Professional Documents
Culture Documents
Sljod v22 p99 100
Sljod v22 p99 100
Uploaded by
Bhagya HettiarachchiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Sljod v22 p99 100
Sljod v22 p99 100
Uploaded by
Bhagya HettiarachchiCopyright:
Available Formats
Pityriasis rubra pilaris like eruption following Sinopharm - SARS COVID 19 vaccine 99
Pityriasis rubra pilaris like eruption following Sinopharm – SARS
COVID-19 vaccine
K H Samarasinghe 1, T Janani2 , C N Gunasekera 3
Sri Lanka Journal of Dermatology, 2021, 22: 99-100
Introduction
Pityriasis rubra pilaris (PRP) is an uncommon
papulosquamous disorder, first described in 1835 by
Claudia’s Tarral 1 which is characterized by the
presence of follicular papules on an erythematous
base and orange-red plaques with islands of sparing.
Six main types of PRP are described with classic
adult being the most commonly seen variant. PRP
can be associated with infections, autoimmunity,
malignancies, drugs, etc. However, most cases are
idiopathic.
Up to now, very few vaccines induced PRP are repor-
ted in the literature. (For MMR, DPT and Influenza
vaccines4).
Recently a few case reports have been reported
following COVID-19 vaccination involving Covi-
shield and Pfizer vaccines, but non following
Sinopharm vaccine to the best of our knowledge. Figure 1.
Herein we report the first case of PRP-like eruption
following Sinopharm COVID-19 vaccination.
Case report
A 46-year-old previously healthy Sri Lankan male
presented with rapidly developing erythematous skin
lesions over the body which first appeared 2 weeks
after the first dose of Sinopharm COVID-19 vaccine
and had worsening of the rash following the second
dose taken 4 weeks after the first dose. There was no
history suggesting any infection, significant ultra-
violet exposure, drugs, or family history of similar
skin rashes.
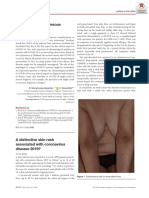
On examination, there were multiple orange-red,
scaly papules and plaques coalescing to form large
plaques with characteristic islands of sparing over
the trunk (Figure 1), upper and lower limbs. Bilateral
palmer and planter diffuse keratoderma (Figure 2)
with onycholysis was also noted. Mucosal involve-
ment was seen. General and systemic examinations
were unremarkable. Figure 2.
1
Senior Registrar in Dermatology, 2Registrar in Dermatology, 3Consultant Dermatologist, National Hospital of Sri
Lanka, Colombo.
Vol. 22, 2021
100 K H Samarasinghe, T Janani, C N Gunasekera
Two biopsies that were taken from the trunk and limb Post vaccine-induced PRP, following MMR, DPT and
revealed psoriasiform hyperplasia of the epidermis Influenza vaccines are very rarely reported entity in
with alternative ortho and parakeratosis in a the literature.
“checkerboard pattern” as well as sparse lymphocytic
perivascular infiltrate in papillary dermis favoring Up to now, a few COVID-19 vaccine-induced PRP
the diagnosis of PRP. cases have been reported in the literature towards
Covishield vaccine2 and Pfizer vaccine3. To the best
His basic hematological investigations and sero- of our knowledge, no Sinopharm COVID-19 vaccine-
logical investigations for blood-borne viruses were induced PRP like eruptions have been reported in
normal. Age-appropriate malignancy screening was the literature. Accordingly, this could be the first
unremarkable. reported case of PRP-like eruption induced by the
Sinopharm COVID-19 vaccine.
A diagnosis of PRP triggered following Sinopharm
COVID-19 vaccine was made. This case adds an important potential adverse effect
of the COVID-19 vaccine, especially considering the
He was started on oral acitretin with significant significant morbidity and protracted nature of this
improvement of the skin rash. skin disease.
Hence, awareness of such adverse effects among
Discussion
dermatologists will help with early recognition and
PRP is a papulosquamous disorder with most cases prompt management.
are acquired, however, familial forms associated with
CARD 14 mutation also reported 1. Both men and
References
women are affected equally. There are six main types
identified and out of all, classic adult variant is the 1. Jean L, Bolognia, Julie V. Schaffer, Cerroni L. Dermatology
commonest type. Even though most cases are – fourth edition.
idiopathic, infections, UV exposure, various minor 2. Mukesh K S, Kanika R, Dinesh P. An old entity, a new
trauma to the skin, have been reported to proceed the trigger: post COVID-19 vaccine pityriasis rubra pilaris.
onset of PRP implicating physical trigger or super International Journal of Risk and Safety in Medicine 2021;
antigenic effect as possible etiological factors in some 32(4): 261-4.
cases.
