Professional Documents
Culture Documents
S.1 Competence Based Assessment Examinations Chemistry Theory
S.1 Competence Based Assessment Examinations Chemistry Theory
Uploaded by
Peter Okidi Agara OyoOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
S.1 Competence Based Assessment Examinations Chemistry Theory
S.1 Competence Based Assessment Examinations Chemistry Theory
Uploaded by
Peter Okidi Agara OyoCopyright:
Available Formats
Page 1 of 4
Student’s
Name: ……………………………………………………………..…Stream: …………………….
Uganda Certificate of Education
S.1 COMPETENCE BASED ASSESSMENT
EXAMINATIONS
CHEMISTRY (THEORY)
2 hours
INSTRUCTIONS:
• This paper consists of eight questions.
• Answer all questions in the spaces provided.
• Illustrations in form of drawings should be made where necessary, with a sharp pencil.
For official use only
Number Score comment Teacher’s
1.
2.
3.
4.
1 (a) State two differences between a temporary and permanent change.
(02 marks)
Temporary change permanent change
RENA COLLEGE CHEM DEPT, 2023
Page 2 of 4
(b) Write true or false for each of the following statements. (01 mark @)
(i) Boiling an egg is a temporary change………………………………
(ii) Sublimation of iodine is a permanent change ………………………
(iii) Burning wood is a permanent change……………………………….
(iv) Souring of porridge is a temporary change…………………………
2. Senior two students went to the laboratory and found out that it was filled with the smell of the
laboratory gas. They later discovered that the smell was because one gas tap at the corner of the
laboratory had been left open.
(a) Using kinetic theory of matter, explain why the smell of the gas reached all parts of the
laboratory. (02 marks)
………………………………………………………………………………………
………………………………………………………………………………………
………………………………………………………………………………………
(b) What is the name of the process by which the gas spread to all parts of the laboratory.
(01 mark)
……………………………………………………………………………………… (c)
Different states of matter undergo melting and freezing processes. State the difference and similarity
of the two processes (02 marks)
………………………………………………………………………………………
………………………………………………………………………………………
………………………………………………………………………………………
………………………………………………………………………………………
(d) Which of the two processes;
(i) Absorbs heat? (01mark)
……………………………………………………………………………………
(ii) Releases heat? (01 mark)
………………………………………………………………………………………
3. From the particle theory of matter, all matter is made up of small particles that are in a
constant and random state of motion. A group of senior one students from vision for Africa High
school carried out an investigation about particles of air using a balloon. They inflated it as
shown below;
RENA COLLEGE CHEM DEPT, 2023
Page 3 of 4
(a) Explain how the particles of air inside the balloon create pressure to make it swell
outwards. (02 marks)
………………………………………………………………………………………
………………………………………………………………………………………
………….……………………………………………………………………………
(b) When the students put the balloon under sunshine for some hours, it was observed that
the balloon increased in size. How can you explain this observation using the particle theory of
matter? (03 marks)
………………………………………………………………………………………
………………………………………………………………………………………
………………………………………………………………………………………
……………….………………………………………………………………………
………………………………………………………………………………………
(c). The students left the balloon in the laboratory for seven days, after which they observed
that the balloon had decreased in size. Give an explanation as to why the balloon reduced
in size after seven days. (03 marks)
………………………………………………………………………………………
………………………………………………………………………………………
………………………………………………………………………………………
………………………………………………………………………………………
………………………………………………………………………………………
………………………………………………………………………………………
(d). Give any two differences in the arrangement and behavior of particles in
solids and in gases. (02 marks)
Particles in solids Particles in gases
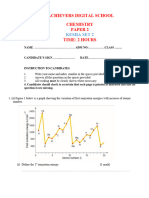
4. The figure below shows the heating and cooling curves for substances X and Y. Study it and
answer the questions that follow.
RENA COLLEGE CHEM DEPT, 2023
Page 4 of 4
(a) What was the original state of substance X and Y? (02 marks)
………………………………………………………………………………………
………………………………………………………………………………………
(b) Explain the shape of the curve for substance X between each of the following parts.
(i) A and B (02 marks)
………………………………………………………………………………………
………………………………………………………………………………………
………………………………………………………………………………………
(ii) B and C (02 marks)
………………………………………………………………………………………
………………………………………………………………………………………
………………………………………………………………………………………
(c) Explain the shape of the curve for substance Y between the following parts.
