Professional Documents
Culture Documents
Reaction Kinetics
Reaction Kinetics
Uploaded by
Hitesh BhavnaniOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Reaction Kinetics
Reaction Kinetics
Uploaded by
Hitesh BhavnaniCopyright:
Available Formats
REACTION KINETICS
Second Order
Odes of reaction
Rate reaction
of
Zero order directly
propo totional
squat
Rate reaction is Rate conc
of independent of
conc reactants
of of a cone
Rate A
cone
Rate A Rate K A
Rate K A Rate K
Time
Rate
First Order
Rate Conc
Rate of reaction is
directly cone a
proportion to conc
of A
cone
Cone
Rate A Rate KLA
Time
Time
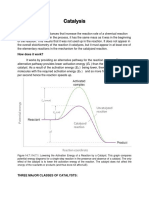
Catalysis
Half Life
Time taken in a reaction
Homogenous catalysis
for
conc to
half ty When reactants and
cataclysts have the
same
phase
conc
tin Heterogenous cataclysis
1ˢᵗ
ty 2ⁿᵈ
ty When reactants and
time
catalysts have different
In conc Time Graphs
phases
zero order 1ˢᵗ t 2nd
tyz ty decrease
y
First order 1ˢᵗ
ty 2nd
tya ty constant
1ˢᵗ t Undergoes diffusion
Second order 2nd
ty t y increases ad soo reaction
y ption
desorption then diffusion
Formula 1st order reaction K
for away from surface
Adsorption Old bonds
breaking
Desorption New bonds form
You might also like
- Accelerated Stability TestingDocument5 pagesAccelerated Stability TestingKevin GaralaNo ratings yet
- Chemical Kinetics 02Document1 pageChemical Kinetics 02confidentialspeech07No ratings yet
- Day 6 - Chemical Kinetics NotesDocument49 pagesDay 6 - Chemical Kinetics NotesDeepak KapaNo ratings yet
- Topic 7 TutorialDocument26 pagesTopic 7 TutorialElsa MahardikaNo ratings yet
- P.R All LecDocument13 pagesP.R All Lecنور عماد احمد /بوليمرات والبتروكيمياوياتNo ratings yet
- Catalysts: Catalyst Stability Assessment in A Lab-Scale Liquid-Solid (LS) Plug-Flow ReactorDocument17 pagesCatalysts: Catalyst Stability Assessment in A Lab-Scale Liquid-Solid (LS) Plug-Flow ReactorMohamed MaherNo ratings yet
- Kinetics NumericalsDocument16 pagesKinetics NumericalsMohnish KodukullaNo ratings yet
- Chemical Equilbrium 2Document4 pagesChemical Equilbrium 2bisenpallavi80No ratings yet
- EquilibriumDocument7 pagesEquilibriumEmily FNo ratings yet
- Chemical KineticsDocument73 pagesChemical Kineticsabcdefghijklmopqrstuvwxyz boyNo ratings yet
- The Rate of ReactionDocument35 pagesThe Rate of ReactionMadhav SethiaNo ratings yet
- Chemical KineticsDocument29 pagesChemical KineticsAditya PandeyNo ratings yet
- Kinetics (Part 2)Document47 pagesKinetics (Part 2)Alya NadhNo ratings yet
- Chemical KineticsDocument9 pagesChemical KineticsMikey Bryant BonbonNo ratings yet
- Qihbn - SN: Product Reaction ProductDocument2 pagesQihbn - SN: Product Reaction ProductChitraNo ratings yet
- Adobe Scan Nov 19, 2023Document1 pageAdobe Scan Nov 19, 2023beautysharma36583No ratings yet
- Levenspiel Bischoff 1959Document4 pagesLevenspiel Bischoff 1959mauroogidoNo ratings yet
- Lecture (4) : Reaction RatesDocument21 pagesLecture (4) : Reaction RatesAhmed AbdullaNo ratings yet
- Chemical Kinetics 2023Document17 pagesChemical Kinetics 2023Rudra YadavNo ratings yet
- Lecture - 16-Enzyme Kinetics and Catalysis 1Document36 pagesLecture - 16-Enzyme Kinetics and Catalysis 1Nagarjuna VuchuruNo ratings yet
- Edmondshtepani Co2 Eor Casper 2012Document54 pagesEdmondshtepani Co2 Eor Casper 2012xin shiNo ratings yet
- Damped Harmonic OscillatorDocument3 pagesDamped Harmonic OscillatorMd Chand AsiqueNo ratings yet
- 1 - Topik Ke 2 - TRK PDFDocument11 pages1 - Topik Ke 2 - TRK PDFcahyoNo ratings yet
- Chemical Kinetics (Revision Notes) - RemovedDocument7 pagesChemical Kinetics (Revision Notes) - Removedsaapldesign1 1No ratings yet
- Chemical EquilibriumDocument4 pagesChemical Equilibriumprachibansal2405No ratings yet
- Beer's Law VerifyDocument6 pagesBeer's Law VerifyVishwesh SNo ratings yet