3. Hunjan MK, Roberts C, Karim S, Hague J. Pityriasis
During this COVID-19, pandemic vaccination against rubra pilaris like eruption following administration of the
SARS COVID-19 is being carried out worldwide with BNT 163b2 [Pfizer -BioNTech] m RNA COVID-19 vaccine,
Clinical and experimental dermatology/BAD August
many of us encountering a wide variety of cutaneous
2021.
adverse effects.The majority of them are minor
cutaneous adverse effects like injection site reactions, 4. Naciri B, Schmutz L, Pityriasis rubra pilaris after
urticaria, urticarial vasculitis, pain, pruritus, morbilli- vaccination, Annales de Dermatology et de venereology
form eruptions, pityriasis rosea like reactions, etc. 2011: 753-6.
Sri Lanka Journal of Dermatology
You might also like
- Sep2020-Review and ClassificationDocument23 pagesSep2020-Review and ClassificationSAJIN ALEXANDERNo ratings yet
- Spectrum - of - Clinicopathologic - Findings - In.1 (1Document11 pagesSpectrum - of - Clinicopathologic - Findings - In.1 (1tzuskyNo ratings yet
- Impact of COVID-19 On Autoimmune Blistering DiseasesDocument10 pagesImpact of COVID-19 On Autoimmune Blistering DiseasesNadhifa FauziahNo ratings yet
- COVID-19 Cutaneous Manifestations: Simplifying The ConfusionDocument2 pagesCOVID-19 Cutaneous Manifestations: Simplifying The Confusionanis utamiNo ratings yet
- Cutaneous Signs in COVID-19 Patients: A ReviewDocument6 pagesCutaneous Signs in COVID-19 Patients: A Reviewϟ Orbi SinkaNo ratings yet
- Spectrum of Clinicopathologic Findings in COVID-19-induced Skin LesionsDocument9 pagesSpectrum of Clinicopathologic Findings in COVID-19-induced Skin LesionsggenesisNo ratings yet
- 2021-Clinical and Pathologic Correlation of Cutaneous COVID-19 Vaccine Reactions Including V-REPP-A Registry-Based StudyDocument9 pages2021-Clinical and Pathologic Correlation of Cutaneous COVID-19 Vaccine Reactions Including V-REPP-A Registry-Based Studyseguridadyambiente641No ratings yet
- Skin Manifestations of COVID-19 in Children Part 1Document7 pagesSkin Manifestations of COVID-19 in Children Part 1Alfi RahmatikaNo ratings yet
- Diffuse Lepromatous Leprosy in An Hiv-Positive PatientDocument7 pagesDiffuse Lepromatous Leprosy in An Hiv-Positive PatientIJAR JOURNALNo ratings yet
- Lessons From Dermatology About Inflammatory Responses in Covid-19Document18 pagesLessons From Dermatology About Inflammatory Responses in Covid-19ϟ Orbi SinkaNo ratings yet
- Jurnal Reading Kulit (Jenifer Johana Paath 201383019)Document34 pagesJurnal Reading Kulit (Jenifer Johana Paath 201383019)Mega BagdadNo ratings yet
- The Role of Emergency Radiology in COVID-19Document13 pagesThe Role of Emergency Radiology in COVID-19ViviApriliaNo ratings yet
- Pityriasis Rosea-Like Eruption Due To Pneumococcal Vaccine in A Child With Nephrotic SyndromeDocument3 pagesPityriasis Rosea-Like Eruption Due To Pneumococcal Vaccine in A Child With Nephrotic SyndromeBudiMulyanaNo ratings yet
- Malignant Syphilis As The Presenting Complaint of Advanced HIVDocument4 pagesMalignant Syphilis As The Presenting Complaint of Advanced HIVManuel López FuentesNo ratings yet
- Prospects For Sars-Cov-2 Diagnostics, Therapeutics and Vaccines in AfricaDocument15 pagesProspects For Sars-Cov-2 Diagnostics, Therapeutics and Vaccines in AfricaÃßD ÔûñîNo ratings yet
