings yet
- s71500 Cpu 1511t 1 PN Manual en-US en-USDocument53 pagess71500 Cpu 1511t 1 PN Manual en-US en-USCriveanu Ionut LucianNo ratings yet
- Siemens 828 Alarms ManualDocument1,280 pagesSiemens 828 Alarms Manualwolf.ds91No ratings yet
- 4SI SIRIUS en-USDocument28 pages4SI SIRIUS en-USJesus Alberto RIVERA /CALVEK AUTOMATIONNo ratings yet
- Et200sp Ai 4xu I 2 Wire ST Manual en-US en-US PDFDocument42 pagesEt200sp Ai 4xu I 2 Wire ST Manual en-US en-US PDFWhite TigerNo ratings yet
- WAGO 750 530 en 2Document12 pagesWAGO 750 530 en 2Letkuan NexusNo ratings yet
- K210611 PDFDocument11 pagesK210611 PDFHyd HydNo ratings yet
- HIMA 2006 Functional Safety A Practical ApproachDocument21 pagesHIMA 2006 Functional Safety A Practical ApproachEduardo Lamar MarinasNo ratings yet
- Um Oisplus E001Document132 pagesUm Oisplus E001Alejandro Correa MorenoNo ratings yet
- AI Breakcold - Infusing Humanity into Artificial IntelligenceFrom EverandAI Breakcold - Infusing Humanity into Artificial IntelligenceNo ratings yet
- Hybrid Electric & Alternative Automotive Propulsion: Low Carbon TechnologiesFrom EverandHybrid Electric & Alternative Automotive Propulsion: Low Carbon TechnologiesNo ratings yet
- Aloka Co LTD Market Share AnalysisDocument5 pagesAloka Co LTD Market Share AnalysisPaulo DoresNo ratings yet
- Olympus Compatibility ListDocument6 pagesOlympus Compatibility ListPaulo DoresNo ratings yet
- Very Very Poor Ultrasound ImageDocument1 pageVery Very Poor Ultrasound ImagePaulo DoresNo ratings yet
- eDMS Exam Answer 2002Document1 pageeDMS Exam Answer 2002Paulo DoresNo ratings yet
- SMS 2.0 User Manual - 20160829 - V1.02Document36 pagesSMS 2.0 User Manual - 20160829 - V1.02Paulo DoresNo ratings yet
- SP-0900-V6.0-2.0 - Iec 2-37Document8 pagesSP-0900-V6.0-2.0 - Iec 2-37Paulo DoresNo ratings yet
- SMS 2.0 User Manual - 20160829 - V1.01Document37 pagesSMS 2.0 User Manual - 20160829 - V1.01Paulo DoresNo ratings yet
- Alpha5 M02296Document1 pageAlpha5 M02296Paulo DoresNo ratings yet
- Addition NL1203Document1 pageAddition NL1203Paulo DoresNo ratings yet
- 2009-01-10 - Source of Electromagnetic Interference - Servo MotorDocument1 page2009-01-10 - Source of Electromagnetic Interference - Servo MotorPaulo DoresNo ratings yet
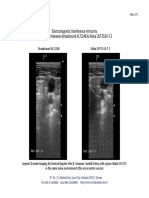
- 2008-04-25 - EII Comparison - Broadsound AL7L24B & Aloka UST-5524-7.5Document1 page2008-04-25 - EII Comparison - Broadsound AL7L24B & Aloka UST-5524-7.5Paulo DoresNo ratings yet
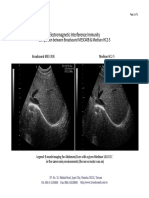
- 2009-05-02 - EII Comparison - Broadsound ME3C40B & Medison HC2-5Document1 page2009-05-02 - EII Comparison - Broadsound ME3C40B & Medison HC2-5Paulo DoresNo ratings yet
- 2008-12-19 - EII Comparison - Broadsound AL3C79B & Aloka UST-979-3.5Document1 page2008-12-19 - EII Comparison - Broadsound AL3C79B & Aloka UST-979-3.5Paulo DoresNo ratings yet
- AMBIVisionPriceList200703Confidential ScannersDocument1 pageAMBIVisionPriceList200703Confidential ScannersPaulo DoresNo ratings yet
- 2008-12-13 - EII Comparison - Broadsound HTC314G & Hitachi EUP-C314GDocument1 page2008-12-13 - EII Comparison - Broadsound HTC314G & Hitachi EUP-C314GPaulo DoresNo ratings yet
- Technology Corp., LTD: AV-7001V/7002V Veterinary Handheld Pulse OximeterDocument3 pagesTechnology Corp., LTD: AV-7001V/7002V Veterinary Handheld Pulse OximeterPaulo DoresNo ratings yet
- Ambisea - Electrocardigrafo - 1Ch - 3Ch - Folha A4Document1 pageAmbisea - Electrocardigrafo - 1Ch - 3Ch - Folha A4Paulo DoresNo ratings yet
- Ambisea - Electrocardigrafo - 1Ch - 3Ch - Folha A4.odgDocument1 pageAmbisea - Electrocardigrafo - 1Ch - 3Ch - Folha A4.odgPaulo DoresNo ratings yet