Professional Documents
Culture Documents
ASSIGNMENT
ASSIGNMENT
Uploaded by
nur farhana0 ratings0% found this document useful (0 votes)
3 views1 pageOriginal Title
ASSIGNMENT (1)
Copyright
© © All Rights Reserved
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
Download as doc, pdf, or txt
0 ratings0% found this document useful (0 votes)
3 views1 pageASSIGNMENT
ASSIGNMENT
Uploaded by
nur farhanaCopyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
Download as doc, pdf, or txt
You are on page 1of 1
ASSIGNMENT
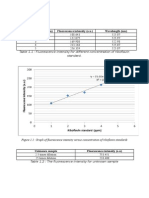
To four 10.0 mL aliquots of water sample were added 1.50, 3.00 and 4.50 mL of
standard NaF solution containing 15.0 ppb. Exactly 3.00 mL of solution containing an
excess of Al-acid Alizarin Garnet R complex, a strongly fluorescent complex, were
added to each and solutions were diluted to 50.00 mL. The fluorescent intensity of the
four solutions plus a blank were as
mL Sample mL of Standard F‒ Meter Reading
10.00 0.00 65
10.00 1.50 52
10.00 3.00 39
10.00 4.50 27
(i) Explain the chemistry of the analytical method.
(ii) Plot the data.
(iii) By least squares compute the regression parameters of the linear equation
relating the decrease in fluorescence to the volume of standard reagent.
(iv) Calculate the standard deviation of the slope.
(v) Calculate the concentration of F‒ in ppb in the sample.
You might also like
- O Level Biology Practice Questions And Answers EnzymesFrom EverandO Level Biology Practice Questions And Answers EnzymesRating: 5 out of 5 stars5/5 (1)
- Chapter 16 Aqueous Ionic Equilibrium: Chemistry: A Molecular Approach, 3e (Tro)Document38 pagesChapter 16 Aqueous Ionic Equilibrium: Chemistry: A Molecular Approach, 3e (Tro)Lilian WeitzelNo ratings yet
- Acid Base Titration Lab H2so4 + Naoh AP Chem 2Document3 pagesAcid Base Titration Lab H2so4 + Naoh AP Chem 2Neen Naaz100% (1)
- A-MDEA Analytical MethodsDocument8 pagesA-MDEA Analytical MethodsSHYAMKANHAIYA100% (3)
- Advanced Pharmaceutical analysisFrom EverandAdvanced Pharmaceutical analysisRating: 4.5 out of 5 stars4.5/5 (2)
- Experiment 4Document2 pagesExperiment 4MirraDeJesusNo ratings yet
- Eplerenone (2765)Document2 pagesEplerenone (2765)Mulayam Singh YadavNo ratings yet
- Problem Set 1Document3 pagesProblem Set 1Lu JunqueiraNo ratings yet
- Acid-Base Equilibrium FR WorksheetDocument4 pagesAcid-Base Equilibrium FR WorksheetKen RubioNo ratings yet
- Determination of Aspirin by Indirect Titration: J. Elijah, J. Paderes, S. NotaDocument4 pagesDetermination of Aspirin by Indirect Titration: J. Elijah, J. Paderes, S. NotaSteffi Grace NotaNo ratings yet
- Buffer Equilibrium FR WorksheetDocument11 pagesBuffer Equilibrium FR WorksheetKen RubioNo ratings yet
- Lecture1 CH317 2020 Fall Statistics Part4 GGclasssDocument21 pagesLecture1 CH317 2020 Fall Statistics Part4 GGclasssshanicejames7867No ratings yet
- Ion-Selective Electrode Determination of Fluoride Ion: Chemistry 321L ManualDocument5 pagesIon-Selective Electrode Determination of Fluoride Ion: Chemistry 321L Manuallsueyin100% (1)
- TB 85titration 61edfa70ef6ee4.61edfa733da046.15966159Document33 pagesTB 85titration 61edfa70ef6ee4.61edfa733da046.15966159任思诗No ratings yet
- Acid Base Tit RationsDocument2 pagesAcid Base Tit RationsIra MunirahNo ratings yet
- LorazepamDocument2 pagesLorazepamAlexi Del Castillo MustaineNo ratings yet
- Titration Lab ReportDocument13 pagesTitration Lab Reportapi-341133750No ratings yet
- 21Rifampicin-Revision - QAS11-44 - FINAL - PDF: Uji Asetat A. Uji Dengan H SO EncerDocument1 page21Rifampicin-Revision - QAS11-44 - FINAL - PDF: Uji Asetat A. Uji Dengan H SO EncerTita DiarniNo ratings yet
- Chem 26 Experiment 4Document10 pagesChem 26 Experiment 4Ayn Forest JoanNo ratings yet
- Experiment No. 1-2-3 - 4 Winter SemDocument19 pagesExperiment No. 1-2-3 - 4 Winter SemAgnivesh SharmaNo ratings yet
- Expt-4 PotentiometryDocument3 pagesExpt-4 PotentiometryAhyessa CastilloNo ratings yet
- Analysis of Solvent Systems Used For The Removal of Water Formed Deposits 2Document6 pagesAnalysis of Solvent Systems Used For The Removal of Water Formed Deposits 2Shad AhmadNo ratings yet
- Titration Curves of Acid-Base and Determination of PH and Concentration From The Equivalence PointDocument24 pagesTitration Curves of Acid-Base and Determination of PH and Concentration From The Equivalence PointMiguel Angel Roldan MartinNo ratings yet