(i) Q and R (02 marks)
………………………………………………………………………………………
………………………………………………………………………………………
………………………………………………………………………………………
(ii) S and T (02 marks)
………………………………………………………………………………………
………………………………………………………………………………………
END
RENA COLLEGE CHEM DEPT, 2023
You might also like
- S.3 ChemistryDocument9 pagesS.3 ChemistryDaniel ComboniNo ratings yet
- S2 Phy Eot 3 Kamota 2023 PDFDocument8 pagesS2 Phy Eot 3 Kamota 2023 PDFamirimagsNo ratings yet
- S.2 Mid 2 Cem 2023Document4 pagesS.2 Mid 2 Cem 2023Maama PhionaNo ratings yet
- Chemistry PP2 2023Document13 pagesChemistry PP2 2023shiklemeNo ratings yet
- April Holiday AssignmentDocument13 pagesApril Holiday AssignmentKevin KiplangatNo ratings yet
- Form 3 - Chemistry - Question PaperDocument14 pagesForm 3 - Chemistry - Question Papermiles computersNo ratings yet
- Kcse Chem Pp1 Replica 2023.newDocument115 pagesKcse Chem Pp1 Replica 2023.newshantelmutuku322No ratings yet
- f3 Chem Qs Code 01 - Hawai (Cbe)Document6 pagesf3 Chem Qs Code 01 - Hawai (Cbe)consolatajaokoNo ratings yet
- Chemistry - Chemistry Form 1 - Question PaperDocument13 pagesChemistry - Chemistry Form 1 - Question PapermwendwavalerieNo ratings yet
- Uganda Certificate of Education: End of Term One Exams 2017Document9 pagesUganda Certificate of Education: End of Term One Exams 2017Daniel MarkNo ratings yet
- S6 183 1588122697Document16 pagesS6 183 1588122697malingaisrealNo ratings yet
- Physics Sample Questions For New Curriculum Paper 1 and 2Document27 pagesPhysics Sample Questions For New Curriculum Paper 1 and 2amkun001100% (2)
- Biology - Paper 2 - Question PaperDocument10 pagesBiology - Paper 2 - Question PaperNZURE NJOKANo ratings yet
- British International College: Year 11 Half Term Assessment ChemistryDocument9 pagesBritish International College: Year 11 Half Term Assessment ChemistryHarry SonNo ratings yet
- Form 2 2023 End T3 Chemistry QN - TeacherDocument12 pagesForm 2 2023 End T3 Chemistry QN - TeacherDaniel KibetNo ratings yet
- Prep 1 Practical Exam Second Term 2022Document5 pagesPrep 1 Practical Exam Second Term 2022mostafa -shNo ratings yet
- Grade 11 Science Seminar Paper Part 2 2020Document14 pagesGrade 11 Science Seminar Paper Part 2 2020My WorksNo ratings yet
- Chemistry Form 3 2024 - Question PaperDocument8 pagesChemistry Form 3 2024 - Question Paperwinfredmwende44No ratings yet
- Maple Leaf International School: Final Examination 2021 Subject: Chemistry Paper: 2 Class: Ix TIME: 1 Hour 15 MinutesDocument12 pagesMaple Leaf International School: Final Examination 2021 Subject: Chemistry Paper: 2 Class: Ix TIME: 1 Hour 15 MinuteszareenNo ratings yet
- Mebu Chem S.3 2023 - 081850Document10 pagesMebu Chem S.3 2023 - 081850jacklinejoggo565No ratings yet
- Chemistry Paper TWODocument12 pagesChemistry Paper TWOMBUGUA GRAPHICSNo ratings yet
- Chemistry Mock 2021 Al p2Document14 pagesChemistry Mock 2021 Al p2zealdajunior4No ratings yet
- Kesha Set & 3Document35 pagesKesha Set & 3wanyoike2023No ratings yet
- Bio Pp3 Success Path Mock 2023Document6 pagesBio Pp3 Success Path Mock 2023DenisNo ratings yet
- f4 Chem Mid-Term 1 ExamsDocument34 pagesf4 Chem Mid-Term 1 ExamsAjuluNo ratings yet
- Chem Form 3 End Term 1 QZDocument16 pagesChem Form 3 End Term 1 QZadriankammau007No ratings yet
- f1 Endterm 1 Series 2Document60 pagesf1 Endterm 1 Series 2abu326274No ratings yet
- Physics F1 AssignmentDocument11 pagesPhysics F1 Assignmentskynet48cyberNo ratings yet