- Erythema Multiforme Eruption Due To SARS-COV 2Document3 pagesErythema Multiforme Eruption Due To SARS-COV 2Irzam PratamaNo ratings yet
- Clinical Infection in Practice: Aafiya Ambereen, Sajjad A. Rahman, Suhailur Rehman, Kamran Zaidi, S.H. ArifDocument6 pagesClinical Infection in Practice: Aafiya Ambereen, Sajjad A. Rahman, Suhailur Rehman, Kamran Zaidi, S.H. Ariffrans lazioNo ratings yet
- Urticarial Exanthem As Early Diagnostic Clue For COVID-19 InfectionDocument2 pagesUrticarial Exanthem As Early Diagnostic Clue For COVID-19 Infectionandini senjaNo ratings yet
- COVID-19 and Dermatology: A Comprehensive Guide For DermatologistsDocument7 pagesCOVID-19 and Dermatology: A Comprehensive Guide For DermatologistsAna Karina Alvarado OsorioNo ratings yet
- Clinical and Histological Characterization of Vesicular COVID-19 Rashes: A Prospective Study in A Tertiary Care HospitalDocument18 pagesClinical and Histological Characterization of Vesicular COVID-19 Rashes: A Prospective Study in A Tertiary Care HospitalANA KARINA ALVARADO OSORIONo ratings yet
- Corynebacterium Ulcerans Cutaneous Diphtheria: Grand RoundDocument8 pagesCorynebacterium Ulcerans Cutaneous Diphtheria: Grand RoundRafa NaufalinNo ratings yet
- "Virtopsy" Virtual Alternative For Post-Mortem Investigation in Pandemic Era of Covid-19: Diagnostic Approach To Reduce The Menace of Contagion: - A ReviewDocument6 pages"Virtopsy" Virtual Alternative For Post-Mortem Investigation in Pandemic Era of Covid-19: Diagnostic Approach To Reduce The Menace of Contagion: - A ReviewInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Cutaneous Manifestations of COVID-19: Review ArticleDocument9 pagesCutaneous Manifestations of COVID-19: Review ArticleMudassar SattarNo ratings yet
- COVID-19, Leprosy, and Neutrophils: Neglected Tropical DiseasesDocument5 pagesCOVID-19, Leprosy, and Neutrophils: Neglected Tropical Diseasesanis utamiNo ratings yet
- Skin Lessions Covid-19Document15 pagesSkin Lessions Covid-19JUAN PABLO DE JESUS CASTRO MORANo ratings yet
- COVID-19: Pathophysiology of Acute Disease 1: SeriesDocument21 pagesCOVID-19: Pathophysiology of Acute Disease 1: SeriesMyatnoe KhinNo ratings yet
- Pi Is 0190962214011566Document2 pagesPi Is 0190962214011566LewishoppusNo ratings yet
- Cutaneus Manifestation of Coronavirus Disease 2019Document7 pagesCutaneus Manifestation of Coronavirus Disease 2019Maria AgathaNo ratings yet
- Adult Measles - Case Reports of A Highly Contagious DiseaseDocument4 pagesAdult Measles - Case Reports of A Highly Contagious DiseaseEchi KaruniaNo ratings yet
- COVID-19 and Multiorgan ResponsDocument21 pagesCOVID-19 and Multiorgan ResponssamuelNo ratings yet
- Coronavirus (COVID-19) OutbreakDocument5 pagesCoronavirus (COVID-19) OutbreakPrima Sarana MedikaNo ratings yet
- COVID-19 Pandemic and The Skin What Should Dermato PDFDocument13 pagesCOVID-19 Pandemic and The Skin What Should Dermato PDFEka Putri Intan SariNo ratings yet
- Follow Up of Skin Lesions During The Evolution of COVID 19 A CaseDocument4 pagesFollow Up of Skin Lesions During The Evolution of COVID 19 A CaseZihan ZetiraNo ratings yet
- Novel CoronavirusDocument7 pagesNovel CoronavirusMustafa KhudhairNo ratings yet
- Childhood Erythrodermic Lichen Planus Pemphigoides After Nonavalent Human Papillomavirus VaccinationDocument3 pagesChildhood Erythrodermic Lichen Planus Pemphigoides After Nonavalent Human Papillomavirus Vaccinationnurul hidayatiNo ratings yet