- Pericyclic Reactions, 1Document7 pagesPericyclic Reactions, 1Aparna SahaNo ratings yet
- Chapter 6 KineticDocument11 pagesChapter 6 KineticPHƯƠNG ĐẶNG YẾNNo ratings yet
- Ap ChemDocument6 pagesAp ChemholaholaholacarlottaNo ratings yet
- 0324 CHDocument3 pages0324 CHtxiaohe0604No ratings yet
- Ideal - Batch and Semi - Batch Reactors Design 2. 1 The Ideal Batch ReactorDocument11 pagesIdeal - Batch and Semi - Batch Reactors Design 2. 1 The Ideal Batch ReactorDagim HailuNo ratings yet
- SY - PP II - Drug StabilityDocument49 pagesSY - PP II - Drug StabilityKevalNo ratings yet
- Chemistry Class 12 FormulasDocument46 pagesChemistry Class 12 FormulasabdulbasithmaricarNo ratings yet
- 1a. Chemical Reaction EngineeringDocument18 pages1a. Chemical Reaction Engineeringchedraemar06No ratings yet
- Chapter4 OCWDocument11 pagesChapter4 OCWعوض أمحمدNo ratings yet
- Design EquationDocument35 pagesDesign EquationPMNo ratings yet
- Rate of Reaction: Appearance of Products (P) (P2) - (P1)Document3 pagesRate of Reaction: Appearance of Products (P) (P2) - (P1)Pardeep KumarNo ratings yet
- 30 Minutes Chemical KineticsDocument24 pages30 Minutes Chemical Kineticscool wingsNo ratings yet
- Chemical Reaction Engineering (CRE) Is TheDocument83 pagesChemical Reaction Engineering (CRE) Is TheLe Anh QuânNo ratings yet
- Kinetika Kimia Pada Laju ReaksiDocument25 pagesKinetika Kimia Pada Laju ReaksiWardahNo ratings yet
- KineticsDocument41 pagesKineticsSafwan ShahidNo ratings yet
- R DC /DT: Formed I: Fluid Reacting of e Unit Volum On BasedDocument8 pagesR DC /DT: Formed I: Fluid Reacting of e Unit Volum On BasedMahfuzur Rahman SiddikyNo ratings yet
- CE312 Lecture1617 Fall 20-21Document12 pagesCE312 Lecture1617 Fall 20-21PRATEEK SHARMANo ratings yet
- Chemical Kinetics: - : Types of Chemical ReactionsDocument12 pagesChemical Kinetics: - : Types of Chemical ReactionsmanishNo ratings yet
- Topik 1: Chemical Reaction Engineering (CRE) Is TheDocument34 pagesTopik 1: Chemical Reaction Engineering (CRE) Is TheRani PutriaNo ratings yet
- Previous Lecture: Review The General Mole Balance Equations For Ideal ReactorsDocument14 pagesPrevious Lecture: Review The General Mole Balance Equations For Ideal ReactorsAmroKashtNo ratings yet
- Design of Catalytic Micro Trickle Bed Reactors: NomenclatureDocument29 pagesDesign of Catalytic Micro Trickle Bed Reactors: NomenclatureFahad kamranNo ratings yet
- Teknik Reaksi Kimia I: by HaryantoDocument23 pagesTeknik Reaksi Kimia I: by HaryantoHarymsl MslNo ratings yet
- Lecture 2 - Chapter 1-Mole BalanceDocument40 pagesLecture 2 - Chapter 1-Mole BalanceNizam JumadiNo ratings yet
- Unit 4 PHYSICS IBDocument3 pagesUnit 4 PHYSICS IBCamila S.No ratings yet
- CHE 102 Formula SheetsDocument3 pagesCHE 102 Formula SheetsMarjuk MahfuzNo ratings yet
- Science: Approaches To Uncertainty EvaluationDocument12 pagesScience: Approaches To Uncertainty EvaluationSaqib KazmiNo ratings yet
- Chemical Reactor Design Chemical Reactor Design: Y W L Youn-Woo LeeDocument41 pagesChemical Reactor Design Chemical Reactor Design: Y W L Youn-Woo LeePara DiseNo ratings yet
- Lecture 6 0Document9 pagesLecture 6 0Pankaj Kumar SainiNo ratings yet
- Online QuizDocument16 pagesOnline QuizPhyo Marn Hone LinNo ratings yet
- Goc and Isomerism 1 YleLP2BEVwSg6aryDocument18 pagesGoc and Isomerism 1 YleLP2BEVwSg6aryzaviaisra1508No ratings yet
- Lecture2 Chapter1 Molebalancepart1Document42 pagesLecture2 Chapter1 Molebalancepart1Nur SafiahNo ratings yet
- Lecture 4 - Rate Law and StoichiometryDocument32 pagesLecture 4 - Rate Law and StoichiometryNizam JumadiNo ratings yet
- Aromatic Anti and Non QuestionsDocument12 pagesAromatic Anti and Non Questionsc5x4xfmjcsNo ratings yet