- S.3 Eot Chem WMSSDocument8 pagesS.3 Eot Chem WMSSDaniel ComboniNo ratings yet
- Chemistry Exam Term 2 2013Document11 pagesChemistry Exam Term 2 2013Chanell M.No ratings yet
- S6 Aceiteka 2023 Chemistry P1Document16 pagesS6 Aceiteka 2023 Chemistry P1kundukefa25No ratings yet
- SCLP Samaj School Year 10 Chemistry Revision WorksheetDocument11 pagesSCLP Samaj School Year 10 Chemistry Revision WorksheetHarshil PatelNo ratings yet
- Bio F2Document11 pagesBio F2Qiash JontezNo ratings yet
- F1 ChemDocument9 pagesF1 ChemgabriellaacholaNo ratings yet
- Shapta Chemistry Uce 2024 2Document12 pagesShapta Chemistry Uce 2024 2thebeatzugNo ratings yet
- Form 2 - Chemistry - Question PaperDocument12 pagesForm 2 - Chemistry - Question PaperMejah GeoffreyNo ratings yet
- s.5 Chem 1 E.O.TDocument11 pagess.5 Chem 1 E.O.TW. Joseph the chemistNo ratings yet
- S4 24 1587601547Document13 pagesS4 24 1587601547Daniel MarkNo ratings yet
- Chem 1 Pre RegeDocument14 pagesChem 1 Pre RegeFIDEL RONEL OTIENONo ratings yet
- Biology Paper 2Document18 pagesBiology Paper 2dragon labNo ratings yet
- Biology - F2 - Cycle 2 Term 2 2023Document8 pagesBiology - F2 - Cycle 2 Term 2 2023profitableagripoultryNo ratings yet
- Form 2 Chem End Term 1 2024Document11 pagesForm 2 Chem End Term 1 2024blueivyl872No ratings yet
- Biology Form Two - Question PaperDocument12 pagesBiology Form Two - Question PaperVernonNo ratings yet
- Kcse Extra-County Mocks s1Document235 pagesKcse Extra-County Mocks s1micah isabokeNo ratings yet
- ChemistryDocument10 pagesChemistrygithukucharles.gcNo ratings yet
- Nairobi School: NAME .Adm No Stream .Document12 pagesNairobi School: NAME .Adm No Stream .Ferdnard WanjalaNo ratings yet
- FORM 1 2023 END T3 CHEMISTRY QN - TEACHER - CO - .KE - SET - ADocument9 pagesFORM 1 2023 END T3 CHEMISTRY QN - TEACHER - CO - .KE - SET - AYussuf HirowNo ratings yet
- A Level Chemistry Paper 1 Set 17Document16 pagesA Level Chemistry Paper 1 Set 17Lutaaya Paul BamutaliraNo ratings yet
- 6 Chem IDocument16 pages6 Chem IW. Joseph the chemistNo ratings yet
- Form 4 Biology - Paper 2 - Question PaperDocument11 pagesForm 4 Biology - Paper 2 - Question PaperraymurigiNo ratings yet
- Chem F1 Kibos Sec Et2 2023Document12 pagesChem F1 Kibos Sec Et2 2023Maureen MwendeNo ratings yet
- S.2 Chem Mid Term TrinityDocument10 pagesS.2 Chem Mid Term TrinityARYATIJUKA FELIXNo ratings yet
- Chemistry P1Document13 pagesChemistry P1zachaeusNo ratings yet
- Form 4 Paper 2Document13 pagesForm 4 Paper 2gerald2.njoruNo ratings yet
- Matigo s2 Biology Assessment 2022Document14 pagesMatigo s2 Biology Assessment 2022Aristotle MuhaweNo ratings yet
- Chemistry Paper 1Document12 pagesChemistry Paper 1MBUGUA GRAPHICSNo ratings yet
- Form 3 - Biology - Question PaperDocument11 pagesForm 3 - Biology - Question Papermiles computersNo ratings yet
- The Maseno School Mock Examinations Biology P1Document12 pagesThe Maseno School Mock Examinations Biology P1Charles NjithiNo ratings yet
- Test 14 Paper OneDocument18 pagesTest 14 Paper OneZziwa ReaganNo ratings yet
- Fourier Analysis: SignalsDocument38 pagesFourier Analysis: SignalsPeter Okidi Agara OyoNo ratings yet
- Chapter 10. Sequences, Etc.: Upper BoundDocument35 pagesChapter 10. Sequences, Etc.: Upper BoundPeter Okidi Agara OyoNo ratings yet
- Hash PDFDocument7 pagesHash PDFPeter Okidi Agara OyoNo ratings yet
- Analyzing Code with Θ, O and Ω: 1 DefinitionsDocument18 pagesAnalyzing Code with Θ, O and Ω: 1 DefinitionsPeter Okidi Agara OyoNo ratings yet