- Tropicalmed 07 00283 v2Document15 pagesTropicalmed 07 00283 v2St. MatthewGalnayonMa. Alyza KaeNo ratings yet
- Jessica Le - Covid-19Document3 pagesJessica Le - Covid-19api-523306558No ratings yet
- A Systematic Review of Asymptomatic Infections With COVID-19Document5 pagesA Systematic Review of Asymptomatic Infections With COVID-19Delfina RubioNo ratings yet
- A Distinctive Skin Rash LTEDocument2 pagesA Distinctive Skin Rash LTEeva yustianaNo ratings yet
- 2 PBDocument4 pages2 PBPororo KewrenNo ratings yet
- Case Report Rickettsiae Final 3 AuthorsDocument11 pagesCase Report Rickettsiae Final 3 AuthorsNaresh GadaganiNo ratings yet
- Perioperative Management of Suspected/ Confirmed Cases of COVID-19Document13 pagesPerioperative Management of Suspected/ Confirmed Cases of COVID-19Dimensi FKUNHASNo ratings yet
- Perioperative Management of Suspected/ Confirmed Cases of COVID-19Document13 pagesPerioperative Management of Suspected/ Confirmed Cases of COVID-19fahadNo ratings yet
- Atow 422 01 PDFDocument13 pagesAtow 422 01 PDFfahadNo ratings yet
- Covid19 PDFDocument6 pagesCovid19 PDFJeremy Kartika SoeryonoNo ratings yet
- IPArchCytolHistopatholRes 6-2-135 139Document5 pagesIPArchCytolHistopatholRes 6-2-135 139FeliciaDewiNo ratings yet
- Final CCHF Updated Guidelines 2019Document25 pagesFinal CCHF Updated Guidelines 2019dr sarangNo ratings yet
- Ivermectina - Profilaxis Covid-19 - DR HirschDocument8 pagesIvermectina - Profilaxis Covid-19 - DR HirschAlheni Fabiola Miranda GomezNo ratings yet
- Kollipara2016 LeishmaniasisDocument12 pagesKollipara2016 Leishmaniasiseva yustianaNo ratings yet
- Leprosy (Hansen's Disease) : 1. Case Definition 2. Reporting RequirementsDocument8 pagesLeprosy (Hansen's Disease) : 1. Case Definition 2. Reporting RequirementsNinel López VillarrealNo ratings yet
- Am J Otolaryngol: Vural FidanDocument2 pagesAm J Otolaryngol: Vural FidanDhyo Asy-shidiq HasNo ratings yet
- Articulo 3-EritemaDocument4 pagesArticulo 3-EritemaMariuxi CastroNo ratings yet
- 32138+post MP AllDocument26 pages32138+post MP AllAndi ToniNo ratings yet
- Lupus Pernio Like - Covid 19Document7 pagesLupus Pernio Like - Covid 19Laura Ximena TorresNo ratings yet
- تقرير وبائياتDocument6 pagesتقرير وبائياتMustafa KhudhairNo ratings yet
- Mucor in A Viral Land: A Tale of Two Pathogens.: 10.4103/ijo - IJO - 3774 - 20Document3 pagesMucor in A Viral Land: A Tale of Two Pathogens.: 10.4103/ijo - IJO - 3774 - 20nNo ratings yet
- Cutaneous Manifestation of COVID-19 in Images: A Case: JeadvDocument2 pagesCutaneous Manifestation of COVID-19 in Images: A Case: Jeadveva yustianaNo ratings yet
- COVID-19 and Cutaneous Manifestations: ReferencesDocument1 pageCOVID-19 and Cutaneous Manifestations: Referenceseva yustianaNo ratings yet
- Investigatory Project Sample SurveyDocument65 pagesInvestigatory Project Sample SurveySnekha TNo ratings yet
- COVID-19 LEGACY: SARS-CoV-2 clinical trials, vaccines trials and bioethicsFrom EverandCOVID-19 LEGACY: SARS-CoV-2 clinical trials, vaccines trials and bioethicsNo ratings yet
- Mapeh 7 Reading Materials 9 (Health)Document13 pagesMapeh 7 Reading Materials 9 (Health)Jeff LacasandileNo ratings yet
- English G8-Q4-L3 ModuleDocument16 pagesEnglish G8-Q4-L3 ModuleKatrina CloresNo ratings yet