- Chemistry Important TopicsDocument4 pagesChemistry Important TopicswerwerNo ratings yet
- FunctionsDocument19 pagesFunctionsHitesh BhavnaniNo ratings yet
- WWW Igcseict Info Theory 7 - 2 Bank Index HTMLDocument4 pagesWWW Igcseict Info Theory 7 - 2 Bank Index HTMLHitesh BhavnaniNo ratings yet
- WWW Igcseict Info Theory 7 - 2 Book Index HTMLDocument2 pagesWWW Igcseict Info Theory 7 - 2 Book Index HTMLHitesh BhavnaniNo ratings yet
- WWW Ictlounge Com HTML Microprocessor - Controlled - Devices HTMDocument6 pagesWWW Ictlounge Com HTML Microprocessor - Controlled - Devices HTMHitesh BhavnaniNo ratings yet
- WWW Igcseict Info Theory 7 - 2 Expert Index HTMLDocument2 pagesWWW Igcseict Info Theory 7 - 2 Expert Index HTMLHitesh BhavnaniNo ratings yet
- Presentación 2 - Cinética PDFDocument7 pagesPresentación 2 - Cinética PDFDanny GarcíaNo ratings yet
- Mikales Menten Great With Green Book AlsoDocument8 pagesMikales Menten Great With Green Book AlsoMOSES MILLERNo ratings yet
- All 1B CSM PDFDocument486 pagesAll 1B CSM PDFNikka Lopez100% (2)
- Limiting and Excess ReactantsDocument23 pagesLimiting and Excess ReactantsJaymar VeroyNo ratings yet
- L9b Selectivity Example ProblemsDocument24 pagesL9b Selectivity Example ProblemsMeghna SheoranNo ratings yet
- Exercises - EnzymesDocument9 pagesExercises - EnzymesBug AphidNo ratings yet
- 3 Enzyme KineticsDocument22 pages3 Enzyme KineticsLee Ping Shin100% (1)
- Enzyme Activity Online LabDocument5 pagesEnzyme Activity Online LabJohn BuzzerioNo ratings yet
- Biochemistry 9th Edition Campbell Test BankDocument30 pagesBiochemistry 9th Edition Campbell Test Bankspawnerminutiaxae7n100% (34)
- Chapter 10 Chemical Kinetics II PDFDocument130 pagesChapter 10 Chemical Kinetics II PDFJuanCarlosVazquezLira100% (3)
- Unit 5Document51 pagesUnit 5leetianyi34No ratings yet
- Lecture 14 Thursday 2/21/08: Pseudo Steady State Hypothesis (PSSH)Document20 pagesLecture 14 Thursday 2/21/08: Pseudo Steady State Hypothesis (PSSH)Pinjala AnoopNo ratings yet
- CATALYSISDocument7 pagesCATALYSISJudy Ann Leyco BartolataNo ratings yet
- CRE-CIA 1-A QUESTION Paper 2023 OddDocument1 pageCRE-CIA 1-A QUESTION Paper 2023 OddA.RAJKUMAR (HICET) HICET STAFFCHEMNo ratings yet
- E 199 Sol 2Document13 pagesE 199 Sol 2David Alemán Sánchez0% (1)
- Unit 4 Chemical KineticsDocument11 pagesUnit 4 Chemical Kineticsnadeemnagthan008No ratings yet
- Formative 2 Ch. 17.2 Reaction Rates Classwork 3Document6 pagesFormative 2 Ch. 17.2 Reaction Rates Classwork 3Rama wedianNo ratings yet
- Homework 3 KeyDocument2 pagesHomework 3 Keychip_daleNo ratings yet
- Ankyl Halogenua Phan Ung The Nucleophil Va Tach LoaiDocument103 pagesAnkyl Halogenua Phan Ung The Nucleophil Va Tach LoaiQuang Cường HoàngNo ratings yet
- Chapter 2. ExerciseDocument5 pagesChapter 2. Exercisedhuy2399No ratings yet
- MCQS CH 3 Bio Part 1 1Document2 pagesMCQS CH 3 Bio Part 1 1All kinds of information on channelNo ratings yet
- 2.01 Surface Kinetics - Unimolecular Reactions PDFDocument33 pages2.01 Surface Kinetics - Unimolecular Reactions PDFMelchiNo ratings yet
- Auto Catalytic Reactions PresentationDocument15 pagesAuto Catalytic Reactions PresentationAmad AmadNo ratings yet
- Rangkuman TRK (Deva Punya)Document4 pagesRangkuman TRK (Deva Punya)gamalielNo ratings yet
- 8.3 Factors Affecting RXN RateDocument36 pages8.3 Factors Affecting RXN RateNur AleyaNo ratings yet
- Reaction Kinetics: The EssentialsDocument12 pagesReaction Kinetics: The EssentialsJohn Carlo MacalagayNo ratings yet
- rr320802 Chemical Reaction Engineering IDocument8 pagesrr320802 Chemical Reaction Engineering ISRINIVASA RAO GANTANo ratings yet
- 03 Enzyme KineticsDocument48 pages03 Enzyme KineticsCester Gale DucusinNo ratings yet
- Chem Chap 4 PDFDocument62 pagesChem Chap 4 PDFNur Husnina HussinNo ratings yet