- Potentiometric Determination of Phosphoric Acid in Unknown SampleDocument7 pagesPotentiometric Determination of Phosphoric Acid in Unknown SamplekahullanyNo ratings yet
- Potentiometric Determination of Phosphor PDFDocument7 pagesPotentiometric Determination of Phosphor PDFFlex GodNo ratings yet
- CHM 221 Review 2Document1 pageCHM 221 Review 2Bryan LeNo ratings yet
- General Chemistry Lab Study GuideDocument2 pagesGeneral Chemistry Lab Study GuideSannam Sara ElazariNo ratings yet
- AP DPS On Buffer Capacity 8.10 - 2022Document3 pagesAP DPS On Buffer Capacity 8.10 - 2022chrisddonald2No ratings yet
- Experiment No. 3 Volumetric TransferDocument14 pagesExperiment No. 3 Volumetric TransferJoemar SubongNo ratings yet
- Lab Lecture 1Document13 pagesLab Lecture 1shiamNo ratings yet
- Concentration)Document3 pagesConcentration)CoibaNo ratings yet
- LEC Quiz 3Document1 pageLEC Quiz 3GNo ratings yet
- Buffers&titrationsquestions ReviewDocument6 pagesBuffers&titrationsquestions Reviewapi-279595789No ratings yet
- Determination of Acidity and Alkalinity: Experiment No: DateDocument5 pagesDetermination of Acidity and Alkalinity: Experiment No: DateNanditha ANo ratings yet
- Titrations in Analytical ChemistryDocument30 pagesTitrations in Analytical Chemistrynorsiah67% (3)
- TB 82pHandpOHofstrongacidandbase 61edf8eca0b257.61edf8edc066f0.51892966Document6 pagesTB 82pHandpOHofstrongacidandbase 61edf8eca0b257.61edf8edc066f0.51892966任思诗No ratings yet
- Quinine FluorescenceDocument4 pagesQuinine FluorescenceDrGajanan VaishnavNo ratings yet
- Experiment No. 5 Spectrophotometric Determination of Phosphate in DetergentDocument2 pagesExperiment No. 5 Spectrophotometric Determination of Phosphate in DetergentIanaNo ratings yet
- Acesulfame Potassium USPDocument2 pagesAcesulfame Potassium USPДарія ОсадчаNo ratings yet
- Experiment 9 (UV-Vis) - Lab ManualDocument2 pagesExperiment 9 (UV-Vis) - Lab ManualJoseph JoeNo ratings yet
- Review Unit 10 Test CHP 17Document13 pagesReview Unit 10 Test CHP 17TechnoKittyKittyNo ratings yet
- Titration - Questions 1 PDFDocument17 pagesTitration - Questions 1 PDFsaha khanNo ratings yet
- Redox Titration PracticalDocument8 pagesRedox Titration Practicaltabbykaranja080No ratings yet
- تقرير 4Document10 pagesتقرير 4ليث علي احمد حريفشNo ratings yet
- Acesulfame PotassiumDocument2 pagesAcesulfame PotassiumJhoan Custodio MancillaNo ratings yet
- Weak Acid Strong Base Titration Teacher NotesDocument4 pagesWeak Acid Strong Base Titration Teacher Notesrasoulinima539No ratings yet
- © Ncert Not To Be Republished: Hemical QuilibriumDocument7 pages© Ncert Not To Be Republished: Hemical QuilibriumSoumik MukhopadhyayNo ratings yet
- Applied Chemistry Lab - CHEM-320: Lab Report # 10 (Semester 6 2021) Submission Date: 30-May, 2021Document6 pagesApplied Chemistry Lab - CHEM-320: Lab Report # 10 (Semester 6 2021) Submission Date: 30-May, 2021AeeshaNo ratings yet
- Total AlkalinityDocument7 pagesTotal Alkalinityfakher adnanNo ratings yet
- ProcedureDocument3 pagesProcedureroshanjimaxoutNo ratings yet
- CHM256 - Tutorial 5Document2 pagesCHM256 - Tutorial 5Fatimah Azzahrah0% (1)
- Results For Experiment 3Document5 pagesResults For Experiment 3Syahriezan HaminNo ratings yet
- Soal + Pembahasan Volumetri UI 2023Document6 pagesSoal + Pembahasan Volumetri UI 2023Muhammad Ihsanul FikryNo ratings yet
- Btech Lab Manual With Assignments - 240303 - 163040Document34 pagesBtech Lab Manual With Assignments - 240303 - 163040devrajmaji457No ratings yet
- 2,4 Dichlorobenzyl AlcoholDocument2 pages2,4 Dichlorobenzyl AlcoholsamanehNo ratings yet
- 695 FullDocument3 pages695 FullZoya ShaikhNo ratings yet
- Ibuprofen (Pharmacopoea)Document3 pagesIbuprofen (Pharmacopoea)Titis Adisti HapsariNo ratings yet
- Spectrophotometric Determination of Iron in Aqueous Solutions As A Complex of 1,10-PhenanthrolineDocument4 pagesSpectrophotometric Determination of Iron in Aqueous Solutions As A Complex of 1,10-PhenanthrolineJaimie LojaNo ratings yet
- Ap11 Chemistry Form B q5Document8 pagesAp11 Chemistry Form B q5jessieNo ratings yet
- Practical Manual of Analytical ChemistryFrom EverandPractical Manual of Analytical ChemistryRating: 4.5 out of 5 stars4.5/5 (3)