- Homework 1 SolutionsDocument6 pagesHomework 1 SolutionsPeter Okidi Agara OyoNo ratings yet
- Nyquist Plots For EDLCDocument13 pagesNyquist Plots For EDLCLoga NathanNo ratings yet
- Reynold NumberDocument5 pagesReynold NumberFita Desti SenjaNo ratings yet
- 1945 - Magnesium Chloride MSDS PDFDocument6 pages1945 - Magnesium Chloride MSDS PDFLuqman HidayatNo ratings yet
- EXAMPLESeparations Pre Lab AX05Document8 pagesEXAMPLESeparations Pre Lab AX05midnightmagicpandaNo ratings yet
- Carbonyl Compounds SheetDocument133 pagesCarbonyl Compounds Sheetadityavaish739No ratings yet
- Chemical BondingDocument18 pagesChemical Bondingteacher zaneNo ratings yet
- China DME OutlookDocument31 pagesChina DME OutlookRamaOktavianNo ratings yet
- Hydro CarbonsDocument10 pagesHydro CarbonsLok Jun Hao100% (1)
- Introduction To Optical Waveguides: Dr. Lukas ChrostowskiDocument66 pagesIntroduction To Optical Waveguides: Dr. Lukas ChrostowskiTiewsoh LikyntiNo ratings yet
- Monoethanolamine - Boletim Técnico PDFDocument2 pagesMonoethanolamine - Boletim Técnico PDFFlavio Jorge Miranda PimentelNo ratings yet
- 19 10th Science Question Bank English MediumDocument12 pages19 10th Science Question Bank English Mediumvadivel.km1527No ratings yet
- 8 Vertical Stresses Below Applied LoadsDocument13 pages8 Vertical Stresses Below Applied LoadsSaša MarinNo ratings yet
- Iec Hazardous Locations Certification DocumentsDocument2 pagesIec Hazardous Locations Certification DocumentsbubuluqNo ratings yet
- Safety Data Sheet Shift: Revision Date: 17/11/2017 Revision: 8Document8 pagesSafety Data Sheet Shift: Revision Date: 17/11/2017 Revision: 8Umair ShafiqueNo ratings yet
- GC SpecsDocument5 pagesGC Specskaysb786133No ratings yet
- TPPG Broschuere TEREZ LFT EN LoResDocument17 pagesTPPG Broschuere TEREZ LFT EN LoResmakoyNo ratings yet
- Lecture 3Document32 pagesLecture 3Oussema Ben KasdallahNo ratings yet
- Solstice N40 TDS 141216 VF PDFDocument2 pagesSolstice N40 TDS 141216 VF PDFnaren233No ratings yet
- Suzuky ReactionDocument13 pagesSuzuky ReactionAlbornoz JuanNo ratings yet
- Ultratech Cement Opc 53 PDFDocument1 pageUltratech Cement Opc 53 PDFAquib khanNo ratings yet
- Ial WPH04 01 Oct19Document28 pagesIal WPH04 01 Oct19Happy AyichNo ratings yet
- Technical Data SheetDocument3 pagesTechnical Data SheetAdrián SánchezNo ratings yet
- Expression of Biological InformationDocument7 pagesExpression of Biological InformationNur Amalina KhozariNo ratings yet
- Respiration Is of Two TypesDocument3 pagesRespiration Is of Two TypesKyng GamariNo ratings yet
- 2598-Article Text-6884-1-10-20230703Document9 pages2598-Article Text-6884-1-10-20230703nuriskaNo ratings yet
- Full Download Book Advances in Synthesis Gas Methods Technologies and Applications Volume 3 Syngas Products and Usages PDFDocument41 pagesFull Download Book Advances in Synthesis Gas Methods Technologies and Applications Volume 3 Syngas Products and Usages PDFchristopher.vale567100% (29)
- Solar Powered Desalination System Using Fresnel LensDocument7 pagesSolar Powered Desalination System Using Fresnel LensjonaNo ratings yet
- PD 1185Document32 pagesPD 1185Vholts Villa VitugNo ratings yet
- Bio Tec EcoPureBiodegradationFAQDocument6 pagesBio Tec EcoPureBiodegradationFAQH. Ali ArvasNo ratings yet
- Aurora: AE MB-72 SeriesDocument2 pagesAurora: AE MB-72 SeriesNatalia IniotakiNo ratings yet