- SARS-CoV-2 Viremia Is Associated With Distinct Proteomic Pathways and Predicts COVID-19 OutcomesDocument13 pagesSARS-CoV-2 Viremia Is Associated With Distinct Proteomic Pathways and Predicts COVID-19 OutcomesYoga Nugraha WNo ratings yet
- Vinmec Services Marketing ReportDocument32 pagesVinmec Services Marketing ReportNgô Hồng Giang 2M-20ACN100% (1)
- Introduction To CovidDocument3 pagesIntroduction To CovidJohn Leonard AbarraNo ratings yet
- HealthiansDocument8 pagesHealthianstanvi tanviNo ratings yet
- Health 8 Q3 Module 4 Week 4Document15 pagesHealth 8 Q3 Module 4 Week 4chingloycasicasNo ratings yet
- 13mar2020 Week11Document16 pages13mar2020 Week11cerejagroselhaNo ratings yet
- The Impact of Sex and Gender in The Covid-JessaDocument10 pagesThe Impact of Sex and Gender in The Covid-JessaDENMARKNo ratings yet
- 1099 Tax Credit For LandscapersDocument1 page1099 Tax Credit For LandscapersCraig Pisaris-HendersonNo ratings yet
- Verify Gov SG PDFDocument4 pagesVerify Gov SG PDFJanice JuarezNo ratings yet
- Evaluation of Platelet Count and Platelet Indices in Covid-19 InfectionDocument6 pagesEvaluation of Platelet Count and Platelet Indices in Covid-19 InfectionIJAR JOURNALNo ratings yet
- MD Tanjil 22Document1 pageMD Tanjil 22wasi Wasikhan890yahoo.comNo ratings yet
- Beacon Askari O Level School: Dear Parents/Guardians, (ملاسلا مکیلع)Document1 pageBeacon Askari O Level School: Dear Parents/Guardians, (ملاسلا مکیلع)Hiba AnwerNo ratings yet
- Covid Treatment Guidelines For Homeopathy PractitionersDocument9 pagesCovid Treatment Guidelines For Homeopathy PractitionersQuality Glass Industries Pvt LtdNo ratings yet
- English 10 Week 2 - Task 6-7Document2 pagesEnglish 10 Week 2 - Task 6-7Glenda FernandoNo ratings yet
- 57-Article Text-231-1-10-20220829Document4 pages57-Article Text-231-1-10-20220829Đơn TThiNo ratings yet
- Proposal BHS InggrisDocument6 pagesProposal BHS InggrisRadeva EdelinaNo ratings yet
- Colorado Department of Public Health and Environment: November 14 Public-Health Order UpdateDocument9 pagesColorado Department of Public Health and Environment: November 14 Public-Health Order UpdateMichael_Roberts2019No ratings yet
- 2186 457 PB 1Document118 pages2186 457 PB 1EtikNo ratings yet
- CertificateDocument1 pageCertificateRamdhun SinghNo ratings yet
- Abstracts From The 6th International Conference On Prevention & Infection Control (ICPIC 2021)Document140 pagesAbstracts From The 6th International Conference On Prevention & Infection Control (ICPIC 2021)Karen RodriguezNo ratings yet
- Motivation During PandemicDocument14 pagesMotivation During PandemicMaria Karen VillapeñaNo ratings yet
- Certificate For COVID-19 Vaccination: Beneficiary DetailsDocument1 pageCertificate For COVID-19 Vaccination: Beneficiary DetailsShristi GuptaNo ratings yet
- The Lancet JournalDocument2 pagesThe Lancet JournalAleaAVKNo ratings yet
- Learner'S Activity Sheet Grade12 - Inquiries, Investigations, and ImmersionsDocument5 pagesLearner'S Activity Sheet Grade12 - Inquiries, Investigations, and ImmersionsValencia John EmmanuelNo ratings yet
- EUA Ihealth RapidTestAg ifuHT - 0 - 0Document14 pagesEUA Ihealth RapidTestAg ifuHT - 0 - 0thehumansyndromeNo ratings yet
- Maklumat Vaksinasi: Vaccination DetailsDocument2 pagesMaklumat Vaksinasi: Vaccination DetailsWana IrzuanaNo ratings yet
- Sema4 COVID 19 FAQDocument2 pagesSema4 COVID 19 FAQEduardo MongeNo ratings yet
- DOH AprilDocument4 pagesDOH AprilMingNo ratings yet